Abstract
Context: Epidioxy sterols and sterols with special side chains, such as hydroperoxyl sterols, usually obtained from marine natural products, are attractive for bioactivities.
Objective: To isolate and screen bioactive and special sterols from China Sea invertebrates.
Materials and methods: Two hydroperoxyl sterols (1 and 2) from the sponge Xestospongia testudinaria Lamarck (Petrosiidae), three epidioxy sterols (3–5) from the sea urchin Glyptocidaris crenularis A. Agassiz (Glyptocidaridae), sponge Mycale sp. (Mycalidae) and gorgonian Dichotella gemmacea Milne Edwards and Haime (Ellisellidae) and an unusual sterol with 25-acetoxy-19-oate (6) also from D. gemmacea were obtained and identified. Using high-throughput screening, their bioactivities were tested toward Forkhead box O 3a (Foxo3a), 3-hydroxy-3-methylglutaryl CoA reductase gene fluorescent protein (HMGCR-GFP), nuclear factor kappa B (NF-κB) luciferase, peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α), protein-tyrosine phosphatase 1B (PTP1B), mitochondrial membrane permeabilization (MMP) and adenosine monophosphate-activated protein kinase.
Results: Their structures were determined by comparing their nuclear magnetic resonance data with those reported in the literature. Three epidioxy sterols (3–5) showed inhibitory activities toward Foxo3a, HMGCR-GFP and NF-κB-luciferase with the IC50 values 4.9–6.8 μg/mL. The hydroperoxyl sterol 29-hydroperoxystigmasta-5,24(28)-dien-3-ol (2) had diverse inhibitory activities against Foxo3a, HMGCR-GFP, NF-κB-luciferase, PGC-1α, PTP1B and MMP, with IC50 values of 3.8–19.1 μg/mL.
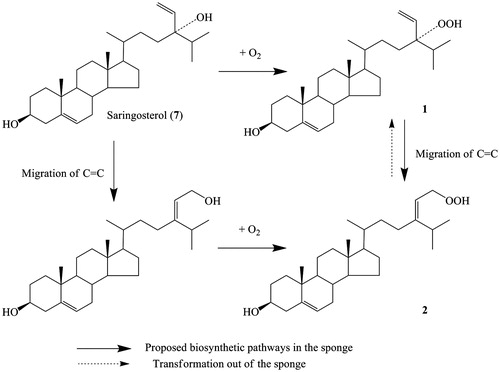
Discussion and conclusion: The bioactivities of 3–5 showed that 5α,8α-epidioxy is the active group. Otherwise, the most plausible biosynthesis pathway for 1 and 2 in sponge involves the abstraction of an allylic proton by an activated oxygen, such as O2, along with migration of carbon–carbon double bond. Therefore, the bioactive and unstable steroid should be biosynthesized in sponge under a special ecological environment to act as a defensive strategy against invaders.
Introduction
Marine invertebrates produce a series of bioactive secondary metabolites that are accumulated in their bodies or are released to their surroundings as chemical defense strategies; these metabolites exhibit activities against numerous disease targets (Paul & Ritson-Williams, Citation2008). Numerous new sterols have been identified in marine organisms, especially in marine sponges and corals (Blunt et al., Citation2012; Sarma et al., Citation2005). Some of these sterols possessing unique side chains and polyoxygenated core structures have been well characterized with their unusual functional groups (D’Auri et al., Citation1993; Feng et al., Citation2005).
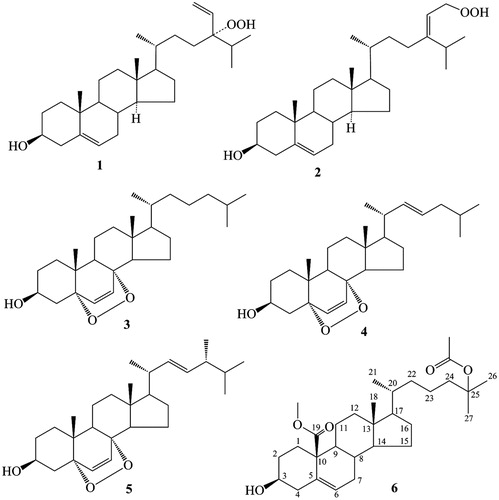
In our search for bioactive constituents in marine invertebrates from the China Sea, we identified six (1–6; ) sterols from sponges, corals, sea urchins and other marine invertebrates. The bioactivities of these sterols were determined through bioactivity tests using high-throughput screening (HTS). We also briefly discussed their structure–activity relationship and bioconversion.
Materials and methods
General experimental procedures
Optical rotation values and nuclear magnetic resonance (NMR) spectra were measured using a Perkin Elmer 341 Polarimeter (Norwalk, CT) and a Bruker AVANCE-500 spectrometer (Basel, Switzerland), respectively. Silica gel (100–200, 300–400 mesh, Qingdao Marine Chemical Group Co., Qingdao, China) and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used for column chromatography. Silica gel column chromatography was performed using the Büchi Sepacore (C-615/605) system (Postfach, Switzerland).
Animal material
The sponge Xestospongia testudinaria Lamarck (Petrosiidae) was collected at a depth of 7–10 m off the coast of Sanya (South China Sea), Hainan, China, in May 2008. The sponge Mycale sp. (Mycalidae) was collected from the same coast of Sanya in May 2010. Dr. Kyung Jin Lee (Wildlife Genetic Resources Center, National Institute of Biological Resources, Environmental Research Complex, Incheon, Korea) identified these two types of sponge. The sea urchin Glyptocidaris crenularis A. Agassiz (Glyptocidaridae) was collected at a depth of 10–50 m off the coast of Dalian (Yellow sea of China), Liaoning, China, in May 2008 and was identified by Dr. Jian Wu, Dalian Marie Fisheries Company. The gorgonian Dichotella gemmacea Milne Edwards and Haime (Ellisellidae) was collected from Meishan Island, Hainan, China, in April 2009 (7–10 m depth) and identified by Prof. Hui Huang (South China Sea Institute of Oceanology, CAS). The vouchers of these four animals (Xt200805, Ms201005, GC200805 and M090405) were deposited in the Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, CAS.
Isolation of compounds
The extraction and isolation of 1–3 were prepared according to our previous study (Zhou et al., Citation2010, Citation2011). The sponge Mycale sp. (2 kg, wet wt.) was extracted three-times with 75% alcohol, and the combined alcohol extracts were concentrated in vacuo. The residue was partitioned between H2O and CHCl3, followed by partitioning of the CHCl3 layer between 90% EtOH and petroleum ether (PE), to yield 90% EtOH fraction (9.3 g). The 90% EtOH fraction was chromatographed on a silica gel column using CHCl3/MeOH gradient to obtain subfractions 1–8. Subfraction 3 (CHCl3:MeOH 50:1) was chromatographed on Sephadex LH-20 column [CHCl3:MeOH (1:1)] to obtain 4 (29 mg). The fresh gorgonian D. gemmacea (4 kg, wet wt.) was extracted twice with 95% EtOH and once with CHCl3:MeOH (1:1). After the solvent was evaporated in vacuo, the combined residue was suspended in H2O and partitioned with EtOAc and n-BuOH to provide the EtOAc extract (18.0 g) and the n-BuOH extract (5.0 g), respectively. The EtOAc extract was chromatographed on a silica gel column (300–400 mesh) and then eluted with a gradient of PE/Me2CO (50:1–0:100) to yield subfractions 1–9. Subfraction 4 (PE:Me2CO 5:1) was chromatographed on Sephadex LH-20 [CHCl3:MeOH (1:1)] and silica gel CC, followed by elution with CHCl3:MeOH 20:1, to obtain 5 (21 mg) and 6 (18 mg).
Assays for bioactivities
Bioactivity assays were performed by the National Center for Drug Screening, the State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences using HTS (Zhu et al., Citation2010). Previously reported procedures were followed for assaying the bioactivity against Forkhead box O 3a (Foxo3a; Zanella et al., Citation2008), 3-hydroxy-3-methylglutaryl CoA reductase gene fluorescent protein (HMGCR-GFP; Schulz et al., Citation2012), nuclear factor kappa B (NF-κB) luciferase (Kang et al., Citation2009), peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α; Arany et al., Citation2008), protein-tyrosine phosphatase 1B (PTP1B; Shi et al., Citation2008), mitochondrial membrane permeabilization (MMP; Farrelly et al., Citation2001) and adenosine monophosphate-activated protein kinase (AMPK; Anderson et al., Citation2004).
3β-Hydroxy-cholest-5-en-25-acetoxy-19-oate (6)
Compound 6 was obtained as a white powder [[α]D−58.0 (c 0.05, CHCl3); ESI-MS m/z 511 [M+Na]+]. shows the list of 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data.
Table 1. 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data of 6.
Results and discussion
Compounds 1 and 2 were isolated from the South China Sea sponge X. testudinaria and identified as 24-hydroperoxy-24-vinylcholesterol and 29-hydroperoxystigmasta-5,24(28)-dien-3-ol, respectively (Zhou et al., Citation2011). Compound 3 was obtained from the sea urchin G. crenularis and determined as 5α,8α-epidioxycholest-6-en-3β-ol (Zhou et al., Citation2010). Compound 4 isolated from the sponge Mycale sp. was identified as 5α,8α-epidioxycholest-6,22-dien-3β-ol by comparing the 1H NMR and 13C NMR data with those reported in the literature (Gauvin et al., Citation2000). Compounds 5 and 6 were obtained from the gorgonian D. gemmacea and identified as 5α,8α-epidioxy-ergosta-6,22-dien-3β-ol and 3β-hydroxy-cholest-5-en-25-acetoxy-19-oate, respectively. Compound 5 was determined by comparing the 1H and 13C NMR data with those reported in the literature (Ioannou et al., Citation2009). Compound 6 was found for the first time by Wang’s group (Changyun Wang, Ocean University of China) in the same gorgonian and was reported only in the master’s degree dissertation of Liu (Citation2008). This compound was determined by comparing the 1H and 13C NMR data with those reported in the aforementioned dissertation.
Using HTS, the bioactivities of the obtained sterols toward several targets, such as Foxo3a, HMGCR-GFP, NF-κB-luciferase, PGC-1α, PTP1B, MMP and AMPK, were evaluated. Sterols with concentrations of 2–50 μg/mL were active against some biological targets, and the IC50 values are listed in .
Table 2. Inhibitory effects of marine sterols against different biological targets.
The bioactivities of 3–5 toward Foxo3a, HMGCR-GFP and NF-κB-luciferase indicated that 5α,8α-epidioxy is the active group and that the branched chain affects the intensity of the bioactivities. 5α,8α-Epidioxysterols, which are proposed to be formed by the photooxidation reaction of conjugated Δ5,7-sterols (Aknin et al., Citation2010), reportedly inhibit the growth of many human and murine cancer cell lines (Ioannou et al., Citation2009). The IC50 values (4.9–6.8 μg/mL) of the inhibitory activities against HMGCR-GFP showed that 5α,8α-epidioxysterols 3–5 may be effective for cholesterol metabolism disorder. In this study, compound 6 is the only active steroid against AMPK, a new therapeutic target in metabolic and cardiovascular diseases.
Cytotoxicity is the most frequently reported activity of hydroperoxysterols (Sheu et al., Citation1996, Citation1997a,Citationb). In this study, 2 had diverse bioactivities, whereas 1 was only active to NF-κB-luciferase. However, 2 was not stable under laboratory environment. The thin-layer chromatography assay showed that 2 can transform to 1 at room temperature and even at 4 °C. Compounds 1 and 2 are two uncommon oxidized steroids containing a hydroperoxyl group in the branched chain. They may have the same precursor, saringosterol (7), which is also contained in this sponge (Zhou et al., Citation2011). The most plausible biosynthetic pathway for 1 and 2 in sponge involves the abstraction of an allylic proton by an activated oxygen, such as O2, along with migration of the carbon–carbon double bond (). Several naturally occurring hydroperoxy steroids have been isolated from tunicates (Sung et al., Citation2007), seaweeds (Teixeira et al., Citation2006), algae (Sheu et al., Citation1997a,Citationb) and some plants (Kato et al., Citation1996), supporting the assumption that these compounds are not artifacts. We propose that the bioactive and unstable steroid can be biosynthesized in sponge under a special ecological environment to act as a defensive strategy against invaders.
Declaration of interest
The authors report no conflicts of interest.
This work was financially supported by National Key Basic Research Program of China (973)’s Project (2010CB833800 and 2011CB915503), National High Technology Research and Development Program of China (863 Program, 2012AA092104), National Natural Science Foundation of China (31270402, 20902094, 30973679 and 21172230) and Knowledge Innovation Program of Chinese Academy of Sciences (SQ201019).
Acknowledgements
The authors would like to thank the National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, for performing the bioactivity assays.
References
- Aknin M, Gros E, Vacelet J, et al. (2010). Sterols from the Madagascar sponge Fascaplysinopsis sp. Mar Drugs 8:2961–75
- Anderson SN, Cool BL, Kifle L, et al. (2004). Microarrayed compound screening (microARCS) to identify activators and inhibitors of AMP-activated protein kinase. J Biomol Screen 9:112–21
- Arany Z, Wagner BK, Ma Y, et al. (2008). Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. Proc Natl Acad Sci USA 105:4721–6
- Blunt JW, Copp BR, Keyzers RA, et al. (2012). Marine natural products. Nat Prod Rep 29:144–222
- D’Auri MV, Minale L, Riccio R. (1993). Polyoxygenated steroids of marine origin. Chem Rev 93:1839–95
- Farrelly E, Amaral MC, Marshall L, Huang SG. (2001). A high-throughput assay for mitochondrial membrane potential in permeabilized yeast cells. Anal Biochem 293:269–76
- Feng R, Cui JG. (2005). Polyhydroxy sterols with different structures from marine organisms. Chemistry, 68:1–8
- Gauvin A, Smadja J, Aknin M, et al. (2000). Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can J Chem 78:986–92
- Ioannou E, Abdel-Razik AF, Zervou M, et al. (2009). 5α,8α-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids 74:73–80
- Kang MI, Henrich CJ, Bokesch HR, et al. (2009). A selective small-molecule nuclear factor-kappaB inhibitor from a high-throughput cell-based assay for activator protein-1 hits. Mol Cancer Ther 8:571–81
- Kato T, Frei B, Heinrich M, Sticher O. (1996). Antibacterial hydroperoxysterols from Xanthosoma robustum. Phytochemistry, 41:1191–5
- Liu HY. (2008). The allelopathic substances from gorgonian Dichotella gemmacea (valenciennes) and soft coral Sinularia sp. from the South China Sea (In Chinese) [dissertation]. Shandong, China: Master’s degree dissertation of Ocean University of China, 25–8
- Paul VJ, Ritson-Williams R. (2008). Marine chemical ecology. Nat Prod Rep 25:662–95
- Sarma NS, Karishna MSR, Rao SR. (2005). Sterol ring system oxidation pattern in marine sponges. Mar Drugs 3:84–111
- Schulz MM, Reisen F, Zgraggen S, et al. (2012). Phenotype-based high-content chemical library screening identifies statins as inhibitors of in vivo lymphangiogenesis. Proc Natl Acad Sci USA 109:E2665–74
- Sheu JH, Huang SY, Duh CY. (1996). Cytotoxic oxygenated desmosterols of the red alga Galaxaura marginata. J Nat Prod 59:23–6
- Sheu JH, Huang SY, Wang GH, Duh CY. (1997a). Study on cytotoxic oxygenated desmosterols isolated from the red alga Galaxaura marginata. J Nat Prod 60:900–3
- Sheu YH, Wang GH, Sung PJ, et al. (1997b). Cytotoxic sterols from the formosan brown alga turbinaria ornata. Planta Med 63:571–2
- Shi L, Yu HP, Zhou YY, et al. (2008). Discovery of a novel competitive inhibitor of PTP1B by high-throughput screening. Acta Pharmacol Sin 29:278–84
- Sung PJ, Lin MR, Chen JJ, et al. (2007). Hydroperoxysterols from the tunicate Eudistoma sp. Chem Pharm Bull 55:666–8
- Teixeira VL, Barbosa JP, Rocha FD, et al. (2006). Hydroperoxysterols from the Brazilian brown seaweeds Dictyopteris justii and Spatoglossum schroederi (Dictyotales): A defensive strategy against herbivory. Nat Prod Comm 1:293–7
- Zanella F, Rosado A, García B, et al. (2008). Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. ChemBioChem 9:2229–37
- Zhou XF, Lu YN, Lin XP, et al. (2011). Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities. Chem Phys Lipids 164:703–6
- Zhou XF, Xu TH, Wen KW, et al. (2010). New N-acyl taurine from the sea urchin Glyptocidaris crenularis. Biosci Biotech Bioch 74:1089–91
- Zhu Y, Zhang Z, Zhang M, et al. (2010). High throughput screening for bioactive components from traditional Chinese medicine. Comb Chem High T Scr 13:837–48