Abstract
Context: A number Hypericum species are well known for their therapeutic efficacy and use in traditional medicine. The various species of Hypericum have been traditionally used for the treatment of wounds, eczema, burns, trauma, rheumatism, neuralgia, gastroenteritis, ulcers, hysteria, bedwetting and depression.
Objective: This study evaluated the in vitro antioxidant, antibacterial and phytochemical properties of essential oils of Hypericum helianthemoides (Spach) Boiss., Hypericum perforatum L. and Hypericum scabrum L. (Hypericaceae) collected from alpine region of Southwest Iran.
Materials and methods: The essential oils obtained from dried flowering aerial parts of three Hypericum species were analyzed by gas chromatography and gas chromatography/mass spectrometry to determine chemical compositions. The antibacterial activity of essential oils within concentration ranges from 16 to 500 µg/mL was individually evaluated against Bacillus cereus, Listeria monocytogenes. Proteus vulgaris and Salmonella typhimurium. The 1,1-diphenyl-2-picrilhydrazyl (DPPH) radical scavenging activity of essential oils was determined using DPPH assay.
Results: Essential oil yield of H. helianthemoides. H. scabrum and H. perforatum were 0.12, 0.20 and 0.21 mL/100 g dried material, respectively. The major constituents of the essential oils were α-pinene (12.52–49.96%), β-pinene (6.34–9.70%), (E)-β-ocimene (4.44–12.54%), β-caryophyllene (1.19–5.67%), and germacrene-D (2.34–6.92%). The essential oils of three Hypericum species indicated moderate-to-good inhibitory activities against four bacteria, especially against L. monocytogenes.
Discussion and conclusion: The essential oils of the three studied Hypericum species sourced in alpine region of West Iran were rich in monoterpene and sesquiterpenes hydrocarbons. Among the three tested species, the essential oil of H. scabrum showed the highest antibacterial and antioxidant activities.
Introduction
Hypericum L., an important genus in the family Hypericaceae, includes 484 species of herbs, shrubs and trees (Crockett & Robson, Citation2011). This genus, which grows in temperate regions, includes many species with traditional value as medicinal plants for treating wounds, eczema and burns (Yazaki & Okada, Citation1994). Many Hypericum species are used as treatment of trauma, rheumatism, neuralgia, gastroenteritis, ulcers, hysteria, bedwetting and depression (Miller, Citation1998). Hypericum species have also been used for sedative, anti-inflammatory and antiseptic effects (Baytop, Citation1984; Mukherjee & Suresh, Citation2000; Ozturk et al., Citation2002).
Primary constituents of Hypericum species include hypericin, tannins, flavonoids, phenolic acids, quercitrin, hyperoside, isoquercitrin, chlorogenic acid and rutin (Barnes et al., Citation2001; Dall'Agnol et al., Citation2003). Bioactivity properties of the plants include antimicrobial (Crockett, Citation2010; Dall'Agnol et al., Citation2003; Jayasuriya et al., Citation1989), anticancer (Agostinis et al., Citation2002), antidepressant (Butterweck et al., Citation2002), antiviral (Meruelo et al., Citation1988), antioxidant (Cakir et al., Citation2003), cytotoxic (Jayasuriya et al., Citation1989) and antifungal activities (Cakir et al., Citation2005; Fenner et al., Citation2005).
The essential oils of Hypericum species grown in different regions of the world have been extensively examined by gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS). For example, Hypericum scabrum L. and Hypericum perforatum L. in Turkey (Cakir et al., Citation1997; Erken et al., Citation2001), Hypericum olympicum L. and H. perforatum in Serbia (Gudzic et al. Citation2001) and H. scabrum and H. perforatum in Uzbekistan (Baser et al., Citation2002). Typical essential oil constituents for Hypericum species include the monoterpenes α- and β-pinene, limonene and myrcene; the sesquiterpenes β-caryophyllene and caryophyllene oxide; and hydrocarbons such as n-decane, C16- and C29 alkanes and C24, C26 and C28 alkanols (Crockett, Citation2010; Nahrstedt & Butterweck, Citation1997). A study by Javidnia et al. (Citation2008) indicated that the major constituent of H. scabrum collected from Fars (South Iran) was α-pinene, while the main constituents of Hypericum helianthemoides (Spach) Boiss., collected also from Fars (South Iran), were β-caryophyllene (23.3%) and spathulenol (17.4%). Motavalizadehkakhky (Citation2012) reported the main constituents of essential oils of H. scabrum, H. hyssopifolium Chaix, H. helianthemoides and H. perforatum flowers collected from North-East Iran (Khorasan) were α-pinene and β-caryophyllene.
Species of this genus growing wild in western and northern parts of Iran include H. helianthemoides. H. perforatum and H. scabrum, which are perennial herbs, widely distributed at high altitude regions in Iran (Rechinger, Citation1963–1998). This study was designed to elucidate the chemical compositions, antibacterial and antioxidant activities of the essential oils of H. helianthemoides. H. scabrum and H. perforatum growing wild in Bakhtiari Zagros Mountains in Iran.
Materials and methods
Plant materials
Inflorescences of H. helianthemoides. H. scabrum and H. perforatum were collected during the mid-flowering stage from five plants of each species in three replications in July, 2012, the Bakhtiari Zagros Mountains (latitude 31° 20′ to 31° 50′; longitude 50° 50′ to 51° 05′; 2700 to 3000 m above sea level), Chaharmahal va Bakhtiari province, Iran. Plant identities were confirmed by Prof. V. Mozaffarian, and voucher specimens have been placed in the Herbarium of Research Center of Agriculture and Natural Resources, Chaharmahal va Bakhtiari, Iran (H. helianthemoides 1334; H. perforatum 1335; and H. scabrum. 1336).
The fresh samples of H. helianthemoides. H. scabrum and H. perforatum were dried inside for five days at room temperature (25 ± 5 °C), and ground to fine a powder using Moulinex food processor (Paris, France) and passed through a 20 mesh sieve to remove large pieces of debris. The ground samples were subsequently dried to a constant weight over a desiccant (Na2SO4) at room temperature (30 °C). The essential oil was extracted from 30 g of sample of tissue in 300 mL of water contained in a 500 mL flask and heated by heating jacket at 100 °C for 3 h in a Clevenger-type apparatus according to the British Pharmacopoeia (British Pharmacopoeia Commission, Citation1988). The collected essential oil was dried over anhydrous sodium sulfate and stored at 4 °C until analyzed.
Identification of the oil constituents
Composition of the essential oils were determined by GC and GC/MS. GC analysis was done on an Agilent Technologies 7890 GC equipped with flame ionization detector and a HP-5MS 5% capillary column (30.00 m × 0.25 mm, 0.25 µm film thicknesses). The carrier gas was helium at a flow of 0.8 mL/min. Initial column temperature was 60 °C and programmed to increase at 4 °C/min to 280 °C. The split ratio was 40:1. The injector temperature was set at 300 °C. The purity of helium gas was 99.999%, and 0.1 µL samples were injected manually in the split mode.
GC/MS analysis was done on the mentioned Agilent Technologies 5975 mass system. Mass spectra were recorded at 70 eV. Mass range was from m/z 50–550. Constituents were identified by comparison of their Kovats index (KI) relative to C5–C24 n-alkanes obtained on a nonpolar DB-5MS column by comparison of the KI, provided in the literature, by comparison of the mass spectra with those recorded by the NIST 08 (National Institute of Standards and Technology, Gaithersburg, MD) and ChemStation Data System (WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany). The individual constituents were identified by retention indices and compared with constituents known from the literature (Adams, Citation2007; McLafferty, Citation2009). The percentage composition was computed from the GC peak areas without using any correction factors.
Antibacterial test
The four bacteria used as test organisms were as follows: Bacillus cereus. Listeria monocytogenes. Proteus vulgaris and Salmonella typhimurium. The bacteria strains were obtained from Food Microbiology Laboratory, Veterinary Medicine Faculty (I.A.U.), Iran. Bacterial strains were identified using polymerase chain reaction-restriction fragment length polymorphism and conventional morphological and biochemical tests. The density of bacteria culture required for the test was adjusted to 1.0 McFarland standards (1.0 × 107 colony-forming units (CFU)/mL) and measured using a spectrophotometer (Eppendorf AG, Hamburg, Germany). The minimum inhibitory concentration (MIC) values were evaluated using the broth serial dilution method according to standard methods (CLSI, Citation2012). Bacterial strains were cultured overnight at 37 °C in Muller Hinton broth (MHB). The essential oils and antimicrobial standards (ampicillin, ciprofloxacin and flumequine) dissolved in 5% dimethyl sulfoxide were first diluted to the highest concentration (500 µg/mL) to be tested, and then series of two-fold dilutions were made in a concentration range from 16 to 500 µg/mL in 10 mL sterile test tubes containing MHB. After incubation at 37 °C for 24 h, absorbance at 630 nm was used as a measurement of bacterial growth using a spectrophotometer (Zampini et al., Citation2005). The minimum bactericidal concentration (MBC) of essential oil were determined according to the MIC values, i.e., 5 µL from MIC tubes were transferred to agar plates and incubated at 37 °C for 24 h. The MBC was referred to the minimum concentration of essential oil with no viable bacteria. Experiments were performed at three different times.
Antioxidant test
The 1,1-diphenyl-2-picrilhydrazyl (DPPH) radical scavenging activity of essential oils was determined using the method proposed by Hung et al. (Citation2005). The essential oils at different concentrations (16–500 µg/mL) were mixed with the same volume of 0.2 mM methanol solution of DPPH. The disappearance of DPPH by essential oils after 30 min of incubation at room temperature was determined spectrophotometrically at 515 nm. Methanol was used to zero the spectrophotometer. The absorbance of the DPPH radical without antioxidant, i.e., the control, was measured daily. The amount of sample necessary to decrease the absorbance of DPPH by 50% (IC50) was calculated graphically. The percentage inhibition was calculated according to the equation:
where AC(0) is the absorbance of the control at t = 0 min; and AA(t) is the absorbance of the antioxidant at t = 30 min. The food preservative butylated hydroxyanisole was used as positive control.
Statistical analyses
The data were statistically analyzed by SPSS (19.0) software (SPSS Inc., Chicago, IL) using a completely randomized design. Means of the traits were separated by Duncan’s multiple range test at p ≤ 0.05 level.
Results and discussion
Oil yield
All essential oils extracted from the flowering aerial parts of H. helianthemoides. H. scabrum and H. perforatum produced a clear, yellow liquid. An analysis of variance indicated no significant differences in oil yields obtained from the three Hypericum species. The essential oil yields were 0.12, 0.20 and 0.21 mL/100 g dry matter for H. helianthemoides. H. scabrum and H. perforatum, respectively (). Hypericum species are generally classed as essential oil-poor plants (oil yields generally <1%, w/w) (Roth, Citation1990). Gudzic et al. (Citation2001) reported the oil yield of H. perforatum collected from Serbia was 0.32% (w/w). The yield of the oils extracted from Hypericum species was: 0.97% for leaves and 1.30% (w/w) for flowers of H. androsaemum L. from Iran (Morteza-Semnani & Saeedi, Citation2005), 0.10% (w/w) for H. perfoliatum and 0.13% for H. tomentosum L. (Hosni et al., Citation2008) and 0.2% for H. scabrum and 0.1% (w/w) for H. perforatum from Uzbekistan (Baser et al., Citation2002). Javidnia et al. (Citation2008) reported the oil yields of H. scabrum. Hypericum dogonbadanicum Assadi, H. helianthemoides and H. hirtellum (Spach) Boiss., collected from south Iran (Fars province), were 0.05, 0.10, 0.06 and 0.07%.
Table 1. Identified constituents of the essential oils from the three studied Hypericum species.
Chemical compositions
In total, 33, 31 and 48 volatile constituents were identified representing 95, 96 and 89% of total volatiles in the essential oils of H. helianthemoides. H. scabrum and H. perforatum, respectively (). The main constituents in the volatile oil of H. helianthemoides were α-pinene (31.9 ± 1.9%), (E)-β-ocimene (12.5 ± 1.0%), β-phellandrene (8.4 ± 1.1%), β-pinene (6.3 ± 0.4%), β-caryophyllene (5.7 ± 0.4%) and germacrene-D (4.3 ± 0.7%). Javidnia et al. (Citation2008) identified 71 constituents in the volatile oil of H. helianthemoides from south Iran. The major constituents were β-caryophyllene (23.3%), spathulenol (17.4%), 14-hydroxy-9-epi-(E)-caryophyllene (15.6%) and α-pinene (6.7%) (Javidnia et al., Citation2008). A report by Ferretti et al. (Citation2005) indicated the main constituent in the oil of H. richeri Vill. from Italy was (E)-β-ocimene (19.5%). The major constituents in the volatile oil of H. scabrum were α-pinene (50.0 ± 7.6%), β-pinene (9.7 ± 3.7%), limonene (6.6 ± 2.9%), (E)-β-ocimene (5.6 ± 1.8%) and carvacrol (5.8 ± 3.1%). Results of a study by Javidnia et al. (Citation2008) indicated that the main constituent in H. scabrum oil was α-pinene (59.3%); our results are in agreement with this report. Results from other studies (Javidnia et al., Citation2008; Sajjadi et al., Citation2001) indicated the major constituents of H. dogonbadanicum oils were α-pinene, β-pinene, limonene and camphene. The main constituents in the volatile oil of H. perforatum were α-pinene (12.5 ± 1.0%), β-pinene (8.3 ± 1.7%), undecane (7.0 ± 0.5) and germacrene-D (6.9 ± 0.1%). Many studies on the essential oil content of H. perforatum indicate the enormous variability inherent in the volatile chemistry of this species (Crockett, Citation2010).
Our results showed significant differences among the three studied Hypericum species for percentages of α-pinene, β-myrcene, limonene, α-terpinene, (E)-β-ocimene and germacrene-D, while no significant differences among the three studied Hypericum species for percentages of β-pinene and carvacrol were apparent (). The highest percentage of α-pinene was observed in H. scabrum, while the lowest percentage was obtained from H. perforatum (). Similarly, α-pinene is the major constituents in other Hypericum species (Baser et al., Citation2002; Couladis et al., Citation2001; Crockett et al., Citation2007; Javidnia et al., Citation2008; Nogueira et al., Citation2008; Yuce & Bagci, Citation2012). Akhbari et al. (Citation2012) reported that the content of α-pinene differs greatly between the essential oil extracted from flowers (70.2%) and fruit (25.4%) of both H. perforatum and H. scabrum growing wild in Iran. An examination of H. androsaemum by Giuliani et al. (Citation2010) indicated that the marginal glands of leaves contained β-caryophyllene and germacrene-B as the dominant volatile constituents, while the laminar glands contained mainly β-pinene and limonene.
Generally, monoterpene hydrocarbons (31–81%) and sesquiterpenes hydrocarbons (6.2–32.3%) were the main chemical groups in the volatile oils from the three Hypericum species tested in this study (). Guedes et al. (Citation2012) reported the main essential oil constituents identified in 40 species of Hypericum were monoterpenoids (α-pinene and β-pinene) and sesquiterpenoids (E-caryophyllene, germacrene-D, caryophyllene oxide, spathulenol and globulol) constituents. Characterization of essential oil from species of the Hypericum genus revealed the presence of monoterpenoid and sesquiterpenoid constituents, as well as alkanes and aldehydes, as the main constituents in the most of the plants (Mathis & Ourisson, Citation1964a,Citationb,Citationc). Our results are in agreement with those of others reporting monoterpenes as the main constituents.
Figure 1. Comparison of main chemical groups (%) of the essential oils of the three studies Hypericum species.
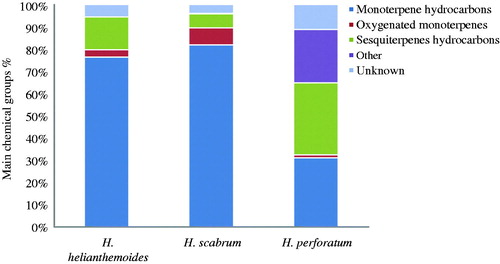
A comparison of our results with the previous reports on the chemical composition of Hypericum species suggests differences in the volatile composition of the plant material could be attributed to genetic (genus, species, sub species and ecotype), chemotype, distinct environmental and climatic conditions, seasonal sampling periods, geographic origins, plant populations, vegetative plant phases and extraction and quantification methods (Ghasemi Pirbalouti et al., Citation2013; Guedes et al., Citation2004; Petrakis et al., Citation2005; Radusiene et al., Citation2005; Southwell & Bourke, Citation2001; Teixeira et al., Citation2013).
Antibacterial and antioxidants activities
An antibacterial test of the essential oils from the three Hypericum species indicated relatively high inhibitory activities against four pathogenic bacteria tested (). The MICs of the essential oils were within concentration ranges from 62 to 250 µg/mL and the respective MBCs were from 250 to 500 µg/mL. The essential oil from H. scabrum had higher inhibitory activity against bacteria than the essential oil from the other two Hypericum species. The antibacterial activity of H. scabrum oil could be attributed to the relatively high level of α-pinene, a constituent with known antimicrobial properties (Stojkovic et al., Citation2008). Lipophilic constituents, including terpenoid derivatives, have been shown to disrupt cellular membranes in bacteria and fungi, thus inhibiting cellular respiration and ionic transport (Hayouni et al., Citation2008). The mechanisms by which essential oil can inhibit microorganisms vary. In some cases, it may be due to the hydrophobicity of the chemical (oil), which penetrates into the lipid bilayer of the cell membrane and makes the cells more permeable, leading to leakage of vital cell contents (Burt, Citation2004). The essential oil constituents move into the membrane, causing swelling and reducing membrane function that leads to cell death (Holly & Patel, Citation2005). Antimicrobial activity of essential oils may be due to the presence of synergy between the major constituents and other constituents of the oils leading to various degrees of antimicrobial activity.
Table 2. MICs and MBCs (µg/mL) of the essential oils of the three studied Hypericum species against four food-borne pathogens.
Free radicals cause auto-oxidation of unsaturated lipids in food (Kaur & Perkins, Citation1991), and the antioxidant activity of essential oils could be attributed to their hydrogen donating ability. Antioxidant properties are very important in counteracting the deleterious role of free radicals in foods or biological systems. The antioxidant activity of essential oils of the three studied Hypericum species are expressed as IC50. A low IC50 value indicates an active ability of the oil to act as a DPPH scavenger (). The highest antioxidant activity of H. scabrum oil could be attributed to the relatively high level of α-pinene.
Figure 2. Antioxidant activity of the essential oils of the three studies Hypericum species using DPPH assay.
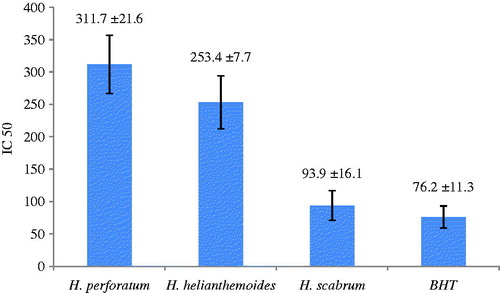
In conclusion, a comparison of our results with other reports on essential oil constituents and biological activities of H. helianthemoides. H. perforatum and H. scabrum demonstrated these species have considerable variation in essential oil compositions and biological activities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adams RP. (2007). Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream (IL): Allured
- Agostinis P, Vantieghem A, Merlevede W, De WPAM. (2002). Hypericin in cancer treatment: More light on the way. Int J Biochem Cell Biol 34:221–41
- Akhbari M, Batooli H, Mozdianfard M. (2012). Comparative study of composition and biological activities of SDE prepared essential oils from flowers and fruits of two Hypericum species from central Iran. Nat Prod Res 26:193–202
- Barnes J, Anderson LA, Phillipson JD. (2001). St John's wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol 53:583–600
- Baser KHC, Ozek T, Nuriddinov HR, Demirci AB. (2002). Essential oils of two Hypericum species from Uzbekistan. Chem Nat Compd 38:54–7
- Baytop T. (1984). Therapy with Medicinal Plants in Turkey. Istanbul, Turkey: Istanbul Univ. Pub. No. 3255, 166–7
- British Pharmacopoeia Commission. (1988). British Pharmacopoeia. Vol. 4. London: HMSO, 137–8
- Burt S. (2004). Essential oils: Their antibacterial properties and potential applications in foods. Int J Food Microbiol 94:223–53
- Butterweck V, Bockers T, Korte B, et al. (2002). Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res 930:21–9
- Cakir A, Duru ME, Harmandar M, et al. (1997). Comparison of the volatile oils of Hypericum scabrum L. and Hypericum perforatum L. in Turkey. Flavour Frag J 12:285–7
- Cakir A, Mavi A, Yildirim A, et al. (2003). Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J Ethnopharmacol 87:73–83
- Cakir A, Kordali S, Kilic H, Kaya E. (2005). Antifungal properties of essential oils and crude extracts of Hypericum linarioides. Biochem Syst Ecol 33:245–56
- CLSI (Clinical and Laboratory Standards Institute). (2012). Performance Standards for Antimicrobial Disks Susceptibility Tests: Approved Standards. 11th edn. M02-A11. Wayne (NJ): CLSI
- Couladis M, Baziou P, Petrakis PV, Harvala C. (2001). Essential oil composition of Hypericum perfoliatum L. growing in different locations in Greece. Flavour Fragr J 16:204–6
- Crockett S, Demirci B, Baser K, Khan I. (2007). Analysis of the volatile constituents of five African and Mediterranean Hypericum L. (Clusiaceae, Hypericoideae) species. J Essen Oil Res 19:302–6
- Crockett SL. (2010). Essential oil and volatile components of the genus Hypericum (Hypericaceae). Nat Prod Commun 5:1493–506
- Crockett SL, Robson NKB. (2011). Taxonomy and chemotaxonomy of the genus Hypericum. Medicinal and aromatic plant science and biotechnology. Global Sci Books 5:1–13
- Dall'Agnol R, Ferraz A, Bernardi AP, et al. (2003). Antimicrobial activity of some Hypericum species. Phytomedicine 10:511–16
- Erken S, Malyer H, Demirci F, et al. (2001). Chemical investigations on some Hypericum species growing in Turkey-I. Chem Nat Compd 37:434–8
- Fenner R, Sortino M, Kuze Rates SM, et al. (2005). Antifungal activity of some Brazilian Hypericum species. Phytomedicine 12:236–40
- Ferretti G, Maggi F, Tirillini B. (2005). Essential oil composition of Hypericum richeri Vill. from Italy. Flavour Fragr J 20:295–8
- Ghasemi Pirbalouti A, Hashemi M, Taherian Ghahfarokhi F. (2013). Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind Crop Prod 48:43–8
- Giuliani C, Pellegrino RM, Tirillini B, Bini LM. (2010). The role of secreting structures position on the leaf volatile organic compounds of Hypericum androsaemum. Nat Prod Commun 5:107–10
- Gudzic B, Dordevic S, Palic R, Stojanovic G. (2001). Essential oils of Hypericum olympicum L. and Hypericum perforatum L. Flavour Frag J 16:201–3
- Guedes AP, Amorim LR, Vicente A, Fernandes-Ferreira M. (2004). Variation of the essential oil content and composition in leaves from cultivated plants of Hypericum androsaemum L. Phytochem Anal 15:146–51
- Guedes AP, Franklin G, Fernandes-Ferreira M. (2012). Hypericum sp.: Essential oil composition and biological activities. Phytochem Rev 11:127–52
- Hayouni EA, Bouix M, Abedrabba M, et al. (2008). Mechanism of action of Melaleuca armillaris (Sol. ex Gaertu) Sm. essential oil on six LAB strains as assessed by multiparametric flow cytometry and automated microtiter based assay. Food Chemistry 111:707–18
- Holly RA, Patel D. (2005). Improvement in shelf life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Chem 22:273–92
- Hosni K, Msaaˆda K, Ben Taaˆrit M, et al. (2008). Essential oil composition of Hypericum perfoliatum L. and Hypericum tomentosum L. growing wild in Tunisia. Ind Crops Prod 27:308–14
- Hung D, Ou B, Prior RL. (2005). The chemistry behind antioxidant capacity assay. J Agric Food Chem 53:1841–56
- Javidnia K, Miri R, Soltani M, et al. (2008). Essential oil composition of four Hypericum species from Iran. Chem Nat Compd 44:374–7
- Jayasuriya H, McChesney JD, Swanson SM, Pezzuto JM. (1989). Antimicrobial and cytotoxic activity of rottlerin-type compounds from Hypericum drummondi. J Nat Prod 52:325–31
- Kaur H, Perkins J. (1991). The free radical chemistry of food additives. In: Aruoma OI, Halliwell B, eds. Free Radicals and Food Additives. London, UK: Taylor and Francis Ltd, 17–35
- Mathis C, Ourisson G. (1964a). Etude chimio-taxonomique du genre Hypericum: II. Identification de constituants de diverses huiles essentielles d’Hypericum. Phytochemistry 3:115–31
- Mathis C, Ourisson G. (1964b). Etude chimio-taxonomique du genre Hypericum: III. Répartition des carbures satures et des monoterpe`nes dans les huiles essentielles d’Hypericum. Phytochemistry 3:133–41
- Mathis C, Ourisson G. (1964c). Etude chimio-taxonomique du genre Hypericum: IV. Repartition des sesquiterpenes, des alcools monoterpeniques et des aldehydes satures dans les huiles essentielles d’Hypericum. Phytochemistry 3:377–8
- McLafferty FW. (2009). Wiley Registry of Mass Spectral Data. 9th ed. Hoboken (NJ): Wiley Inc
- Meruelo D, Lavie G, Lavie D. (1988). Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci USA 85:5230–4
- Miller ND. (1998). St John's wort (Hypericum perforatum): Clinical effects on depression and other conditions. Alter Med Rev 3:18–26
- Morteza-Semnani K, Saeedi M. (2005). The essential oil composition of Hypericum androsaemum L. leaves and flowers from Iran. Flavour Fragr J 20:332–4
- Motavalizadehkakhky AR. (2012). Antimicrobial activity and chemical composition of essential oils of four Hypericum from Khorasan, Iran. J Med Plant Res 6:2478–87
- Mukherjee PK, Suresh B. (2000). The evaluation of wound healing potential of Hypericum hookerianum leaf and stem extracts. J Alter Med Complem 6:61–9
- Nahrstedt A, Butterweck V. (1997). Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry 30:129–34
- Nogueira T, Marcelo-Curto MJ, Figueiredo AC, et al. (2008). Chemotaxonomy of Hypericum genus from Portugal: Geographical distribution and essential oils composition of Hypericum perfoliatum. Hypericum humifusum. Hypericum linarifolium and Hypericum pulchrum. Biochem Syst Ecol 36:40–50
- Ozturk B, Apaydýn S, Goldeli E, et al. (2002). Hypericum triquetrifolium Turra. extract exhibits anti-inflammatory activity in the rat. J Ethnopharmacol 80:207–9
- Petrakis PV, Couladis M, Roussis V. (2005). A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochem Syst Ecol 33:873–98
- Radusiene J, Judzentiene A, Bernotiene G. (2005). Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochem Syst Ecol 33:113–24
- Rechinger KH. (1963–1998). Flora Iranica. Akademische Druck-U. Graz, Austria: Verlagsanstalt, 49, 13–15
- Roth L. (1990). Hypericum, hypericin: Botanik, Inhaltstoffe, Wirkung. Landsberg, Germany: Ecomed Verlagsgesellschaft GmbH
- Sajjadi SE, Rahiminezhad MR, Mehregan I, Poorassar A. (2001). Constituents of essential oil of Hypericum dogonbadanicum Assadi. J Essent Oil Res 13:43–4
- Southwell IA, Bourke CA. (2001). Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 56:437–41
- Stojkovic D, Sokovic MD, Glamoclija J, et al. (2008). Susceptibility of three clinical isolates of Actinomodura madurae to alpha-pinene, the bioactive agent of Pinus pinaster turpentine oil. Arch Biol Sci 60:697–701
- Teixeira B, Marques A, Ramos C, et al. (2013). Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric 93:2707--14
- Yazaki K, Okada T. (1994). Medicinal and aromatic plants VI. In: Y.P.S. Bajaj, ed. Biotechnology in Agriculture and Forestry. Vol. 26. Berlin: Springer-Verlag, 167–78
- Yuce E, Bagci E. (2012). The essential oils of the aerial parts of two Hypericum taxa (Hypericum triquetrifolium and Hypericum aviculariifolium subsp. depilatum var. depilatum (Clusiaceae)) from Turkey. Nat Prod Res 26:1985–90
- Zampini IC, Vattuone MA, Isla MI. (2005). Antibacterial activity of Zuccagnia punctata Cav. ethanolic extracts. J Ethnopharmacol 102:450–6

