Abstract
Context: The health benefits and medicinal properties of herbal food products are known since antiquity. Fenugreek [Trigonella foenum-graecum Linn. (Fabaceae)], a seed spice used to enhance flavor, color and texture of food, is employed for medicinal purposes in many traditional systems. A number of epidemiological studies and laboratory research have unraveled the biological actions of fenugreek.
Objective: Research on fenugreek in recent years has identified a number of health benefits and physiological attributes in both experimental animals as well as clinical trials in humans. In this study we have reviewed the available scientific literature on fenugreek.
Methods: This review article summarizes and reviews published experimental studies and scientific literature from the databases including PubMed, Google and local library searches.
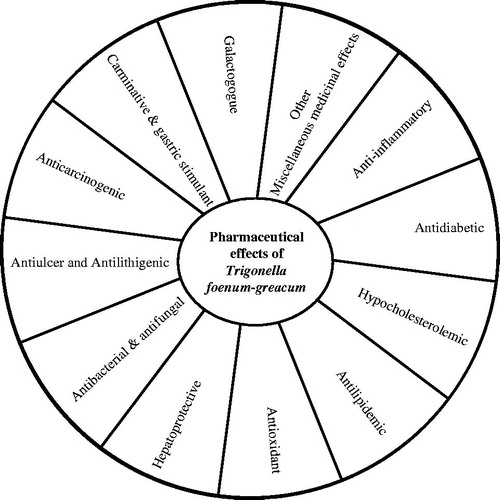
Results: The information available in the literature on the health benefits and pharmaceutical effects of Trigonella accounts for its known medicinal properties and adds new therapeutic effects in newer indications. Besides its known medicinal properties such as carminative, gastric stimulant, antidiabetic and galactogogue (lactation-inducer) effects, newer research has identified hypocholesterolemic, antilipidemia, antioxidant, hepatoprotective, anti-inflammatory, antibacterial, antifungal, antiulcer, antilithigenic, anticarcinogenic and other miscellaneous medicinal effects of fenugreek. Although most of these studies have used whole seed powder or different forms of extracts, some have identified active constituents from seeds and attributed them medicinal values for different indications.
Conclusion: The resarch on Trigonella exhibits its health benefits and potential medicinal properties in various indications and has little or no side effects, suggesting its pharmaceutical, therapeutic and nutritional potential.
Introduction
Use of plant-derived medicinal compounds has been in practice since antiquity in many cultural systems including India, China, Egypt and Middle Eastern countries. In recent times, plant-derived medicinal compounds are being widely used and are suggested by doctors to be used in a number of ailments due to their minimal side effects and numerous positive effects on human health. Out of many such medicinal plants, fenugreek [Trigonella foenum-graecum Linn (Fabaceae)] has recently attracted the attention of scientists from across the globe. Fenugreek is native to Eastern Europe and parts of Asia but now widely cultivated almost all over the world for its leaves and seeds, which are commonly used as leafy vegetables and condiments, respectively (Anonymous, Citation1998; Rastogi & Mehrotra, Citation1990; Srinivasan, Citation2006). Fenugreek plant is an erect annual herb with trifoliate leaves reaching a height of 0.3–0.8 m. The plants bear white or yellow flowers, which give rise to long, slender, yellow to brown pods. At maturity the pods contain hard brown seeds of fenugreek, which is known and utilized for its medicinal use. While the green leaves are used as a vegetable in many societies, the dried leaves are an excellent additive in many food preparations in the Indian subcontinent.
In the ancient Indian traditional system of medicine, Ayurveda, fenugreek has been suggested as an important medicine to treat a variety of digestive and mucosal conditions (Escot, Citation1994/95; Passano, Citation1995). The fenugreek seed has traditionally been used as a carminative, demulcent, expectorant, laxative and stomachic agent. The mature fenugreek seed has many other active components such as amino acids, fatty acids, vitamins and saponins such as disogenin, gitogenin, neogitogenin, homorientin saponaretin, neogigogenin and trigogenin, fibers, flavonoids, polysaccharides, fixed oils and some identified alkaloids, that is, trigonelline and choline (Jayaweera, Citation1981; Yoshikawa et al., Citation1997). The plant has also been employed against diseases such as bronchitis, fever, sore throat, wound, swollen glands, skin irritation, diabetes and ulcers. Recently, due to the widespread use and beneficial properties of fenugreek, many studies have been undertaken to investigate its potential application in health and many common disorders. Such experimental studies examined the effects of various extracts of fenugreek seed in experimental animals in the early to late 1960s (Abdo & Al-Kafawi, Citation1969; Bhatt et al., Citation1958). A huge number of studies have returned positive findings, indicating the efficacy of fenugreek seed as a functional food that can be beneficial in health and disease. Fenugreek is well known for its multiple pharmacological properties including antidiabetic, antioxidative, hypocholesterolemic, antineoplastic, anti-inflammatory, antiulcerogenic, antipyretic, immunomodulatory and antitumor (Dixit et al., Citation2010; Satheeshkumar et al., Citation2010; Xue et al., Citation2011). Different active components of fenugreek seeds have been identified and isolated such as polyphenolic flavonoids which exhibit most common properties, that is, hypoglycemic, hypocholesterolemic, hypotriglyceridemic and antiperoxidative (Gupta & Nair, Citation1999), steroid saponins exhibiting anti-inflammatory and uterus and lactation-stimulating properties (Petit et al., Citation1995), polysaccharides such as galactomannans contains antidiabetic effects (Madar & Shomer, Citation1990) and an amino acid 4-hydroxyisoleucine has been shown to possess insulin-mimetic properties (Broca et al., Citation2004). Here, we provide a review of recent findings showing effects of Trigonella in different diseases () in the experimental studies and in some clinical trials.
Trigonella in health
The hypoglycemic effect of fenugreek is well known in diabetic subjects including experimental animals as well as humans. However, a study by Abdel-Barry et al. (Citation1997) suggests that the aqueous extract of Trigonella leaves given either orally or intraperitoneally possesses a hypoglycemic effect in normoglycemic rats. Further, in another study, when extracts of Trigonella foenum-graecum seeds were fed to normal mice orally, it produced a hypoglycemic effect and reduced the blood glucose levels (Zia et al., Citation2001). These studies suggest that Trigonella could be used as a food supplement to regulated blood glucose even in non-diabetic individuals on calorie-rich diet. Another study suggested the potential of fenugreek as a functional food and nutraceutical, owing to its effects on glycemia and lipidemia (Roberts, Citation2011). Accordingly, when taken post meal, dietary fibers present in the fenugreek seed could regulate the production of cholesterol in the liver. The authors suggest that fenugreek seeds contain 45.4% dietary fiber of which 32% are insoluble fibers and 13.3% are soluble fibers (Roberts, Citation2011). Further, polysaccharides such as galactose and mannose are also found in seeds, which are associated with antiglycemic and anticholesterolemic properties. Galactomannan, a soluble fiber from fenugreek seed, has been reported to reduce postprandial blood glucose response. Srichamroen et al. (Citation2009) showed in an in vitro study, using the segments of jejunum and ileum derived from genetically lean and obese rats, that when incubated with labeled glucose (2 or 32 mmol/L) in the presence of different concentrations of galactomannan ranging from 0.1 to 0.5% (wt/wt) uptake of low or high concentration of glucose significantly and progressively reduced by increasing concentrations of galactomannan in both lean and obese rats, suggesting the use of fenugreek-derived galactomannan for the management of blood glucose. Similarly, due to the presence of galactomannan, the fenugreek-derived dietary fiber could be used in the food industry as an emulsifying and stabilizing agent. In bakery, 8–10% fenugreek dietary fiber supplemented flour is used to produce baked goods such as bread, pizza, muffins and cakes that can be utilized as functional foods to improve the nutritional value of western diets (Roberts, Citation2011). Further, study by Rajalakshmi and Subbulakshmi (Citation1964) suggested that fenugreek supplementation could enhance the biological value of rice and black gram [Phaseolus mungo L. (Fabaceae)] diet. Hooda and Jood (Citation2004) demonstrated that substituting wheat flour with fenugreek flour by 5–20% increased the protein, fat, lysine, minerals and dietary fiber contents proportionately to the level of substitution. The composite flours containing germinated fenugreek flour found to be superior in nutritional quality compared to others suggesting enhanced nutritional value of fenugreek supplementation in diet (Hooda & Jood, Citation2004).
A study by Ikeuchi et al. (Citation2006) showed that fenugreek could be used to enhance the strength and endurance in healthy individuals. They have demonstrated that when mice were given fenugreek seed extract at 300 mg/kg body weight by stomach intubation for 4 weeks, they showed a significant increase in swimming time to exhaustion as compared to the control group who received vehicle (Ikeuchi et al., Citation2006). Further, investigators found that in fenugreek-fed mice blood lactate concentration decreased significantly, whereas non-esterified fatty acid (NEFA) and plasma glucose levels significantly increased after swimming. The authors suggested that improvement in swimming endurance in fenugreek-fed animals could be due to the increase in utilization of fatty acids as an energy source (Ikeuchi et al., Citation2006). Besides its hypocholesterolemic and hypotriglyceridemic role, Trigonella has been shown to decrease dietary fat consumption in humans after repeated administration of a fenugreek seed extract, suggesting reduced spontaneous fat consumption in healthy volunteers (Chevassus et al., Citation2009). Further, in a short-term study, repeated administration of a fenugreek seed extract slightly but significantly decreased dietary fat consumption in healthy overweight subjects (Chevassus et al., Citation2010). These studies indicate that Trigonella consumption caused a modification of feeding behavior in humans, which may help in weight management. This is an important and significant finding with implications in managing body weight, considering the modern sedentary life-style, feeding habits, calorie-rich diet and consequently increasing obesity worldwide.
Modulation of the immune system has been indicated in many diseases including diabetes, cancer, arthritis and other allergic and autoimmune pathogenesis. Bin-Hafeez et al. (Citation2003) demonstrated that fenugreek plant extract could have immunomodulatory effects in mice. Treatment of mice with T. foenum-graecum extract elicited a significant increase in the delayed type of hypersensitivity response, phagocytic index and phagocytic capacity of macrophages as well as in lymphocyte proliferation assay. These findings suggest an immunostimulatory effect of fenugreek in the prevention of a number of diseases (Bin-Hafeez et al., Citation2003).
Since 4-hydroxyisoleucine has been found in fenugreek seed, which has insulin-like effects, Haefele et al. (Citation1997) tried to synthesize 4-hydroxyisoleucine by incubating isoleucine with a cell-free extract from etiolated 6-day-old fenugreek seedlings in the presence of various cofactors. The reaction mixture subjected to HPLC and 4-hydroxyisoleucine has been successfully detected in the mixture. This study indicated that it could be feasible to prepare the fenugreek’s bioactive molecule in the laboratory, which could have far-reaching consequences.
Although fenugreek is contraindicated in pregnancy and believed to cause abortion according to the Ayurvedic tradition (Brinker, Citation1998; Escot, Citation1994/95), post-partum women are encouraged to eat a sweetened paste containing fenugreek seeds to increase lactation as it contains galactogogue properties (Passano, Citation1995; Riordan & Auerbach, Citation1998). In a study by Mital and Gopaldas (Citation1986), the abortive effect of fenugreek could not be substantiated in laboratory animals. However, due to its uterine stimulation properties, which may have suggested its contraindication during pregnancy, the seeds are considered useful as a childbirth aid (Bingel & Farnsworth, Citation1991; Ody, Citation1999). It is suggested that uterine and lactation-stimulating properties of fenugreek could be due to the presence of steroids such as saponins in the seed, which may be related to similar stimulant effect of the hormone oxytocin or similar compounds on uterus and milk ducts (Bingel & Farnsworth, Citation1991).
Fenugreek has also been shown to possess a growth-promoting effect. Saponin I and dioscin derived from its seed showed increased release of rat growth hormone from rat pituitary cells demonstrating for the first time that fenugreek-derived steroidal saponins could stimulate rat growth hormone release in rat pituitary cells (Shim et al., Citation2008).
Although fenugreek seeds are known to have a mastogenic effect and enhance breast size, little is known about its estrogenic effect. Sreeja et al. (Citation2010) demonstrated that chloroform extracts of fenugreek seeds stimulated the proliferation of breast cancer cells, MCF-7. The authors showed its binding to ER which acted as an agonist for ER-mediated transcription, suggesting its estrogenic effect in breast cancer cells. It also induced the expression of estrogen responsive gene pS2 in MCF-7 cells (Sreeja et al., Citation2010).
Trigonella in disease
Antioxidant effects of Trigonella
Being an obligatory aerobe, humans consume a lot of oxygen to live and survive, to derive energy by the oxidation of food molecules. This oxidation process is tightly regulated; however, some spills do occur due to many factors. Additionally, the body has devised a method where it utilizes reactive oxygen species as a communicator of important messages to regulate biochemical and molecular events in cells. The excessive production of oxidants in body is normally taken care of by the native antioxidant mechanisms; however, in certain conditions these mechanisms become overwhelming for the cells leading to inflammation, tissue damage and disease. A number of antioxidants have been tried clinically to prevent oxidative stress-induced pathogenesis, but they have not been successful because they tend to become pro-oxidant at the clinically effective doses. This has lead to the search of plant-derived antioxidants, which are also non-toxic. A number of plant-derived antioxidants are flavonoids, which have been shown to be effective in biological systems in reducing oxidative stress. For example, treatment with Trigonella seed has been shown to restore the altered activity of cellular antioxidant enzymes including superoxide dismutase (SOD), glutathione reductase (GR), catalase and glutathione peroxidase (GPx) in tissue such as heart, muscle and brain during diabetes (Baquer et al., Citation2011). Further, Annida et al. (Citation2005) showed that supplementation of fenugreek leaves reduces oxidative stress in streptozotocin-induced diabetic rats. The antioxidant effect, determined by measuring thiobarbituric acid-reactive substances (TBARS) and reduced glutathione (GSH) levels and activities of catalase and SOD in liver, heart and kidney in diabetic rats, showed that in diabetic rats supplementation of fenugreek leaf powder, on the one hand, significantly lowered lipid peroxidation and, on the other hand, significantly increased the antioxidant system (Annida et al., Citation2005). In another study, daily oral administration of Trigonella seed-derived soluble dietary fiber (SDF) to type-2 diabetic rats for 28 days enhanced total antioxidant status besides decreasing serum glucose (Hannan et al., Citation2007). However, it is not clear whether the antioxidative effect of SDF is secondary to decreased glucose levels.
Kaviarasan et al. (Citation2004) reported that polyphenol-rich fenugreek seeds extract significantly reduced H2O2-induced oxidative modifications in normal and diabetic human erythrocytes, suggesting potent antioxidant properties of the fenugreek seeds. Another study examined the antioxidant properties of germinated fenugreek seeds, which are considered to be more beneficial than dried seeds by providing essential amino acids, especially leucine, lysine and tryptophan (Mansour & El-Adawy, Citation1994). Further, seed germination activates many proteolytic and lipid degrading enzymes that help improve protein digestibility as well as fat absorption capacity (Mansour & El-Adawy, Citation1994) because germination leads to decreased levels of total unsaturated fatty acids, total lipid, triglycerides, phospholipids and unsaponifiable matter while those of saturated fatty acids are increased (El-Mahdy & El-Sebaiy, Citation1983). Dixit et al. (Citation2005) used different fractions of the germinated seeds to determine their antioxidant potential by assaying ferric reduction, radical scavenging by 1,1-diphenyl-2-picrylhydrazyl, ferrylmyoglobin/2,2′azobis-3-ethylbenzthiazoline-6-sulfonic acid, pulse radiolysis, oxygen radical absorbance capacity and inhibition of lipid peroxidation in mitochondrial preparations from rat liver (Dixit et al., Citation2005). An aqueous fraction of fenugreek containing mostly flavonoids and polyphenols exhibited the highest antioxidant activity, suggesting that soaking and subsequent processes unleash the antioxidative effect. As oxidative stress is involved in the development and progression of diabetic nephropathy (DN), Xue et al. (Citation2011) examined antioxidant activity of Trigonella seed aqueous extract in restoring the kidney function of diabetic rats. They observed that treatment with Trigonella extract showed upregulation in the activities of SOD and catalase, and decrease in the concentrations of malondialdehyde (MDA) in the serum and kidney of diabetic rats in addition to decreased levels of 8-hydroxy-2′-deoxyguanosine, a marker of increased oxidative stress, in urine. These findings suggested that supplementation of Trigonella extract significantly increased antioxidant enzymes activities in the kidney thereby conferred protection against functional and morphologic injuries in diabetic kidneys (Xue et al., Citation2011). Similar results were found in another study where Trigonella improved both the TBARS levels and antioxidant enzyme activities in tissues such as heart, kidney and liver, suggesting antioxidant potential of Trigonella against damages caused due to diabetes-induced oxidative stress (Tripathi & Chandra, Citation2009). In a study by Thirunavukkarasu et al. (Citation2003), an aqueous extract of fenugreek seeds showed similar effects on lipid peroxidation and antioxidant status in experimental ethanol toxicity in rats. The ethanol feeding to rats for 60 days resulted in a significant increase in the activities of serum aspartate transaminase, alanine transaminase, alkaline phosphatase along with elevated levels of serum lipid hydroperoxides and TBARS in liver and brain. The activities of antioxidant enzymes such as SOD, catalase, GPx, glutathione-S-transferase (GST) and GR decreased in liver and brain, which accompanied depletion of glutathione, ascorbic acid and α-tocopherol concentrations. Simultaneous administration of aqueous extract of fenugreek seeds with ethanol prevented the enzymatic leakage and the rise in lipid peroxidation and enhanced antioxidant potential. The seeds exhibited appreciable antioxidant properties in vitro comparable with that of GSH and α-tocopherol (Thirunavukkarasu et al., Citation2003). Although the exact nature of the antioxidant constituent in Trigonella seed is not known, these investigators suggested that Trigonella seed-derived flavonoids and polyphenols have antioxidative properties, which have been shown to reduce oxidative stress in different experimental model systems.
Antidiabetic effect of Trigonella
Increased levels of glucose and deranged glucose metabolism are hallmarks of diabetes mellitus (DM). The increased blood glucose is attributed to insulin deficiency or resistance, resulting in diminished glucose utilization in insulin-dependent tissues such as liver and muscle tissues that require insulin for glucose uptake. On the other hand, in insulin-independent tissues such as cardiac tissue, blood vessels, peripheral nerves, renal medulla and lens, there is glucose overutilization due to sustained hyperglycemia. It is well known that regulating blood glucose is the best way to prevent diabetes-related tissue damage and secondary complications such as cardiovascular diseases including micro- and macro-angiopathy, nephropathy, neuropathy and retinopathy. However, controlling blood glucose is difficult and there is no medicine available to achieve this goal. Many investigators have indicated that crude as well as various extractions of Trigonella successfully decreased blood glucose levels in experimental animals as well as human diabetic patients. Out of a number of medicinal properties known of Trigonella, its hypoglycemic or antihyperglycemic effect has been studied the most and has also been utilized by diabetic patients (Bordia et al., Citation1997; Khan et al., Citation2012; Sharma et al., Citation1990).
Several investigators have used various types of Trigonella extracts in the experimental models and have demonstrated their hypoglycemic effect. We have covered the most recent references here. In type-2 diabetic rats daily oral administration of Trigonella seed-derived soluble dietary fiber (SDF) for 28 days decreased serum glucose, increased liver glycogen content and enhanced total antioxidant status; however, serum insulin and insulin secretion remained unaffected (Hannan et al., Citation2007). In cultured 3T3-L1 adipocytes, glucose transport and insulin action increased by Trigonella. These studies suggest that antidiabetic effect of T. foenum-graecum seed-derived SDF is mediated through inhibition of carbohydrate digestion and absorption, and enhanced peripheral insulin action (Hannan et al., Citation2007). It is suggested that SDF fraction suppressed the elevation of blood glucose after oral sucrose ingestion in both non-diabetic and type-2 diabetic rats. Further, intestinal disaccharidase activity and glucose absorption decreased and gastrointestinal motility increased by the SDF fraction (Hannan et al., Citation2007).
Recently, Morani et al. (Citation2012) demonstrated ameliorative effects of Trigonella seed extract on painful peripheral neuropathy in rats. Fenugreek seed-derived fraction, named IND01 by the authors, has been purified and standardized by high-performance liquid chromatography (HPLC) to a marker compound trigonelline. Daily oral administration of IND01 for 15 days restored motor nerve conduction velocity in rats with SNCI. The results from this study suggested a neuroprotective role of Trigonella in painful peripheral neuropathy commonly observed in diabetes (Morani et al., Citation2012).
Kannappan and Anuradha (Citation2009) observed insulin-sensitizing actions of fenugreek seed-derived polyphenols and found it comparable to a well-known diabetic drug metformin in a rat model. In experimental rats fructose-feeding caused increased levels of glucose, insulin, triglycerides (TG) and free fatty acid (FFA), altered insulin sensitivity indices, enzyme activities and reduced glycogen content. Further, fructose-rich diet leads to higher protein tyrosine phosphatase (PTP) and lower protein tyrosine kinase (PTK) activities, suggesting decreased tyrosine phosphorylation status in these rats. Administration of fenugreek seed polyphenolic extract (FPEt) improved insulin sensitivity and tyrosine phosphorylation status in fructose-fed animals compared to metformin-treated rats. This indicates that FPEt improved insulin signaling and sensitivity and thereby promoted the cellular actions of insulin (Kannappan & Anuradha, Citation2009).
Increased glucose level has a causal correlation with atherosclerotic changes in diabetic patients. A beneficial vascular effect of aqueous leaf extract of Trigonella has previously been reported; Mahdavi et al. (Citation2008) examined how Trigonella leaf extract may affect the vascular pathophysiology in diabetic rats. The diabetic rats received Trigonella extract (200 mg/kg; i.p.) every other day for 1 month and contractile reactivity of the thoracic aorta to KCl and noradrenalin and relaxation response to acetylcholine (ACh) was determined. A significant increase in the maximum contractile response to KCl and Na and a significant decrease in maximum relaxation due to ACh in diabetic rats have been observed as compared to controls. The treatment with Trigonella extract significantly improved these changes, suggesting its ameliorative effects on the vascular system in diabetic rats (Mahdavi et al., Citation2008).
The preceding evidence indicates that Trigonella not only regulates glucose levels but also blocks the development of secondary diabetic complications. These effects, however, could be associated with better control of blood glucose, which prevents the development of secondary complications. Thus, it appears that antidiabetic effects of Trigonella may emanate from its hypoglycemic properties.
Antilipidemic effect of Trigonella
Increasing incidence of obesity and subsequent development of many lifestyle pathogenesis constituting metabolic syndrome is a major health concern globally. Obesity is correlated with modern urban lifestyle that involves lack of physical activity and sedentary lifestyle and a calorie-rich diet, which leads to accumulation of excess fat as well as deranged fat metabolism. It is believed that if levels of lipids, especially LDL-cholesterol and triglycerides, are controlled, it can markedly prevent many chronic inflammatory diseases that emanate from obesity related low-grade inflammation. Trigonella has been shown to regulate the lipid levels in experimental models (Bordia et al., Citation1997). Fenugreek showed lower serum TG and total cholesterol and hepatic lipid concentrations (Annida et al., Citation2004; Hannan et al., Citation2003; Raju & Bird, Citation2006). Fenugreek given at a dose of 2.5 g twice daily for 3 months to healthy individuals showed no affect on the blood lipids and fasting or postprandial blood sugar. However, fenugreek administered in similar fashion to coronary artery disease (CAD) patients with or without type-2 diabetes significantly decreased blood lipids, total cholesterol and triglycerides, without affecting the HDL-cholesterol (Bordia et al., Citation1997). In another study, Trigonella-derived SDF showed a beneficial effect on dyslipidemia and a tendency to inhibit platelet aggregation in type-2 diabetic rats (Hannan et al., Citation2003). Atherogenic lipids, that is, triglycerides, cholesterol and LDL-cholesterol, have been found to decrease significantly in SDF-fed rats, while HDL-cholesterol increased. The hepatic lipid-lowering effect of fenugreek seeds can be attributed to its role in modulating the activity of several glucose and lipid metabolism enzymes (Raju et al., Citation2001; Yadav et al., Citation2004) or to its ability to enhance biliary cholesterol excretion (Stark & Madar, Citation1993).
The pathophysiology of many diseases of aging brain involves lipid peroxides (LPO) derived from lipid membrane and cholesterol metabolism. In an experimental model of AlCl3-fed rats, simultaneous supplementation of fenugreek seeds powder or extract for 5 months enhanced the levels of LPO in posterior brain, liver and plasma, along with lactate dehydrogenase (LDH) activities, whereas total cholesterol, TG and LDL-cholesterol levels reversed, suggesting an antiperoxidative role in the brain which may be attributed to its modulatory effect on plasma lipid metabolism (Belaïd-Nouira et al., Citation2012).
Further, feeding ethanol extract of Trigonella seed to hypercholesterolemic rats showed 18 to 26% reduced plasma cholesterol levels and lowered concentrations of liver cholesterol, suggesting its hypocholesterolemic effect. The authors suggested that saponin-like active components in the ethanol extract of fenugreek seeds may have interacted with bile salts in the digestive tract and modified lipid metabolism (Stark & Madar, Citation1993). Fenugreek seed-derived steroid saponins also modified feeding behavior by enhancing food consumption and motivation to eat, and reduce plasma cholesterol levels (Roberts, Citation2011). Furthermore, in a recent study, fenugreek has been demonstrated to decrease the hepatic TG and total cholesterol levels dose-dependently and increase the excretion of cholesterol and total bile acids into the feces (Muraki et al., Citation2011). Saponins are known to affect lipid metabolism, including liver and plasma TG and plasma cholesterol concentrations (Li et al., Citation2008; Trinh et al., Citation2007). Fenugreek seeds contain saponins along with various alkaloids, flavonoids and polyphenols. Although the molecular mechanism of how fenugreek regulates lipid metabolism is still unclear, Uemura et al. (Citation2011) showed that Trigonella seed-derived saponins, diosgenin, inhibited the accumulation of TG and the expression of lipogenic genes in a hepatic cell line (HepG2 cells) by inhibiting the transactivation of liver-X-receptor-α and suggested that fenugreek could ameliorate dyslipidemia by decreasing the hepatic lipid content in diabetic mice via diosgenin. Roberts (Citation2011) further elaborated the potential of fenugreek as a functional food and nutraceutical and its effects on glycemia and lipidemia and attributed hypocholesterolemia to the gum contained in fenugreek seed.
Anticancer effect of Trigonella
Cancer is one of the leading causes of mortality worldwide. Conventional therapeutic modalities only extend the patient’s lifespan by a few months or years but cause serious side effects. In alternative medicine, fruits and vegetables or their active ingredients are recommended for prevention of cancer. Increased efforts to utilize alternative concepts or approaches to prevent cancer are underway. In this effort, many studies have demonstrated the protective effect of fenugreek seeds in experimental models of cancer using cell lines or experimental animals. Trigonella foenum-graecum, traditionally used to treat disorders such as diabetes, high cholesterol, wounds, inflammation and gastrointestinal ailments, has been recently demonstrated to possess anticarcinogenic potential. Hibasami et al. (Citation2003) demonstrated that fenugreek-derived compound protodioscin displayed a growth inhibitory effect against HL-60 cells by inducing apoptotic changes. Amin et al. (Citation2005) also showed that fenugreek seed extract significantly inhibited 7,12-dimethylbenz(α)anthracene-induced mammary hyperplasia and decreased its incidence in rats and suggested that fenugreek’s anti-breast cancer protective effects could be due to increased apoptosis. Further, alcoholic whole plant extracts of Trigonella foenum-graecum showed in vitro cytotoxicity against different human cancer cell lines such as IMR-32, a neuroblastoma cell line, and HT29, a cancer cell line (Verma et al., Citation2010). Sebastian and Thampan (Citation2007) examined the effect of aqueous and ethanol extracts of fenugreek on the growth of MCF-7 cells, an estrogen receptor positive breast cancer cell line, and reported that the ethanol extract of fenugreek decreased cell viability and induced early apoptotic changes such as inversion of phosphatidylserine and decreased mitochondrial membrane potential. Further, degradation of DNA into fragments comprising multiples of approximately 180–200 base pair has also been observed. Cell cycle analysis revealed a sub-G1 apoptotic population along with cell cycle arrest at G2/M phase in fenugreek extract-treated cells implicating the role of fenugreek extract-induced apoptosis in its anticancer role (Sebastian & Thampan, Citation2007). Further, according to the study by Shabbeer et al. (Citation2009), treatment with fenugreek extract showed growth inhibitory effects on breast, pancreatic and prostate cancer cell lines but primary prostate or immortalized prostate cells remained unaffected. Inhibition of cancer cell growth by Trigonella is attributed to its ability to induce cell death, despite simultaneous upregulation of growth stimulatory pathways in normal cells.
In the Ehrlich ascites carcinoma (EAC) model in Balb-C mice, Trigonella seed extract showed an antineoplastic effect. Intra-peritoneal administration of the alcohol extract of the Trigonella seed before as well as after inoculation of EAC cell in mice decreased tumor cell growth by more than 70% as compared to the control not treated with the extract (Sur et al., Citation2001). Prabhu and Krishnamoorthy (Citation2010) also demonstrated anticancer activity of the ethanol extract of Trigonella in EAC cells induced cancer in Swiss albino mice. The mice inoculated with EAC and treated with Trigonella leaf extract showed increased lifespan in comparison with the tumor control, suggesting anticancer activity of fenugreek leaf extract in animal models (Prabhu & Krishnamoorthy, Citation2010). The Trigonella extract also produced a significant anti-inflammatory effect and enhanced both the peritoneal exudate cell and macrophage cell counts (Verma et al., Citation2010). Antioxidant properties of fenugreek seed and its ability to modulate hepatic oxidative stress is implicated in 1,2-dimethylhydrazine (DMH)-induced colon cancer in Wistar rats. The DMH-treated rats showed 100% colon tumor incidence accompanied by enhanced LPO and decreased GSH content, GPx, GST, SOD and catalase activities in liver. A diet containing fenugreek seed powder reduced colon tumor incidence and LPO in DMH-treated rats and also increased activities of GPx, GST, SOD and catalase in liver (Devasena & Menon, Citation2007).
In another study, diosgenin, a steroid saponin derived from Trigonella, was found to inhibit azoxymethane (AOM)-induced aberrant crypt foci formation, a preneoplastic colonic lesions in F344 rats. The same study also reported Trigonella-derived, diosgenin-induced apoptosis in HT-29 human colon cancer cells by inhibiting bcl-2 and inducing caspase-3 protein expression, suggesting its potential as a colon cancer preventive agent (Raju et al., Citation2004). Further, Li et al. (Citation2010) showed that diosgenin could modulate the STAT3 signaling pathway in hepatocellular carcinoma by suppressing the activation of c-Src, JAK1 and JAK2. Diosgenin also downregulated the expression of various STAT3-regulated genes, inhibited proliferation and potentiated the apoptotic effects of paclitaxel and doxorubicin, suggesting that diosgenin could be a novel and potential treatment option for hepatocellular carcinoma and other cancers (Li et al., Citation2010).
Anti-inflammatory effect of Trigonella
Inflammation, a defensive mechanism of the body, is a complex biological response of a vascularized tissue to potentially harmful external or internal stimuli including pathogens, chemical, foreign agents, xenobiotics and so on. The immune cells such as macrophages, dendritic cells and other leukocytes present in the tissue distinguish foreign molecules from host molecules through the pattern recognition receptors on their membrane and are activated. Upon activation by recognizing as pathogen-associated molecular patterns (PAMPs), the immune cells release inflammatory mediators responsible for the clinical signs of inflammation such as redness, swelling, warmness (heat), pain leading to tissue damage and loss of function. These steps lead to identification of pathogen or foreign substances that are then cleared by immune cells; however, overwhelming inflammatory response is dangerous to tissue and body. Therefore anti-inflammatory substances are immensely important to subside the inflammation. There are many anti-inflammatory agents that are known to reduce the inflammatory response such as corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs). These agents, however, have serious side effects such as gastric erosion and ulcers, exacerbation of asthma, kidney damage and in some cases myocardial infarction. Therefore, identification of natural, plant-based and nontoxic anti-inflammatory is highly important to treat various inflammatory disorders.
Many investigators have demonstrated the ant-inflammatory potential of Trigonella in experimental models. In an in vitro model, a methanol extract of fenugreek seed inhibited the production of phorbol-12-myristate-13-acetate-induced inflammatory cytokines such as tumor necrosis factor (TNF)-α in cultured THP-1 cells (Kawabata et al., Citation2011). In an adjuvant-induced arthritis in albino rats, Suresh et al. (Citation2012) showed that an ethanol extract of Trigonella significantly decreased paw edema and decreased levels of IL-1α, IL-1β, IL-2, IL-6 and TNF-α. The extract also significantly decreased the levels of LPO and increased the SOD and GSH levels in cartilage tissue (Suresh et al., 2012). These results suggested anti-inflammatory and antioxidant activities of Trigonella, which could be responsible for antiarthritic activity.
Vyas et al. (Citation2008) examined analgesic and anti-inflammatory effects of a partially purified fraction of the Trigonella seed extract in a mouse model of chemically (acetic acid) and thermally (hot-plate) induced pain. In comparison to the non-treated group, the Trigonella extract-treated group showed significant dose-dependent analgesic activity against chemically as well as thermally induced pain (Vyas et al., Citation2008). Further, Trigonella seed extract showed significant analgesic and anti-inflammatory activity in the carrageenan-induced rat paw edema as compared to diclofenac sodium, a well-known analgesic. The authors suggested that flavonoid components of fenugreek seeds in aqueous and acidified chloroform fractions could have anti-inflammatory effects as it significantly inhibited carrageenan-induced paw edema (Mandegary et al., Citation2012).
Antibacterial and antifungal effect of Trigonella
Plant-derived compounds with antibacterial and antifungal activities have been identified and reviewed (Ahmad & Beg, Citation2001; Aqil & Ahmad, Citation2007; Gangoué-Piéboji et al., Citation2009; Giordani et al., Citation2002; Khodaie et al., Citation2012; Rabe & van Staden, Citation1997). However, the antibacterial and antifungal role of Trigonella is recently being uncovered. Haouala et al. (Citation2008) prepared aqueous extracts from various plant parts of fenugreek leaves and stems, roots, ground and non-ground seeds in petroleum ether, ethyl acetate and methanol fractions of the aerial parts and determine their antifungal potential against fungal strains including Botrytis cinerea, Fusarium graminearum, Alternaria sp., Pythium aphanidermatum and Rhizoctonia solani. They found that all parts of the fenugreek plant showed antifungal potential and the magnitude of effect depends upon fungal species and plant parts. They further identified that the methanol fraction has the main antifungal activity, which totally inhibited the growth of R. solani and Alternaria sp. This study suggested that fenugreek could be an important source of biologically active compounds useful for developing better and novel antifungal drugs (Haouala et al., Citation2008). Various investigators have also showed effectiveness of Trigonella extracts against Helicobacter pylori (O’Mahony et al., Citation2005; Randhir et al., Citation2004; Randhir & Shetty, Citation2007). In one study, honey samples with highest antibacterial activity against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus shown maximum pollens from Trigonella, among other plants (Mercan et al., Citation2007).
Defensins are small cysteine-rich peptides with potent antifungal activity. Olli and Kirti (Citation2006) have successfully cloned Trigonella-derived cDNA of 225 bp cysteine-rich defensin named Tfgd1. The recombinant protein expressed in E. coli exhibited antifungal activity against the broad range of fungi, R. solani and the peanut leaf spot fungus, Phaeoisariopsis personata.
The methanol soluble fraction of Trigonella extract showed nematicidal activity and caused significant mortality of Meloidogyne javanica larvae (>92%), suggesting the potential use against nematodes (Zia et al., Citation2001).
Hepatoprotective and nephroprotective effects of Trigonella
Hepatotoxicity and chronic liver injury due to various reasons are the major metabolic disorders affecting individuals of all ages (Dhiman & Chawla, Citation2005). Chronic alcoholism is one of the many reasons associated with liver diseases and fibrosis (Bellentani et al., Citation1997; Patsenker et al., Citation2011). Similarly, many allopathic medicines are also known to cause liver damage (Bell & Chalasani, Citation2009; Dhiman et al., Citation2012; Martinez et al., Citation2012; Williams et al., Citation2011). Therefore, there is growing need to utilize traditional knowledge of herbal hepatoprotective agents and develop plant-based nontoxic and clinically safe hepatoprotective medicines (Alvari et al., Citation2012). Many herbal extracts are traditionally used as hepatoprotective agents in the ancient Indian and Chinese systems of medicine (Dhiman et al., Citation2012; Dhiman & Chawla, Citation2005). The digestion-stimulating effect of Trigonella may emanate from its hepatoprotective role. Kaviarasan et al. (2006) showed that in human Chang liver cells EtOH treatment suppressed the Chang liver cells’ growth, induced cytotoxicity, oxygen radical formation and mitochondrial dysfunction, and concentration of oxidized glutathione (GSSG), while decreased the GSH level as compared with normal cells. Incubation of cells with a polyphenolic extract of fenugreek seeds along with EtOH significantly increased cell viability in a dose-dependent manner, reduced lactate dehydrogenase leakage, TBARS formation and normalized the GSH/GSSG ratio. These cytoprotective effects of FPEt are comparable with those of silymarin, a known hepatoprotective agent. Kaviarasan and Anuradha (Citation2007) further examined the hepatoprotective effect of FPEt in vivo using chronic ethanol-induced hepatic injury in a rat model. FPEt administration restored the altered levels of liver function enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), bilirubin and gamma-glutamyl transferase and decreased liver glycogen. FPEt also mitigated the alterations in alcohol-metabolizing and detoxification enzymes such as alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) and the electron transport component cytochrome-c reductase. Treatment of hepatocytes ex vivo with FPEt increased viability and reduced apoptotic nuclei. The administration of fenugreek seed to alcohol-fed rats significantly improved lipid profile and reduced collagen content, aldehyde content and peroxidation, an effect comparable to that of silymarin (Kaviarasan & Anuradha, 2007). Further, in a goat model of H2O2- and CCl4-induced liver damage, ethanol extract of Trigonella leaves showed a significant hepatoprotective effects as evidenced by decreased levels of enzymatic and nonenzymatic antioxidant enzymes (Meera et al., Citation2009). The extract also showed significant antilipid peroxidation effects in vitro, besides exhibiting significant activity in superoxide radical and nitric oxide radical scavenging, indicating their potent antioxidant effects. Raju and Bird (Citation2006) showed that in Ob rats fed with fenugreek-supplemented diets, hepatic triglyceride level and the soluble and bound forms of TNF-α protein significantly decreased in comparison to control, suggesting that dietary fenugreek supplementation could reduce the triglyceride accumulation in the liver, a hallmark feature of hepatic steatosis.
The effect of fenugreek-derived 4-hydroxyisoleucine, a well-known insulinomimetic agent, was examined on liver function and blood glucose in insulin-resistant, fructose-fed rats and streptozotocin-induced diabetic rat. In fructose-fed rats, levels of glucose and liver damage marker AST and ALT significantly elevated compared to controls, and restored to near control values by 4-hydroxyisoleucine (Haeri et al., Citation2009). Further, 4-hydroxyisoleucine improved HDL-cholesterol levels in diabetic rats. The control animals tolerated the prolonged 4-hydroxyisoleucine treatments without any alteration in the levels of glucose or liver damage markers. These results suggest liver-protecting properties of fenugreek-derived 4-hydroxyisoleucine besides its usefulness in insulin resistance (Haeri et al., Citation2009).
Organ toxicity is also caused by commonly used anticancer drugs, for example, cyclophosphamide (CP) by their reactive metabolites such as acrolein and phosphoramide mustard. Bhatia et al. (Citation2006) observed that pretreatment of rats with fenugreek extract restored the CP-induced and l-buthionine-SR-sulfoximine (BSO)-augmented decrease in the activities of GST, GR, GPx, catalase and GSH in urinary bladder of mice. These findings suggest that fenugreek could be consideration in complementary therapy in cancer patients to prevent chemotherapeutic toxicity. Xue et al. (Citation2011) examined antioxidant activity of Trigonella seed aqueous extract in restoring the kidney function of diabetic rats and found that treatment with Trigonella extract ameliorated ultra-morphologic abnormalities in the kidney of diabetic rats, such as uneven thickening of the glomerular base membrane. These findings suggest that Trigonella extract conferred protection against functional and morphologic injury in diabetic kidneys.
Antigastric ulcer and anti-gallstone effect of Trigonella
Fenugreek seeds are commonly used as a condiment to flavor curries and other recipes and are known for nutritive and digestion stimulating properties. In the Indian system of traditional medicine, Ayurveda, fenugreek seeds have been used to treat a number of gastrointestinal disorders (Puri, Citation1998). However, experimental evidence has been lacking. In a recent study, Pandian et al. (Citation2002) showed antiulcer potential of fenugreek seeds. The effect of fenugreek seeds is comparable to omeprazole, a known proton pump blocker used in the treatment of gastrointestinal problems such as gastroesophagel reflux disease, gastric and duodenum ulceration, and gastritis (Pandian et al., Citation2002). In a rodent model of ethanol-induced gastric ulcer, the aqueous extract and a gel fraction isolated from fenugreek seeds showed significant ulcer protective effects that is attribute to its antisecretory action as well as effects on mucosal glycoproteins. Further, ethanol-induced lipid peroxidation and subsequent mucosal injury is prevented by fenugreek seed extract presumably by enhancing antioxidant potential of the gastric mucosa (Pandian et al., Citation2002). Investigators have also shown antioxidant potential of aqueous extract of Trigonella seeds (Anuradha & Ravikumar, Citation1998) and administration of antioxidants is known to inhibit ethanol-induced gastric injury in the rat (Ligumsky et al., Citation1995). Furthermore, the soluble gel fraction of fenugreek seed has been found better than omeprazole in preventing gastric lesion formation. The authors speculated that the polysaccharide composition of the gel and/or the flavonoids could be responsible for the gastroprotective and antisecretory activities of fenugreek seeds (Pandian et al., Citation2002). As fenugreek seeds are known to possess high levels of flavonoids, and Saurez et al. (Citation1996) have demonstrated flavonoids protective role in the mucosa by preventing various necrotic agents induced lesions formation, this speculation could be valid. However, more experimental evidence is warranted.
Further, the antilithogenic effect of dietary fenugreek seeds has been shown by many investigators (Reddy & Srinivasan, Citation2009; Citation2011a,Citationb). Lithogenic conditions were induced in mice by feeding them a high (0.5%) cholesterol diet (HCD) for 10 weeks without or with supplementation of fenugreek (12%) or onion (2%) or both. Fenugreek, onion and their combination reduced the incidence of cholesterol gallstones by 75, 27 and 76%, respectively, showing highest antilithogenic influence of fenugreek alone, and the presence of onion showed no augmentation to this effect. Consequently, the cholesterol/phospholipid ratio reduced significantly in serum, liver and bile. Changes in the hepatic enzyme activities (3-hydroxy-3-methylglutaryl coenzyme A reductase, cholesterol-7α-hydroxylase and cholesterol-27-hydroxylase) induced by HCD showed significant reversal by fenugreek (Reddy & Srinivasan, Citation2009). Further, increased accumulation of fat in the liver and inflammation of the gallbladder membrane produced by HCD decreased significantly by fenugreek as well as its combination with onion (Reddy & Srinivasan, Citation2011a).
As cholesterol gallstones are known to be controlled by pro- and anti-crystallizing factors present in bile, Reddy and Srinivasan (Citation2011b) examined the effect of dietary fenugreek on the composition of bile in rats fed for 10 weeks with a high cholesterol diet. Fenugreek supplementation of HCD decreased the cholesterol, total protein, glycoprotein, lipid peroxides and cholesterol saturation index in bile, and increased the bile flow rate, and cholesterol nucleation time. Fenugreek also significantly increased biliary phospholipid and total bile acid, indicating that beneficial antilithogenic effect of dietary fenugreek could be due to decreased cholesterol content of bile and modulation of the nucleating and antinucleating proteins, which are known to regulate cholesterol crystallization (Reddy & Srinivasan, Citation2011b). As fenugreek seed is known for its hypocholesterolemic properties, dietary supplementation should have a beneficial role in the prevention and treatment of cholesterol gallstones (CGS). Fenugreek seed powder when fed along with lithogenic diet (0.5% cholesterol) for 10 weeks it significantly lowered the incidence of CGS in mice (Reddy & Srinivasan, Citation2009). The antilithogenic influence of fenugreek is attributed to its hypocholesterolemic effect as it significantly decreased serum cholesterol level as well as hepatic cholesterol in these high cholesterol-fed animals.
Miscellaneous effect of Trigonella
There are many other medicinal effects of Trigonella or Trigonella-derived molecules that are not well known or very few studies have reported such properties. For examples, in the study by Hamden et al. (Citation2010), when diabetic rats received fenugreek-derived steroids orally for 30 days, it potentially unregulated the key steroidogenic enzymes activities such as 3-hydroxy-3-methyl-glutaryl-CoA reductase, malic enzyme, 3-β-hydroxysteroid dehydrogenase and glucose-6-phosphate dehydrogenase in testis, which considerably enhanced testosterone and estradiol levels in the plasma as well as testicular glycogen and seminal fructose contents in surviving diabetic rats. Furthermore, fenugreek-derived steroids administration to surviving diabetic rats significantly decreased the sperm shape abnormality and improved the sperm count and protected reproductive systems from menace of high glucose as determined by histological study of testis and epididymis. Additionally, blood glucose level decreased and β-cells of diabetic rats showed a considerable increase of insulin-immunoreactive area (Hamden et al., Citation2010).
In a pilot study, subjects with frequent heartburn, a symptom of indigestion, when given fenugreek fiber product, 30 min before the meals/day for 2 week, showed diminished heartburn severity reducing the use of a mild antacid as a rescue medicine. Moreover, the fenugreek fiber showed effects similar to that of an over-the-counter antacid medication (ranitidine at 75 mg, twice a day), suggesting benefits of fenugreek fiber products in people with certain degrees of heartburn (DiSilvestro et al., Citation2011).
Active ingredients of Trigonella
Most of the herbal products used as medicine are primarily part of our diet and are consumed almost daily as such. The epidemiological studies indicate the health benefit of such a use for the long time in different populations, but to utilize the therapeutic potential of herbal extract the extraction, isolation and characterization is necessary. Further, proper pharmacological and toxicological studies of the active ingredients would lead to their approval for clinical application as therapeutic drugs. A number of active ingredients from fenugreek have been isolated and studied including mostly alkaloids, saponins and flavonoids such as coumarin, fenugreekine, nicotinic acid, sapogenins, phytic acid, scopoletin, trigonelline, disogenin, gitogenin, neogitogenin, homorientin saponaretin, trigogenin and mucilaginous fiber. These constituents have been examined for their potential medicinal values by various investigators as discussed in this review. Isolation of furostanol saponins called trigoneoside Ia, Ib, IIa, IIb, IIIa and IIIb has been reported by Yoshikawa et al. (Citation1997) and Petit et al. (Citation1995). Furostanol saponins have been shown to increase food consumption and induce hypocholesterolemia in experimental diabetic rats (Petit et al., Citation1995). Disogenin (saponin) and trigonelline (alkaloid) inhibit glucose uptake in vitro (Al-Habori et al., Citation2001). Similarly, our group has also shown that Trigonella seed extract modulated the expression of glucose transporter 4 (glut-4) in skeletal muscle (Mohammad et al., Citation2006). An amino acid extracted and purified from Trigonella seed, 4-hydroxyisoleucine, displayed a hypoglycemic and insulinotropic properties both in vitro as well as in vivo (Basch et al., Citation2003; Broca et al., Citation1999). In the Trigonella seed extract at least five flavonoids (namely kaempferol-3-O-glucoside, apigenin-7-O-rutinoside, naringenin, quercetin and vitexin) have been identified using LC-MS/MS. The antiperoxidative action of fenugreek seeds in the brain during diabetes could also be attributed to its hypoglycemic property (Raju & Bird, Citation2006; Siddiqui et al., Citation2005). It is likely that inhibition of LPO and subsequently of LDH could be due to anti-free radical and antioxidant potential of polyphenolic flavonoids of Trigonella seeds emphasized through in vitro and in vivo experiments (Genet et al., Citation2002; Kaviarasan et al., Citation2006, 2007, Citation2008; Kaviarasan & Anuradha, Citation2007). Muraki et al. (Citation2011) determined the effective, safe and tolerable dose of fenugreek extract to be around 2.50% (w/w) in experimental rats.
Potential side effects of Trigonella
Every drug has potential side effects and even herbal products are not completely safe in this regard. Efforts should be taken to avoid making herbal agents panacea for every ailment and there should be scientific evaluation of their beneficial effects in health and disease as well as of their potential side effects. Therefore, there is greater need to study the pharmacological and toxicological effects of herbal products to examine their clinical efficacy and safety. In this regard, side effects of Trigonella and its different preparations have been studied to spell out its potential side effects. Although no major clinical trials have been undertaken to study the use of Trigonella as mainstream or alternative herbal medicine, most of the side effects known today are the result of either user reported symptoms such as stomach upset, diarrhea, or bloating in animal studies (Muraki et al., Citation2011).
Experts advise that some rather serious side effects including signs of low blood sugar such as nervousness, shakiness, fast heartbeat, sweating may occur. Although a very serious allergic reaction to this product is rare, minor rash itching/swelling, especially of the face/tongue/throat, severe dizziness, trouble breathing may occur in some patients. In theory, fenugreek may increase the risk of bleeding. It is likely that fenugreek may lower blood sugar levels. Thus, patients taking oral drugs for diabetes or using insulin should be monitored closely by a health care professional while using fenugreek. There is some evidence that fenugreek may reduce potassium levels in the blood. Although it has not been widely studied in humans, fenugreek may alter the levels of thyroid hormones.
Consumption of fenugreek seeds during pregnancy has been associated with a range of congenital malformations, including hydrocephalus, anencephaly and spina bifida. Khalki et al. (Citation2010) evaluated the potential toxic effects of fenugreek seeds (lyophilized aqueous extract) on pregnant mice and fetal development. The extract was administered to mated female mice during the entire period of pregnancy, at doses of 500 and 1000 mg/kg/day. The mothers showed no obvious symptoms of toxicity, but an increase in the fetal death rate, a decrease in the litter size, and a reduction in the fetal body weight and increase in the incidence of morphological abnormalities observed in offspring (Khalki et al., Citation2010). This study suggested that fenugreek may have deleterious toxic effects on reproductive performance and potential teratogenic effects in fetuses. However, more detailed study is needed to ascertain these findings.
The same group examined longer-term neurobehavioral effects in prenatally exposed mice (Khalki et al., Citation2012). Pregnant females received 0, 500 or 1000 mg/kg/day aqueous extract of fenugreek seed by gavage for the whole period of gestation. The pup’s body weights measured at 1, 7, 14, 21 and 28 days of age and behavior of progenies evaluated three weeks after birth using the open field, the rotarod test and the continuous alternation task by the T-maze. At 28 postnatal day age, brain of progeny was examined histologically. The progeny of exposed mice displayed reduced body weight at birth and reduced brain weight, significant decrease in the locomotor activity and motor coordination (Khalki et al., Citation2012). These results suggest that prenatal exposure of mice to a high dose of fenugreek seeds caused growth retardation and altered neurobehavioral performance in the post-weaning period, though the molecular reasons remain to be determined.
Araee et al. (Citation2009) examined the effect of fenugreek on fetal macroscopic diameters and microscopic bone-marrow cell histological changes in its teratogenic dosages in rats. A positive relationship between the injected drug dosage and fetal mortality rate observed. Among all fetal diameters, ear-to-ear diameter decreased in groups received fenugreek decoction. The severity of stem cell histological changes significantly decreased compared to control, suggesting that fenugreek in teratogenic dosages can decrease the severity of bone marrow cell proliferation and increase fetal mortality rate.
Flammang (Citation2004) investigated genotoxicity potential of a fenugreek seed extract as part of a safety evaluation of novel ingredients. The extract, containing a minimum of 40% 4-hydroxyisoleucine, has been evaluated using the standard tests such as reverse mutation assay; mouse lymphoma forward mutation assay; mouse micronucleus assay as recommended by US Food and Drug Administration (FDA) for food ingredients. Fenugreek seed extract has not been found to be genotoxic under the tested conditions, thus provides support that fenugreek extract supplementation of foodstuffs for people with diabetes is expected to be safe. Muraki et al. (Citation2011) determined the effective, safe and tolerable dose of fenugreek extract to be around 2.50% (w/w) in experimental rats.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdel-Barry JA, Abdel-Hassan IA, Al-Hakiem MH. (1997). Hypoglycaemic and antihyperglycaemic effects of Trigonella foenum-graecum leaf in normal and alloxan induced diabetic rats. J Ethnopharmacol 58:149–55
- Abdo MS, Al-Kafawi AA. (1969). Experimental studies on the effect of Trigonella foenum-graecum. Planta Med 17:14–18
- Ahmad I, Beg AZ. (2001). Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74:113–23
- Al-Habori M, Raman A, Lawrence MJ, Skett P. (2001). In vitro effect of fenugreek extracts on intestinal sodium-dependent glucose uptake and hepatic glycogen phosphorylase A. In J Exp Diabet Res 2:91–9
- Alvari A, Ohadi Rafsanjani MS, Ahmad FJ, Abdin MZ. (2012). Current status and future prospects of hepatoprotective herbal medicines: Contemporary overview into the clinical trials. Rev Recent Clin Trials 7:214–23
- Amin A, Alkaabi A, Al-Falasi S, Daoud SA. (2005). Chemopreventive activities of Trigonella foenum-graecum (Fenugreek) against breast cancer. Cell Biol Int 29:687–94
- Annida B, Stanely Mainzen, Prince P. (2004). Supplementation of fenugreek leaves lower lipid profile in streptozotocin-induced diabetic rats. J Med Food 7:153–6
- Annida B, Stanely Mainzen, Prince P. (2005). Supplementation of fenugreek leaves reduces oxidative stress in streptozotocin-induced diabetic rats. J Med Food 8:382–5
- Anonymous. (1998). The Wealth of India A Dictionary of Indian Raw Materials and Industrial Procedures. New Delhi, India: National Institute of Science and Communication, Council of Scientific and Industrial Research, Vol. X Sp-W
- Anuradha CV, Ravikumar P. (1998). Anti-lipidperoxidative activity of seeds of fenugreek (Trigonella foenum-graecum). Med Sci Res 26:317–21
- Aqil F, Ahmad I. (2007). Antibacterial properties of traditionally used Indian medicinal plants. Methods Find Exp Clin Pharmacol 29:79–92
- Araee M, Norouzi M, Habibi G, Sheikhvatan M. (2009). Toxicity of Trigonella foenum-graecum (Fenugreek) in bone marrow cell proliferation in rat. Pak J Pharm Sci 22:126–30
- Baquer NZ, Kumar P, Taha A, et al. (2011). Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci 36:383–96
- Basch E, Ulbricht C, Kuo G, et al. (2003). Therapeutic applications of fenugreek. Altern Med Rev 8:20–7
- Belaïd-Nouira Y, Bakhta H, Bouaziz M, et al. (2012). Study of lipid profile and parieto-temporal lipid peroxidation in AlCl3 mediated neurotoxicity. Modulatory effect of fenugreek seeds. Lipids Health Dis 11:16–23
- Bell LN, Chalasani N. (2009). Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis 29:337–47
- Bellentani S, Saccoccio G, Costa G, et al. (1997). Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41:845–50
- Bhatia K, Kaur M, Atif F, et al. (2006). Aqueous extract of Trigonella foenum-graecum L. ameliorates additive urotoxicity of buthionine sulfoximine and cyclophosphamide in mice. Food Chem Toxicol 44:1744–50
- Bhatt RH, Khorana ML, Patel JR, et al. (1958). Pharmacological studies of saponins of the fruits of Luffa echinata Roxb. and seeds of Trigonelia foenum-graecum Linn. Indian J Psychol 2:309–21
- Bingel AS, Farnsworth NR. (1991). Higher plants as potential sources of galactagogues. Econ Med Plant Res 6:1–54
- Bin-Hafeez B, Haque R, Parvez S, et al. (2003). Immunomodulatory effects of fenugreek (Trigonella foenum-graecum L.) extract in mice. Int Immunopharmacol 3:257–65
- Bordia A, Verma SK, Srivastava KC. (1997). Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenum-graecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 56:379–84
- Brinker F. (1998). Herb Contradictions and Drug Interactions. 1st ed. Sandy (OR): Eclectic Medical Publications, 70–1
- Broca C, Breil V, Cruciani-Guglielmacci C, et al. (2004). Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab 287:E463–71
- Broca C, Gross R, Petit P, et al. (1999). 4-Hydroxyisoleucine: Experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol 277:617–23
- Chevassus H, Gaillard JB, Farret A, et al. (2010). A fenugreek seed extract selectively reduces spontaneous fat intake in overweight subjects. Eur J Clin Pharmacol 66:449–55
- Chevassus H, Molinier N, Costa F, et al. (2009). A fenugreek seed extract selectively reduces spontaneous fat consumption in healthy volunteers. Eur J Clin Pharmacol 65:1175–8
- Devasena T, Menon PV. (2007). Fenugreek seeds modulate 1,2-dimethylhydrazine-induced hepatic oxidative stress during colon carcinogenesis. Ital J Biochem 56:28–34
- Dhiman A, Nanda A, Ahmad S. (2012). A recent update in research on the antihepatotoxic potential of medicinal plants. Zhong Xi Yi Jie He Xue Bao 10:117–27
- Dhiman RK, Chawla YK. (2005). Herbal medicines for liver diseases. Dig Dis Sci 50:1807–12
- DiSilvestro RA, Verbruggen MA, Offutt EJ. (2011). Anti-heartburn effects of a fenugreek fiber product. Phytother Res 25:88–91
- Dixit P, Ghaskadbi S, Mohan H, Devasagayam TP. (2005). Antioxidant properties of germinated fenugreek seeds. Phytother Res 19:977–83
- Dixit PP, Misar A, Mujumdar AM, Ghaskadbi S. (2010). Pre-treatment of Syndrex protects mice from becoming diabetic after streptozotocin injection. Fitoterapia 81:403–12
- El-Mahdy AR, El-Sebaiy LA. (1983). Changes in carbohydrates of germinating fenugreek seeds (Trigonella foenum-graecum L.). J Sci Food Agric 34:951–6
- Escot N. (1994/95). Fenugreek. Atoms Summar:7–12
- Flammang AM. (2004). Genotoxicity testing of a fenugreek extract. Food Chem Toxicol 42:1769–75
- Gangoué-Piéboji J, Eze N, Ngongang Djintchui A, et al. (2009). The in vitro antimicrobial activity of some medicinal plants against beta-lactam-resistant bacteria. J Infect Dev Ctries 3:671–80
- Genet S, Kale RK, Baquer NZ. (2002). Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum-graecum). Mol Cell Biochem 236:7–12
- Giordani R, Regli P, Buc J. (2002). Antifungal effect of Hevea brasiliensis latex with various fungi. Its synergistic action with amphotericin B against Candida albicans. Mycoses 45:476–81
- Gupta R, Nair S. (1999). Antioxidant flavonoids in common Indian diet. South Asian J Prev Cardiol 3:83–94
- Haefelé C, Bonfils C, Sauvaire Y. (1997). Characterization of a dioxygenase from Trigonella foenum-graecum involved in 4-hydroxyisoleucine biosynthesis. Phytochemistry 44:563–6
- Haeri MR, Izaddoost M, Ardekani MR, et al. (2009). The effect of fenugreek 4-hydroxyisoleucine on liver function biomarkers and glucose in diabetic and fructose-fed rats. Phytother Res 23:61–4
- Hamden K, Jaouadi B, Carreau S, et al. (2010). Potential protective effect on key steroidogenesis and metabolic enzymes and sperm abnormalities by fenugreek steroids in testis and epididymis of surviving diabetic rats. Arch Physiol Biochem 116:146–55
- Hannan JM, Ali L, Rokeya B, et al. (2007). Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr 97:514–21
- Hannan JM, Rokeya B, Faruque O, et al. (2003). Effect of soluble dietary fibre fraction of Trigonella foenum-graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type 2 diabetic model rat. J Ethnopharmacol 88:73–7
- Haouala R, Hawala S, El-Ayeb A, et al. (2008). Aqueous and organic extracts of Trigonella foenum-graecum L. inhibit the mycelia growth of fungi. J Environ Sci (China) 20:1453–7
- Hibasami H, Moteki H, Ishikawa K, et al. (2003). Protodioscin isolated from fenugreek (Trigonella foenum-graecum L.) induces cell death and morphological change indicative of apoptosis in leukemic cell line H-60, but not in gastric cancer cell line KATO III. Int J Mol Med 11:23–6
- Hooda S, Jood S. (2004). Nutritional evaluation of wheat-fenugreek blends for product making. Plant Foods Hum Nutr 59:149–54
- Ikeuchi M, Yamaguchi K, Koyama T, et al. (2006). Effects of fenugreek seeds (Trigonella foenum-greaecum) extract on endurance capacity in mice. J Nutr Sci Vitaminol (Tokyo) 52:287–92
- Jayaweera DMA. (1981). Medicinal Plant: Part III. Peradeniya: Sri Lanka Royal Botanic Garden. pp. 225
- Kannappan S, Anuradha CV. (2009). Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J Med Res 129:401–8
- Kaviarasan S, Anuradha CV. (2007). Fenugreek (Trigonella foenum-graecum) seed polyphenols protect liver from alcohol toxicity: A role on hepatic detoxification system and apoptosis. Pharmazie 62:299–304
- Kaviarasan S, Naik G, Gangabhagirathi R, et al. (2007). In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum-graecum) seeds. Food Chem 103:31–7
- Kaviarasan S, Ramamurty N, Gunasekaran P, et al. (2006). Fenugreek (Trigonella foenum-graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol 41:267–73
- Kaviarasan S, Sundarapandiyan R, Anuradha CV. (2008). Protective action of fenugreek (Trigonella foenum-graecum) seed polyphenols against alcohol-induced protein and lipid damage in rat liver. Cell Biol Toxicol 24:391–400
- Kaviarasan S, Vijayalakshmi K, Anuradha CV. (2004). Polyphenol-rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum Nutr 59:143–47
- Kawabata T, Cui MY, Hasegawa T, et al. (2011). Anti-inflammatory and anti-melanogenic steroidal saponin glycosides from Fenugreek (Trigonella foenum-graecum L.) seeds. Planta Med 77:705–10
- Khalki L, Bennis M, Sokar Z, Ba-M'hamed S. (2012). The developmental neurobehavioral effects of fenugreek seeds on prenatally exposed mice. J Ethnopharmacol 139:672–7
- Khalki L, M'hamed SB, Bennis M, et al. (2010). Evaluation of the developmental toxicity of the aqueous extract from Trigonella foenum-graecum (L.) in mice. J Ethnopharmacol 131:321–5
- Khan V, Najmi AK, Akhtar M, et al. (2012). A pharmacological appraisal of medicinal plants with antidiabetic potential. J Pharm Bioallied Sci 4:27–42
- Khodaie L, Delazar A, Lotfipour F, Nazemiyeh H. (2012). Antioxidant and antimicrobial activity of Pedicularis sibthorpii Boiss and Pedicularis wilhelmsiana Fisch ex. Adv Pharmaceut Bull 2:89–92
- Li F, Fernandez PP, Rajendran P, et al. (2010). Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett 292:197–207
- Li H, Wang QJ, Zhu DN, Yang Y. (2008). A triterpene saponin of Polygala aureocauda Dunn, exerts hypolipidemic effect on hyperlipidemic mice. Phytother Res 22:159–64
- Ligumsky M, Sestieri M, Okon E, Ginsburg I. (1995). Antioxidants inhibit ethanol-induced gastric injury in the rat. Role of manganese, glycine and carotene. Scand J Gastroenterol 30:854–60
- Madar Z, Shomer I. (1990). Polysaccharide composition of a gel fraction derived from fenugreek and its effect on starch digestion and bile acid absorption in rats. J Agric Food Chem 38:1535–39
- Mahdavi MR, Roghani M, Baluchnejadmojarad T. (2008). Mechanisms responsible for the vascular effect of aqueous Trigonella foenum-graecum leaf extract in diabetic rats. Indian J Pharmacol 40:59–63
- Mandegary A, Pournamdari M, Sharififar F, et al. (2012). Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem Toxicol 50:2503–7
- Mansour EH, El-Adawy TA. (1994). Nutritional potential and functional properties of heat treated and germinated fenugreek seeds. Lebensmittel-Wissenschaft und Technologie 27:568–73
- Martinez RM, Nordt SP, Cantrell FL. (2012). Prescription acetaminophen ingestions associated with hepatic injury and death. J Community Health 37:1249–52
- Meera R, Devi P, Kameswari B, et al. (2009). Antioxidant and hepatoprotective activities of Ocimum basilicum Linn. and Trigonella foenum-graecum Linn. against H2O2 and CCl4 induced hepatotoxicity in goat liver. Indian J Exp Biol 47:584–90
- Mercan N, Guvensen A, Celik A, Katircioglu H. (2007). Antimicrobial activity and pollen composition of honey samples collected from different provinces in Turkey. Nat Prod Res 21:187–95
- Mital N, Gopaldas T. (1986). Effect of fenugreek (Trigonella foenum-graecum) seed based diets on the lactational performance in albino rats. Nutrition Rep Int 33:477–84
- Mohammad S, Taha A, Bamezai RN, Baquer NZ. (2006). Modulation of glucose transporter (GLUT4) by vanadate and Trigonella in alloxan-diabetic rats. Life Sci 78:820–4
- Morani AS, Bodhankar SL, Mohan V, Thakurdesai PA. (2012). Ameliorative effects of standardized extract from Trigonella foenum-graecum L. seeds on painful peripheral neuropathy in rats. Asian Pac J Trop Med 5:385–90
- Muraki E, Hayashi Y, Chiba H, et al. (2011). Dose-dependent effects, safety and tolerability of fenugreek in diet-induced metabolic disorders in rat. Lipids Health Dis 10:240–6
- Ody P. (1999). Herbs to avoid during pregnancy. In: Ody P, ed. Herbs for a Healthy Pregnancy. Los Angeles: Keats Publishing, 81
- Olli S, Kirti PB. (2006). Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J Biochem Mol Biol 39:278–83
- O'Mahony R, Al-Khtheeri H, Weerasekera D, et al. (2005). Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol 11:7499–507
- Pandian RS, Anuradha CV, Viswanathan P. (2002). Gastroprotective effect of fenugreek seeds (Trigonella foenum-graecum) on experimental gastric ulcer in rats. J Ethnopharmacol 81:393–7
- Passano P. (1995). The many uses of methi. Manushi November:31–4
- Patsenker E, Stoll M, Millonig G, et al. (2011). Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med 17:1285–94
- Petit PR, Sauvaire YD, Hillaire-Buys DM, et al. (1995). Steroid saponins from fenugreek seeds: Extraction, purification, and pharmacological investigation on feeding behavior and plasma cholesterol. Steroids 60:674–80
- Prabhu A, Krishnamoorthy M. (2010). Anticancer activity of Trigonella foenum-graecum on Ehrlich Ascites carcinoma in Mus musculus system. J Pharm Res 3:1181–3
- Puri D. (1998). Therapeutic potentials of fenugreek. Indian J Physiol Pharmacol 42:423–4
- Rabe T, van Staden J. (1997). Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol 56:81–7
- Rajalakshmi R, Subbulakshmi G. (1964). Effect of fenugreek (Trigonella foenum-graecum Linn.) supplementation on the biological value of rice & black gram (Phaseolus mungo) diet. Indian J Biochem 11:104–6
- Raju J, Bird RP. (2006). Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum-graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obese (Lond) 30:1298–307
- Raju J, Gupta D, Rao AR, et al. (2001). Trigonella foenum-graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem 224:45–51
- Raju J, Patlolla JM, Swamy MV, Rao CV. (2004). Diosgenin, a steroid saponin of Trigonella foenum-graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev 13:1392–8
- Randhir R, Lin YT, Shetty K. (2004). Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac J Clin Nutr 13:295–307
- Randhir R, Shetty K. (2007). Improved alpha-amylase and Helicobacter pylori inhibition by fenugreek extracts derived via solid-state bioconversion using Rhizopus oligosporus. Asia Pac J Clin Nutr 16:382–92
- Rastogi RP, Mehrotra BN. (1990). Compendium of Indian Medicinal Plants. New Delhi: PID. Vol. II. pp. 422
- Reddy RL, Srinivasan K. (2009). Fenugreek seeds reduce atherogenic diet-induced cholesterol gallstone formation in experimental mice. Can J Physiol Pharmacol 87:933–43
- Reddy R, Srinivasan K. (2011a). Dietary fenugreek and onion attenuate cholesterol gallstone formation in lithogenic diet-fed mice. Int J Exp Pathol 92:308–19
- Reddy RR, Srinivasan K. (2011b). Effect of dietary fenugreek seeds on biliary proteins that influence nucleation of cholesterol crystals in bile. Steroids 76:455–63
- Riordan J, Auerbach K. (1998). Human Lactation and Breastfeeding. 2nd ed. Boston (MA): Jones & Bartlett
- Roberts KT. (2011). The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J Med Food 14:1485–9
- Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. (2010). Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum-graecum L. and its constituents. Phytomedicine 17:292–5
- Saurez J, Herrera MD, Marhuenda, E. (1996). Hesperidine and neohesperidin dihydrochalcone on different experimental models of induced gastric ulcer. Phytother Res 10:616–18
- Sebastian KS, Thampan RV. (2007). Differential effects of soybean and fenugreek extracts on the growth of MCF-7 cells. Chem Biol Interact 170:135–43
- Shabbeer S, Sobolewski M, Anchoori RK, et al. (2009). Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol Ther 8:272–8
- Sharma RD, Raghuram TC, Rao NS. (1990). Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr 44:301–6
- Shim SH, Lee EJ, Kim JS, et al. (2008). Rat growth-hormone release stimulators from fenugreek seeds. Chem Biodivers 5:1753–61
- Siddiqui MR, Taha A, Moorthy K, et al. (2005). Amelioration of altered antioxidant status and membrane linked functions by vanadium and Trigonella in alloxan diabetic rat brains. J Biosci 30:483–90
- Sreeja S, Anju VS, Sreeja S. (2010). In vitro estrogenic activities of fenugreek Trigonella foenum-graecum seeds. Indian J Med Res 131:814–19
- Srichamroen A, Thomson AB, Field CJ, Basu TK. (2009). In vitro intestinal glucose uptake is inhibited by galactomannan from Canadian fenugreek seed (Trigonella foenum-graecum L.) in genetically lean and obese rats. Nutr Res 29:49–54
- Srinivasan K. (2006). Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev Int 22:203–24
- Stark A, Madar Z. (1993). The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graecum) on bile acid absorption and cholesterol levels in rats. Br J Nutr 69:277–87
- Sur P, Das M, Gomes A, et al. (2001). Trigonella foenum-graecum (fenugreek) seed extract as an antineoplastic agent. Phytother Res 15:257–9
- Suresh P, Kavitha CN, Babu SM, et al. (2012). Effect of ethanol extract of Trigonella foenum-graecum (fenugreek) seeds on freund's adjuvant-induced arthritis in albino rats. Inflammation 35:1314–21
- Thirunavukkarasu V, Anuradha CV, Viswanathan P. (2003). Protective effect of fenugreek (Trigonella foenum-graecum) seeds in experimental ethanol toxicity. Phytother Res 17:737–43
- Trinh HT, Han SJ, Kim SW, et al. (2007). Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotech 17:1127–33
- Tripathi UN, Chandra D. (2009). The plant extracts of Momordica charantia and Trigonella foenum-graecum have antioxidant and anti-hyperglycemic properties for cardiac tissue during diabetes mellitus. Oxid Med Cell Longev 2:290–6
- Uemura T, Goto T, Kang MS, et al. (2011). Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr 141:17–23
- Verma SK, Singh SK, Mathur A. (2010). In vitro cytotoxicity of Calotropis procera and Trigonella foenum-graecum against human cancer cell lines. J Chem Pharm Res 2:861–5
- Vyas S, Agrawal RP, Solanki P, Trivedi P. (2008). Analgesic and anti-inflammatory activities of Trigonella foenum-graecum (seed) extract. Acta Pol Pharm 65:473–6
- Williams CD, Antoine DJ, Shaw PJ, et al. (2011). Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol 252:289–97
- Xue W, Lei J, Li X, Zhang R. (2011). Trigonella foenum-graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr Res 31:555–62
- Yadav UC, Moorthy K, Baquer NZ. (2004). Effects of sodium-orthovanadate and Trigonella foenum-graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J Biosci 29:81–91
- Yoshikawa M, Murakami T, Komatsu H, et al. (1997). Medicinal Foodstuffs: IV. Fenugreek seeds (1): Structures of trigoneosides Ia, Ib, IIb, IIIa and IIIb new furostanol saponins from the seeds of Indian Trigonella foenum- graecum L. Chem Pharmacol Bull 45:81–7
- Zia T, Hasnain SN, Hasan SK. (2001). Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. (methi) in normal mice. J Ethnopharmacol 75:191–5