Abstract
Context: Keloids and hypertrophic scars are hyperproliferative skin disorders resulting in abnormal wound healing. In the prevention and treatment of keloids and hypertrophic scars, ointments containing heparin and onion extract are very popular. Their therapeutic effects, however, are still controversial and the mechanism of action is not fully understood.
Objective: The aim of this study was to assess the effect of enoxaparin and dry onion extract on proliferation, apoptosis and β1 integrin expression in human fibroblasts.
Materials and methods: Fibroblast human cell lines (46 BR.1 N) were treated for 48 h with various concentrations of enoxaparin sodium (20, 100, 500 µg/mL) and/or onion [Allium cepa L. (Alliaceae)] extract (50, 250, 1000 µg/mL). The cell proliferation was evaluated by [3H]-thymidine incorporation assay. Furthermore, the expression of β1 integrin and apoptosis was determined by flow cytometry.
Results and discussion: The results demonstrate that enoxaparin and onion extract inhibited the proliferation of human fibroblasts. Almost complete inhibition of cell proliferation was achieved by enoxaparin in 500 µg/mL concentration (91.5% reduction). The onion extract at a concentration of 250 µg/mL also strongly inhibited the proliferation of cells (50.8% reduction). Depending on concentration, enoxaparin and onion extract induced apoptosis (500 and 1000 µg/mL, respectively) and, depending on concentration, downregulated the expression of β1 integrin on human fibroblasts.
Conclusion: This work points at possible mechanism of action of enoxaparin and onion extract, when administered in the treatment of patients with keloids and hypertrophic scars.
Introduction
Keloids and hypertrophic scars pose a serious problem for today’s dermatology (Chung et al., Citation2006). They result from abnormal wound healing, after traumatic or surgical injuries, and can be defined as fibroproliferative disorders in which an abnormal balance between proliferation and apoptosis of fibroblasts has been confirmed. Only in keloids, however, this proliferation continues throughout the course of a disease (Luo et al., Citation2001). Fibroblasts are responsible for an excessive deposition of connective tissue in dermis and subcutaneous tissues in such changes. Significant proportion of fibroblasts, higher than in normal tissue, expresses high levels of integrins. This feature allows for a high invasiveness of the infiltrative fibroblasts into tissues (Liu et al., Citation2009; Szulgit et al., Citation2002).
Current therapeutic modalities, used in the prevention and treatment of problematic scars, include various combinations of surgical and laser excisions, steroids and immunomodulators (Zurada et al., Citation2006). Preparations containing an onion extract and heparin are very popular, among others. Efficacy of these treatment modalities is still controversial; however, some studies and clinical practice showed that they exert a positive effect on the treatment of keloids and hypertrophic scars (Draelos, Citation2008; Saulis et al., Citation2002).
Heparin is a sulfated polysaccharide belonging to the family of glycosaminoglycans (GAGs) and has been in use as an anticoagulant agent for 70 years. Recently, low molecular weight derivatives of heparin (LMWH), such as enoxaparin, dalteparin, nadroparin, having similar biological activity as unfractionated heparin, have been more popular in therapy (Garg et al., Citation2005). Clinical studies of thrombosis showed that dalteparin and nadroparin prolong the survival of cancer patients, which could not be explained by the prevention of venous thromboembolism (Hettiarachchi et al., Citation1999; Klerk et al., Citation2005; Lee et al., Citation2005). In contrast to unfractionated heparin, LMWHs can be chosen as more appropriate for transdermal penetration from topical preparations, due to smaller size of molecules (average molecular weight of heparin is 12–15 kDa while enoxaparin is 4500 Da), as opposed to those used in scar treatment.
Onion [Allium cepa L. (Alliaceae)] contains many organosulfur compounds, flavonoids (mainly quercetin in the form of aglycone or sugar conjugates), saponins, vitamins (B1, B2, B6, C, E) and other chemical constituents. Because of these compounds, onion is known to exert a great number of biological activities, such as antibacterial, antiviral, antioxidant and, according to some authors, anticarcinogenic and antimutagenic effects (Corzo-Martínez et al., Citation2007). It is believed that onion extract decreases the histamine level, inflammation and collagen production in abnormal scars (Saulis et al., Citation2002). However, the exact mechanism of onion activity is yet to be discovered.
Our previous studies showed that both enoxaparin and onion extracts may inhibit the production of IL-6 and VEGF in the skin fibroblast cell line (Pikula et al., Citation2009). It is supposed that these cytokines play a pivotal role in the abnormal growth of keloid fibroblasts (Atamas, Citation2002). To elucidate on the mechanisms that may govern the effective treatment of keloids, we investigated the effect the enoxaparin and onion extract have on the proliferation and apoptosis of the skin fibroblast cell line, as well as on the expression of β1 integrin on these cells.
Materials and methods
Examined chemical
Enoxaparin sodium (anti-Factor Xa activity of 119 IU/mg) was a kind gift from Welding (Hamburg, Germany).
Onion extract preparation and characteristics
Peeled fresh onion bulbs (Allii cepae bulbus) were extracted in darkness by maceration with 95% (v/v) ethanol (onion: solvent ratio 1:1 w/w) for 5 days. To obtain a dry extract, the filtered liquid extract was concentrated with a rotary vacuum evaporator to reduce the ethanol content to approximately 20% and spray-dried (B-290 Büchi spray-dryer, Flawil, Switzerland). HPLC separation of flavonoids in onion extracts was accomplished on a Discovery HS C-18 (75 mm × 4.6 mm i.d., 3 µm) column (Supelco, Bellefonte, PA) with a gradient program from 10% to 100% A in A+B mixture (solvent A – acetonitrile/water/formic acid 50/50/0.1, v/v/v; solvent B – water/formic acid 100/0.1, v/v), at temperature 25 °C; injection volume 3 µL, flow rate 1.0 mL/min, tG 50 min, UV detection at 366 nm. The content of quercetin 3,4′-di-O-glucoside was expressed as spireoside (Lanzotti, Citation2006). The quantitative analysis revealed that the most abundant compound in the examined material was spireoside (quercetin 4′-O-glucoside, 9.472 mg/g), but the onion extract also contained another quercetin glucoside (quercetin 3,4′-di-O-glucoside, 3.375 mg/g) and only a small quantity of free quercetin (0.278 mg/g).
Cell culture conditions
Human fibroblast cell line (46 BR.1 N) was obtained from the European Collection of Cell Cultures (ECACC). The cell line was originally derived from the skin of an anonymous individual with hypogammaglobulinemia and was transformed with the plasmid pSV3neo, and now is immortal. Fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, Steinheim, Germany), with 4500 mg/L glucose, 584 mg/L l-glutamine, sodium pyruvate and sodium bicarbonate. The medium contained 15% FCS and was supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich). Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. Cells for experiments were seeded at a density of 1 × 104/cm2 in 60 mm diameter (growth surface area 21 cm2) culture dishes (Corning) or in 96-well plates and grown for 24 h in medium with FCS (15%). Thereafter, the cells were washed in PBS, the medium was changed to DMEM without serum and the examined compounds were added in the appropriate final concentrations. Cells were then cultured for 48 h. After this time, the intensity of apoptosis, the expression of β1 integrin and thymidine incorporation were measured. All solutions of the investigated agents, used in the experiments, were prepared with phosphate-buffered saline (PBS) solution (pH 7.4) in sterile conditions.
Thymidine incorporation assay
Cells were seeded in a 96-well plate at a density of 5000 cells/well, in DMEM containing 15% FCS and incubated for 24 h. After this time, the cells were washed with PBS, the medium was changed to DMEM without serum, containing appropriate concentrations of onion extract and/or enoxaparin and incubated for 48 h at 37 °C. 0.25 μCi of [3H]thymidine was added for the last 16 h of the culture. Thereafter, cells were washed with phosphate-buffered saline (PBS), treated with trypsin for 20 min and harvested with a cell harvester (Scatron, Norway). The incorporated radioactivity of cell suspension obtained after trypsin treatment was measured using a liquid scintillation counter (Beckman, Germany).
Flow cytometric analysis of integrin β1 expression
Flow cytometry analysis of β1 integrin expression required 2 × 105 cells/sample. Fibroblasts cultured in medium, as described above, were suspended in PBS with 0.1% bovine serum albumin (BSA) containing APC-conjugated anti-integrin β1 antibody (IgG1, clone MAR4, Becton Dickinson, Franklin Lakes, NJ). After 1 h of incubation, the cells were washed, re-suspended in PBS and analyzed in LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ). There were 15 000 events collected from each sample. Fluorescence signal intensities were analyzed with FACSDiva Software (BD Biosciences, Franklin Lakes, NJ) and graphical representations of the signal were prepared with WinMdi 2.8 (kindly provided by Dr. Trotter). Unstained cells and cells stained with isotype control antibody were used as controls. The mean fluorescence of each experiment was calculated by subtracting the mean control fluorescence values from the mean fluorescence with target antibodies.
Analysis of apoptosis by flow cytometry
To identify apoptotic cells, an Annexin V-FITC Apoptosis Detection Kit II (BD Pharmingen, Franklin Lakes, NJ) was used. The assay was performed according to the manufacturer’s suggestions. Cells were washed twice with cold PBS, transferred to 5 mL cytometer tubes (1 × 105 cells in 100 µL) and stained with 5 µL of Annexin V-FITC and 5 µL of propidium iodide. After 15 min of incubation at room temperature, in the dark, 400 µL of the binding buffer were added. Apoptotic cells were assessed by binding Annexin V-FITC to exposed phosphatidylserine residues and propidium iodide (PI) exclusion was used to confirm the integrity of the cell membrane. Samples were analyzed (typically 15 000 cells per sample) with a LSRII cytometer (Becton Dickinson), equipped with a laser with the emission wavelength of 488 nm. Cells that did not stain for Annexin V-FITC and PI were considered viable; cells positive for Annexin V-FITC and negative for PI were considered as early stages of apoptosis; double-positive cells were considered as late stages of apoptosis and events single-positive for PI were considered as the cells with a disintegrated cell membrane and cell nuclei.
Statistics
Data were computed using the software Statistica 8.0 (Statsoft, Poland). The analysis of data obtained from the experiments was based on the ANOVA test, as indicated by data distribution. When more than two groups were evaluated, the analysis of variance (ANOVA) followed by the lowest significant difference post-hoc tests were performed. p < 0.05 was recognized as significant.
Results
Onion extract and enoxaparin inhibited proliferation of dermal fibroblasts
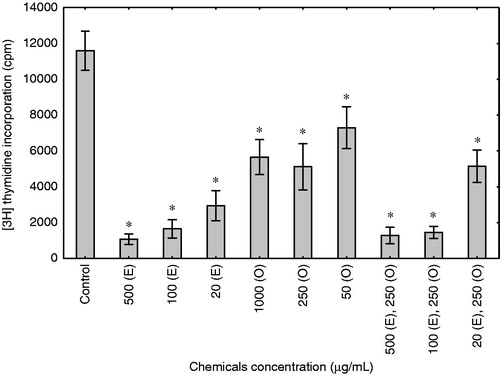
The 3H-thymidine incorporation assay was used to measure the effects of enoxaparin and onion extract on the proliferation of human fibroblast cells. The treatment of skin fibroblasts for 48 h with those substances resulted in a significant inhibition of cell proliferation (). When compared to control cells, enoxaparin induced three- to five-fold inhibition of cell proliferation (p < 0.0001). The strongest effect was observed in cells treated with enoxaparin at the highest concentration (500 µg/mL), and lower for 250 and 50 µg/mL. The onion extract in concentrations of 1000, 250 and 50 µg/mL also inhibited the proliferation of skin fibroblasts (p < 0.0001). However, this effect of onion extract was significantly lower than the one observed for enoxaparin. Among different concentrations of enoxaparin and onion extract added together, the strongest inhibitory effect was observed in the combination of 500 µg/mL of enoxaparin with 250 µg/mL of onion extract as well as for 100 µg/mL of enoxaparin and 250 µg/mL of onion extract (p < 0.0001). Interestingly, the onion extract and enoxaparin, when added together, did not exert a synergic effect on the inhibition of proliferation.
Figure 1. Enoxaparin and onion extract inhibited proliferation of fibroblast cells. Thymidine assay was used to measure DNA synthesis in human fibroblasts cultured for 48 h in response to vehicle control (control), different concentrations of enoxaparin (E), onion extract (O) and combinations of enoxaparin and onion extract. All assays were performed in triplicate in the culture of fibroblast cell line. Data are presented as a mean 3H-thymidine incorporation ± SD. *p < 0.0001 compared with untreated cells.
Enoxaparin and onion extract changed β1 integrin expression in skin fibroblasts
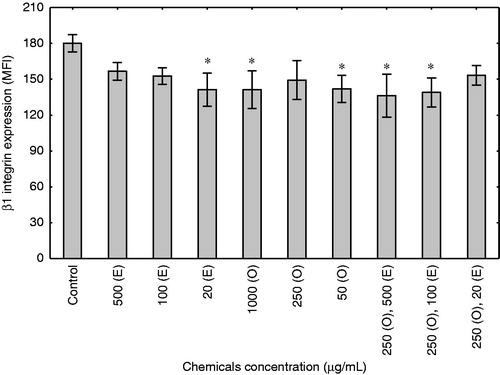
To define the effect of enoxaparin and onion extract on the expression of β1 integrin, we performed flow cytometry analyses of fibroblasts cultured for 48 h in serum-free medium, in the presence of different concentrations of the examined agents. As shown in , enoxaparin significantly decreased the expression of β1 integrin only in the lowest concentration of 20 µg/mL (p < 0.05). A similar effect was observed when cells were cultured with onion extract in the concentration of 1000 and 50 µg/mL (p < 0.05). The combination of enoxaparin and onion extract also resulted in a significant decrease in the expression of β1 integrin (p < 0.05), for the concentrations of 250 and 500 as well as 250 and 100 µg/mL of onion and enoxaparin, respectively.
Figure 2. Effect of enoxaparin and onion extract on β1 integrin expression in human fibroblasts. The figure illustrates flow cytometry differences of mean fluorescence intensity ± SD of triplicate values. Cells for the experiments were cultured for 48 h in response to different concentrations of enoxaparin (E), onion extract (O) and combinations of onion extract and enoxaparin or vehicle medium (control). *p < 0.05 compared with untreated cells.
Onion extract and enoxaparin-induced apoptosis in dermal fibroblasts only at the highest concentrations
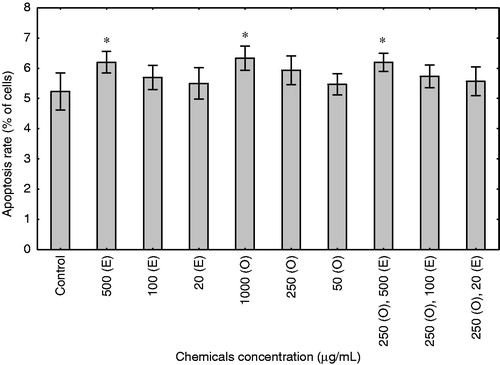
To assess the effect of enoxaparin and onion extract on fibroblast apoptosis, the cells were treated with these compounds for 48 h in medium without serum. After this time, apoptosis was measured by flow cytometry, counting early apoptotic cells (FITC-Annexin V positive and PI negative) and late apoptotic cells (positive for FITC-Annexin V binding and for PI uptake). As shown in , enoxaparin and onion extract induced apoptosis in the skin fibroblast only at the highest concentrations of 500 and 1000 µg/mL, respectively (p < 0.05). Among different concentrations of enoxaparin and onion extract added together, only the highest concentration of these substances induced apoptosis in fibroblasts (p < 0.05). Interestingly, lower concentrations of these substances, which were shown to inhibit proliferation, did not significantly affect cell viability.
Figure 3. Effect of enoxaparin and onion extract on apoptosis in the culture of human fibroblasts. Cells were stimulated for 48 h with different concentrations of enoxaparin (E), onion extract (O) and combinations of enoxaparin and onion extract. The results are presented as mean rate of apoptosis representing early (FITC-Annexin V positive and PI negative) and late apoptotic cells (positive for FITC-Annexin V binding and for PI uptake) ± SD (n = 3 for each group). *p < 0.05.
Discussion
In this study, we evaluated the effects of enoxaparin and onion extract on a human dermal fibroblast cell line. We showed that these compounds, separately as well as in combination, were highly effective in the inhibition of fibroblast proliferation. This fact, together with the reports that low molecular weight heparin (LMWH) penetrates epidermal barrier, makes this compound a more promising candidate for topical preparations than the unfractionated heparin (UH), whose dermal penetration is doubted or largely restricted to the stratum corneum (Betz et al., Citation2001; Xiong et al., Citation1996). Nevertheless, it should be emphasized that, although both keloids and hypertrophic scars develop as a result of hyperproliferation of dermal fibroblasts, only keloids are characterized by an increased proliferation rate of the fibroblasts (Nakaoka et al., Citation1995). Hence, we assumed that enoxaparin and onion extract may exert a positive effect mainly in the treatment of keloids. Still, we cannot disregard the fact that these substances are useful in the prevention and softening of hypertrophic scars. Apart from antiproliferative effects, the enoxaparin and onion extract inhibited the expression of β1 integrin in selected concentrations. Integrins, as cell-surface molecules, are responsible for cell-extracellular matrix (ECM) interactions. Human dermal fibroblasts utilize β1 integrins mainly for adhesion to fibronectin and collagen fibers (Gailit & Clark, Citation1996; Grinnell, Citation1994). It has been reported that during the formation of scars, dermal fibroblasts have transiently expressed mainly α2 and α5 subunits of integrins and that in turn was associated with the upregulation of β1 subunit. Upregulation of some chains of integrins is also recognized as an important regulator of the deposition of ECM components associated with wound healing (Noszczyk et al., Citation2002; Wang et al., Citation2006). Recently, it has been shown that β1 integrin expressed by fibroblasts is required for fibrogenesis. Interestingly, downregulation of β1 integrin resulted in the resistance to skin scleroderma in the mouse model (Liu et al., Citation2009). In the light of these facts, a decrease in the expression of β1 integrin can be recognized as another therapeutic effect of enoxaparin and onion extract. The study does not address the question whether downregulation of β1integrin is a separate process, or whether it is part of more complex changes, such as apoptosis.
Apoptosis has been found to play an important role in proper wound healing and may signal the end of an inflammatory phase of this process (Brown et al., Citation1997; Desmouliere et al., Citation1995; Kinloch et al., Citation1999). It has been reported that apoptosis-related genes are downregulated in keloids cells (Sayah et al., Citation1999). Hence, our findings that both onion extract and enoxaparin may induce apoptosis in dermal fibroblasts seem to be beneficial during the treatment of keloids. This effect could only regard the highest concentrations of enoxaparin and onion extract used in the experiments, but it should be taken into consideration that immortal cell lines may be more resistant to apoptosis than primary cells. It would be interesting to know the compounds responsible for the above described effects. While enoxaparin has a well-described chemical structure, onion extract is a heterogeneous mixture of different agents. Nevertheless, the quantitative HPLC analysis has confirmed that the examined onion material contained spireoside (quercetin 4′-O-glucoside), another quercetin glucoside (quercetin 3,4′-di-O-glucoside) and free quercetin, which corresponded with the results obtained by other authors (Park et al., Citation2007). Flavonoids, especially quercetin and its glucosides, are known to demonstrate an antioxidant and, according to some authors, an anticarcinogenic activity (Jung et al., Citation2010; Lea et al., Citation2010). Due to such biological activity, they might be responsible for the inhibitory effect of the onion extract on the proliferation of fibroblasts. It has also been proved that quercetin inhibits the proliferation and contraction of fibroblasts isolated from keloids (Phan et al., Citation2003).
Altogether, the study supports, at the biological level, the hypothesis that enoxaparin and onion extract may be beneficial in the treatment of keloids. While we were mainly focused on keloids, it is possible that our findings can also be adapted to the treatment of other dermatologic or systemic lesions in which fibroblasts are activated, such as scleroderma or lichen planus. Indeed, recent reports suggest the efficacy of enoxaparin in the treatment of the latter disease (Pacheco & Kerdel, Citation2001). No doubt, there are limitations in our study. Firstly, a transfected cell line of dermal fibroblasts was used as a model. The response of these cells may differ from primary keloid fibroblasts, especially in relation to the intensity of apoptosis. Secondly, although concentrations of substances used in our experiments were similar to those expected in dermis after a topical treatment, further human skin penetration studies are necessary. Further studies are also required to gain an understanding of the mechanisms of action of enoxaparin and onion on the metabolism of fibroblasts, for example, the production of extracellular matrix.
Conclusions
In summary, the presented data prove that enoxaparin and the onion extract exert a significant inhibitory effect on the human fibroblast proliferation. Taking into account the current use and biological safety of these substances, they may serve as promising medicaments in the prevention of a pathological tissue outgrowth, especially in patients with keloids. However, it is necessary to conduct further research in this field to acquire knowledge about the broader biological effects of the examined substances on the cells isolated from skin lesions. It is also vital to perform in vivo experiments in suitable animal models, followed by the clinical effectiveness assessment.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the Polish Ministry of Science and Higher Education (grant N405 008 32/0528 to M. S.), HOMING program of the Foundation for Polish Science (grant from Iceland, Liechtenstein and Norway through the EEA Financial Mechanism to P. T.) and by the European Union within the framework of the European Social Fund (the system project Pomorskie Voivodeship “InnoDoktorant – Scholarships for PhD students, II edition” to M. Z.).
References
- Atamas SP. (2002). Complex cytokine regulation of tissue fibrosis. Life Sci 72:631–43
- Betz G, Nowbakht P, Imboden R, Imanidis G. (2001). Heparin penetration into and permeation through human skin from aqueous and liposomal formulations in vitro. Int J Pharm 228:147–59
- Brown DL, Kao WW, Greenhalgh DG. (1997). Apoptosis down-regulates inflammation under the advancing epithelial wound edge: Delayed patterns in diabetes and improvement with topical growth factors. Surgery 121:372–80
- Chung VQ, Kelley L, Marra D, Jiang SB. (2006). Onion extract gel versus petrolatum emollient on new surgical scars: A prospective double-blinded study. Dermatol Surg 32:193–7
- Corzo-Martínez M, Corzo N, Villamiel M. (2007). Biological properties of onions and garlic. Trends Food Sci Tech 18:609–25
- Desmouliere A, Redard M, Darby I, Gabbiani G. (1995). Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
- Draelos ZD. (2008). The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J Cosmet Dermatol 7:101–4
- Gailit J, Clark AF. (1996). Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Invest Dermatol 106:102–8
- Garg HG, Linhardt RJ, Hales CA. (2005). Chemistry and Biology of Heparin and Heparan Sulfate. Oxford: Elsevier
- Grinnell F. (1994). Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124:401–4
- Hettiarachchi RJ, Smorenburg SM, Ginsberg J, et al. (1999). Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost 82:947–52
- Jung YH, Heo J, Lee YJ, et al. (2010). Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci 86:351–7
- Kinloch RA, Treherne JM, Furness LM, Hajimohamadreza I. (1999). The pharmacology of apoptosis. Trends Pharmacol Sci 20:35–42
- Klerk CP, Smorenburg SM, Otten HM, et al. (2005). The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 23:2130–5
- Lanzotti V. (2006). The analysis of onion and garlic. J Chromatogr A 1112:3–22
- Lea MA, Ibeh C, Han L, Desbordes C. (2010). Inhibition of growth and induction of differentiation markers by polyphenolic molecules and histone deacetylase inhibitors in colon cancer cells. Anticancer Res 30:311–18
- Lee AY, Rickles FR, Julian JA, et al. (2005). Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol 23:2123–9
- Liu S, Kapoor M, Denton CP, et al. (2009). Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum 60:2817–21
- Luo S, Benathan M, Raffoul W, et al. (2001). Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 107:87–96
- Nakaoka H, Miyauchi S, Miki Y. (1995). Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm Venereol 75:102–4
- Noszczyk B, Klein E, Holtkoetter O, et al. (2002). Integrin expression in the dermis during scar formation in humans. Exp Dermatol 11:311–18
- Pacheco H, Kerdel F. (2001). Successful treatment of lichen planus with low-molecular-weight heparin: A case series of seven patients. J Dermatol Treat 12:123–6
- Park J, Kim J, Kim MK. (2007). Onion flesh and onion peel enhance antioxidant status in aged rats. J Nutr Sci Vitaminol (Tokyo) 53:21–9
- Phan TT, Sun L, Bay BH, et al. (2003). Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: Therapeutic implication for excessive scarring. J Trauma 54:1212–24
- Pikula M, Zebrowska ME, Trzonkowski P, Sznitowska M. (2009). Effects of enoxaparin and onion extract on cytokine production in skin fibroblasts. Centr Eur J Immunol 34:68–71
- Saulis AS, Mogford JH, Mustoe TA. (2002). Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg 110:177–83
- Sayah DN, Soo C, Shaw WW, et al. (1999). Downregulation of apoptosis-related genes in keloid tissues. J Surg Res 87:209–16
- Szulgit G, Rudolph R, Wandel A, et al. (2002). Alterations in fibroblast alpha1 beta1 integrin collagen receptor expression in keloids and hypertrophic scars. J Invest Dermatol 118:409–15
- Wang Z, Fong KD, Phan TT, et al. (2006). Increased transcriptional response to mechanical strain in keloid fibroblasts due to increased focal adhesion complex formation. J Cell Physiol 206:510–17
- Xiong GL, Quan D, Maibach HI. (1996). Effects of penetration enhancers on in vitro percutaneous absorption of low molecular weight heparin through human skin. J Control Release 42:289–96
- Zurada JM, Kriegel D, Davis IC. (2006). Topical treatments for hypertrophic scars. J Am Acad Dermatol 55:1024–31

