Abstract
Context: Polyphenols are naturally occurring compounds found in fruits, vegetables, cereals, and beverages. Polyphenols occupy a unique place in biological science for their pharmacological properties. Gossypol is a polyphenolic compound that has attracted attention because of its biological effects.
Objective: Gossypol is reported to exhibit antifertility, antioxidant, anticancer, antivirus, antiparasitic, and antimicrobial properties and lower plasma cholesterol. These are summarized with attention to the mechanisms of activity.
Methods: This review summarizes the results of studies obtained in a comprehensive search of ScienceDirect, PubMed, Scirus, and Web of Science.
Results and conclusion: The results of these studies provide a comprehensive understanding of the biological action of gossypol and its potential for the prevention of and therapy for resistant tumors and chronic human diseases such as HIV, malaria, and psoriasis.
Introduction
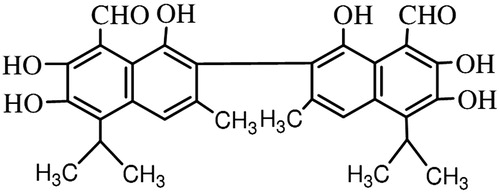
Gossypol [(2,2′-binaphthalene)-8,8′-dicarboxaldehyde, 1,1′,6,6′,7,7′-hexahydroxy-5,5′-diisopropyl-3,3′-dimethyl] is a lipid-soluble polyphenolic compound extracted from the cotton plant (genus Gossypium) and the tropical tree Thespesia populnea (L.) Sol. ex Corrêa, both members of the family Malvaceae (; Jaroszewski et al., Citation1992a; King & de Silva, Citation1968). Gossypol is produced in the plant by the dimerization of two molecules of hemigossypol and is best classified as a dimeric-sesquiterpenoid. Sesquiterpenoids are a class of terpenes having three isoprene units that protect a plant from pathogens and insects (Stipanovic et al., Citation1986).
History
Gossypol was first discovered by Longmore (Citation1886). Marchlewski (Citation1899) purified it via precipitation from an ether solution using acetic acid to produce gossypol acetic. These investigators tried to use gossypol as a dye, but found it to be unstable when exposed to light. The chemical was eventually named gossypol because of its origin from the genus Gossypium and its polyphenolic chemical nature.
A study of couples using crude cottonseed oil for cooking in a region of China during the 1950s showed low birth rates, with men having very low sperm counts and women experiencing amenorrhea. Subsequent studies have confirmed the antifertility effect of gossypol in mammals and humans (Coutinho, Citation2002). In the 1970s, a research team from the Institute of Pharmacology of the Chinese Academy of Medical Sciences began a series of experiments on 10 000 volunteers that continued for over a decade. They found that taking a daily gossypol pill provided reliable male contraception that did not affect their hormonal balance (Taylor et al., Citation1991).
Few side effects other than transient hypocalemia have been reported (Bi et al., Citation1981; Prasad & Diczfalusy, Citation1982; Qian & Wang, Citation1984); this has led to reduced scientific interest in gossypol. Later studies of experimental tumor models indicate that gossypol is a potential antitumor chemotherapeutic agent (Tso, Citation1984; Tuszynski & Cossu, Citation1984).
Chemical properties
The structure of gossypol consists of two naphthalene rings joined by a single internaphthyl bond between the 2- and 2′-carbon atoms. The presence of six phenolic hydroxyl groups and two aldehydic groups makes gossypol chemically reactive (; James, Citation2006).
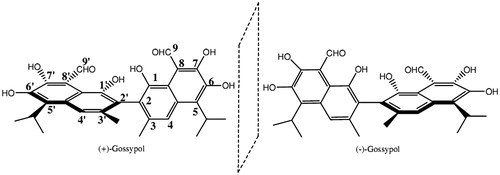
Gossypol is a polyphenolic bissesquiterpene that has been isolated as a racemic mixture from cottonseed. Gossypol exists as (+) and (−) enantiomers because of hindered rotation around the binaphthyl bond (; Freedman et al., Citation2003).
Jaroszewski et al. (Citation1992b) investigated the gossypol racemization energy barrier using a molecular mechanics program and found that racemization of gossypol requires inaccessibly high energy and, thus, the individual enantiomers are optically stable under normal conditions (e.g., ambient temperature and neutral pH).
Gossypol has a complicated reaction chemistry that stems from its different tautomeric forms. Adams et al. (Citation1960) proposed three tautomeric forms – aldehyde, ketone (quinoid), and lactol (hemiacetal) – to explain some of these reactions and their properties and degradation products.
Biological properties
Antifertility/contraceptive
Gossypol is non-steroidal and does not affect hormone levels, but does inhibit sperm production and motility in male animals and humans. It acts as a contraceptive by inhibiting enzyme systems that effect energy metabolism in sperm and spermatogenic cells (Coutinho, Citation2002; Wang et al., Citation2009).
Human lactate dehydrogenase (LDH) has five isoenzymes. Under anaerobic glucose conditions, pyruvate is reduced to lactate by LDH in the presence of NADH. Numerous reports suggest that the antifertility properties of gossypol are associated specifically with the (−)-isomer. (−)-Gossypol is a non-selective competitive inhibitor of NADH binding with LDH. Yu et al. (Citation2001) attribute its antifertility action to inhibition of mitochondrial LDH-C4 (LDH-X), which is present only in the testes and sperm and is essential for energy production. However, the mode of action is complex and involves the inhibition of a number of essential enzyme systems, including ribonucleotide reductase (McClarty et al., Citation1985), malate dehydrogenase (MDH), glyceraldehyde-3-phosphate dehydrogenase (GA3PDH) (Ikeda, Citation1990), and cytoplasmic phospholipase A2 (cPLA2). The latter enzyme plays an important role in the acrosomal reaction during sperm maturation (Dodou, Citation2005; Vainio et al., Citation1985).
Antioxidant properties
Polyphenols are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens. Like many other aromatic phenolic chemicals, gossypol is an effective and potent natural antioxidant (Laughton et al., Citation1989). For example, gossypol was found to protect carotene against preformed fat peroxides in vitro and also act as a carotene protecting antioxidant in vivo (Hove, Citation1944).
Gossypol was reported to inhibit rat liver microsomal peroxidation caused by incubation with ferric/ascorbate (IC50 < 0.1 µM; Hove & Hove, Citation1944). In some cases, the modification of phenolic hydroxyl groups on gossypol significantly decreases the chemical antioxidative abilities of free radical scavenging activity, reducing power assay, DNA damage prevention, and demonstrating that the hydroxyl groups are critical to antioxidation (Wang et al., Citation2008).
Li et al. (Citation2000) found that gossypol in the presence of Fe3+/ascorbate protects supercoiled plasmid DNA from damage in a dose-dependent manner. Dodou et al. (Citation2005) postulated that the antioxidant properties of gossypol may be useful in diseases characterized by lipid oxidative damage, such as psoriasis.
Antitumor properties
Researchers have assessed the anticancer properties of gossypol against many types of cancer cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) or flow cytometry cell-viability assays. The effect on cells of (−)-gossypol at a lower concentrations was more potent in comparison with (+)-gossypol or racemic gossypol (Kitada et al., Citation2003; Liu et al., Citation2002; Oliver et al., Citation2004). The effects included inhibition of cytoplasmic and mitochondrial enzymes involved in energy production (Zhai et al., Citation2006), uncoupling oxidative phosphorylation (Ueno et al., Citation1988) and depletion of cellular adenosine triphosphate (Flack et al., Citation1993).
Gossypol was also shown to inhibit key nuclear enzymes responsible for DNA replication and repair, including DNA polymerase α (Keniry et al., Citation1989) and topoisomerase II, and to block DNA synthesis in HeLa cells (Rosenberg et al., Citation1986). Wang and Rao (Citation1984) reported that inhibition of DNA synthesis was achieved with 10 µM gossypol by blocking the G1/S checkpoint in MCF-7 cells after 24 h of incubation.
Gossypol has shown in vitro to inhibit cell cycling by modulating regulatory proteins Rb and cyclin D1, elevating TGF-β1 gene expression and inhibiting protein kinase C activity (Ligueros et al., Citation1997; Shidaifat, Citation1997; Teng, Citation1995).
Telomerase is a reverse transcriptase which helps to stabilize the length of telomeres. The absence of telomerase activity causes replication senescence and cell death. Gossypol has been shown to induce apoptosis and repress telomerase activity via transcriptional downregulation and posttranslational modification of hTERT in human leukemia cells. Transcriptional downregulation involves the inactivation of c-Myc and posttranslational modification that of Akt (Moon et al., Citation2008a). Gossypol also downregulates the expression of NF-kappaB-regulated gene products, including inhibitors of apoptosis such as the proteins IAP-1, IAP-2, and X-linked IAP. Moon et al. (Citation2008b) suggested that gossypol-induced apoptosis partially involves suppression of NF-kappaB activity.
(−)-Gossypol induces complete cytochrome c release from mitochondria, increases caspase-3 and caspase-9 activity, and causes apoptotic death. Balakrishnan et al. (Citation2008) found that (−)-gossypol acts as a BH3 mimetic and binds to the BH3-binding domain in pro-apoptotic proteins of the Bcl2 family, displacing pro-death partners to induce apoptosis. It enhances the antitumor activity of X-ray irradiation and chemotherapeutic agents such as docetaxel that exert antitumor activity via inhibition of the antiapoptotic protein Bcl-xL and increasing proapoptotic Noxa and Puma (Meng et al., Citation2008; Xu et al., Citation2005).
Antivirus properties
Gossypol has been reported to possess antiviral properties against enveloped viruses, including HIV-1, HSV-2, influenza, and parainfluenza (Lin et al., Citation1993; Vander Jagt et al., Citation2000). Although the compound is significantly less potent than AZT, it lacks the serious side effect on bone marrow toxicity that is associated with AZT treatment. It is not clear whether the inhibition of HIV-1 reverse transcriptase by gossypol is the primary mechanism of action. Lin et al. (Citation1989) have synthesized analogs of gossypol for potential antiviral activity against HIV-1.
Antiparasitic properties
Malaria is a mosquito-borne infectious disease caused by protozoan parasites of the genus Plasmodium. Four species (Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax) can infect and be transmitted by humans (Mendis et al., Citation2001). Gossypol derivatives with ethyl, propyl, or isopropyl side chains and gossylic nitrile 1,1-divalerate have shown stronger inhibition than other gossypol derivatives against the growth of P. falciparum (Razakantoanina et al., Citation2000; Royer et al., Citation1986).
Gossypol also exhibits activity against Endameba histolytica and Trypanosoma cruzi. The molecular mechanism behind gossypol antiparasitic activity could be the selective inhibition of vital and essential enzymes in the anaerobic life cycle of parasites (Gonzalez-Garza et al., Citation1993; Montamat et al., Citation1982).
Antimicrobial properties
Margalith (Citation1967) demonstrated the antibiotic properties of gossypol against sporeformers and lactobacilli by testing its inhibitory effect on microorganisms in cottonseed meal-fed animals. The result showed that gossypol caused a fundamental change in the equilibrium of the microflora of the gastrointestinal tract.
Vadehra et al. (Citation1985) reported that gossypol is a more potent antibacterial agent against Gram positive organisms (Streptococcus spp., Bacillus spp., Staphylococcus aureus) as opposed to Gram-negative bacteria such as Pseudomonas aeruginosa, Salmonella spp., Klebsiella pneumoniae, Shigella spp., Proteus spp., and Escherichia coli. This may originate from structural differences in the cell wall and cell membrane of Gram-positive and Gram-negative groups. For example, Gram positive bacteria have more peptidoglycan in their cell walls and lack the outer membrane found in Gram-negative organisms. This possibly influences the transport of gossypol to its target site.
Plasma cholesterol reduction properties
Cholesterol is a lipid produced by the liver and is vital for normal body function. Elevated levels of low density lipoprotein (LDL) have been linked to an increased risk of heart disease. Shandilya et al. (Citation1982) found that gossypol administered orally at 10 mg/kg/day for 6 months to adult male Cynomolgus monkeys caused a significant decrease in total plasma cholesterol and LDL without a significant decrease in high density lipoprotein (HDL) levels. The possible mechanisms of this action can be attributed to a reduction in intestinal absorption of dietary cholesterol and a decrease in hepatic synthesis of LDL.
Conclusion
The numerous studies outlined above show that gossypol has the potential for prevention and therapy of various cancers and chronic human diseases. Gossypol is a versatile molecule with an abundance of biological properties. It has the potential for use in the development of drugs for disorders as varied as resistant tumors, HIV, malaria, and psoriasis. Further investigation on the mechanisms, the nature of the active compounds and appropriate dose levels are needed for therapeutic exploitation of gossypol.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
- Adams R, Geissman TA, Edwards JD. (1960). Gossypol, a pigment of cottonseed. Chem Rev 60:555–74
- Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. (2008). Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood 112:1971–80
- Bi XF, Ye YX, Yang HF, Zhang ZR. (1981). Preliminary study on gossypol induced hypokalemia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 3:175–78
- Coutinho EM. (2002). Gossypol: A contraceptive for men. Contraception 65:259–63
- Dodou K. (2005). Investigations on gossypol: Past and present developments. Expert Opin Investig Drugs 14:1419–34
- Dodou K, Anderson RJ, Lough WJ, et al. (2005). Synthesis of gossypol atropisomers and derivatives and evaluation of their antiproliferative and antioxidant activity. Expert Opin Investig Drugs 13:4228–37
- Flack MR, Pyle RG, Mullen NM, et al. (1993). Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab 76:1019–24
- Freedman TB, Cao X, Oliviera RV, et al. (2003). Determination of the absolute configuration and solution conformation of gossypol by vibrational circular dichroism. Chirality 15:196–200
- Gonzalez-Garza MT, Matlin SA, Mata-Cardenas B, Said-Fernandez S. (1993). Differential effects of the (+) and (−)-gossypol enantiomers upon Entamoeba histolytica axenic cultures. J Pharm Pharmacol 45:144–5
- Hove EL. (1944). Gossypol as a carotene-protecting antioxidant, in vivo and in vitro. J Biol Chem 156:633–42
- Hove EL, Hove Z. (1944). A method for estimating total fat-soluble antioxidants based on the relation between fat peroxides and carotene destruction. J Biol Chem 156:611–22
- Ikeda M. (1990). Inhibition kinetics of NAD-linked enzymes by gossypol acetic acid. Andrologia 22:409–16
- James AK. (2006). Reaction chemistry of gossypol and its derivatives. JAOCS 83:269–302
- Jaroszewski JW, Strom-Hansen T, Hansen SH, et al. (1992a). On the botanical distribution of chiral forms of gossypol. Planta Med 58:454–8
- Jaroszewski JW, Strom-Hansen T, Hansen LL. (1992b). Optical stability of gossypol. Chirality 4:216–21
- Keniry MA, Hollander C, Benz CC. (1989). The effect of gossypol and 6-aminonicotinamide on tumor cell metabolism: A 31P-magnetic resonance spectroscopic study. Biochem Biophys Res Commun 164:947–53
- King TJ, de Silva LB. (1968). Optically active gossypol from Thespesia populnea. Tet Lett 3:261–3
- Kitada S, Leone M, Sareth S, et al. (2003). Discovery, characterization, and structure–activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 46:4259–64
- Laughton MJ, Halliwell B, Evans PJ, Hoult JR. (1989). Antioxidant and prooxidant actions of the phenolics quercetin, gossypol, and myricetin. Biochem Pharmacol 38:2859–65
- Li A, Bandy B, Tsang SS, Davison AJ. (2000). DNA-breaking versus DNA-protecting activity of four phenolic compounds in vitro. Free Rad Res 33:551–66
- Ligueros M, Jeoun D, Tang B, et al. (1997). Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin D1 transfected human fibrosarcoma cells. Br J Cancer 76:21–8
- Lin TS, Schinazi RF, Zhu J, et al. (1993). Anti-HIV-1 activity and cellular pharmacology of various analogs of gossypol. Biochem Pharmacol 46:251–5
- Lin TS, Schinazi R, Griffith BP, et al. (1989). Selective inhibition of human immunodeficiency virus type 1 replication by the (−) but not the (+) enantiomer of gossypol. Antimicrob Agents Chemother 33:2149–51
- Liu S, Kulp SK, Sugimoto Y, et al. (2002). The (−)-enantiomer of gossypol possesses higher anticancer potency than racemic gossypol in human breast cancer. Anticancer Res 22:33–8
- Longmore J. (1886). Cotton seed oil: Its colouring matter and mucilage, and description of a new method of recovering the loss occurring in the refining process. J Chem Ind 5:200–6
- Marchlewski L. (1899). Gossypol, ein Bestandtheil der Baumwollsamen. J Prakt Chem 60:84–94
- Margalith P. (1967). Inhibitory effect of gossypol on microorganisms. Appl Microbiol 15:952–3
- McClarty GA, Chan AK, Creasy DC, Wright JA. (1985). Ribonucleotide reductase: An intracellular target for the male antifertility agent, gossypol. Biochem Biophys Res Commun 133:300–5
- Mendis K, Sina BJ, Marchesini P, Carter R. (2001). The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64:97–106
- Meng Y, Tang W, Dai Y, et al. (2008). Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther 7:2192–202
- Moon DO, Kim MO, Choi YH, et al. (2008a). Gossypol suppresses telomerase activity in human leukemia cells via regulating hTERT. FEBS Lett 582:3367–73
- Moon DO, Kim MO, Lee JD, Kim GY. (2008b). Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene expression in human leukemia U937 cells. Cancer Lett 264:192–200
- Montamat EE, Burgos C, Gerez de Burgos NM, et al. (1982). Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 218:288–9
- Oliver CL, Bauer JA, Wolter KG, et al. (2004). In vitro effects of the BH3 mimetic, (−)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res 10:7757–63
- Prasad MRN, Diczfalusy E. (1982). Gossypol. Int J Androl 5:53–70
- Qian S-Z, Wang, Z-G. (1984). Gossypol: A potential antifertility agent for males. Ann Rev Pharm Toxicol 24:329–60
- Razakantoanina V, Nguyen Kim PP, Jaureguiberry G. (2000). Antimalarial activity of new gossypol derivatives. Parasitol Res 86:665–8
- Rosenberg LJ, Adlakha RC, Desai DM, Rao PN. (1986). Inhibition of DNA polymerase alpha by gossypol. Biochim Biophys Acta 866:258–67
- Royer RE, Deck LM, Campos NM, et al. (1986). Biologically active derivatives of gossypol: Synthesis and antimalarial activities of peri-acylated gossylic nitriles. J Med Chem 29:1799–801
- Shandilya LN, Clarkson TB, Adams MR, Lewis JC. (1982). Effects of gossypol on reproductive and endocrine functions of male cynomolgus monkeys (Macaca fascicularis). Biol Reprod 27:241–52
- Shidaifat F, Canatan H, Kulp S, et al. (1997). Gossypol arrests human benign prostatic hyperplastic cell growth at G0/G1 phase of the cell cycle. Anticancer Res 17:1003–9
- Stipanovic R, Stoessl A, Stothers JB, et al. (1986). The stereochemistry of the biosynthetic precursor of gossypol. J Chem Soc Chem Commun 2:100–2
- Taylor GT, Griffin MG, Bardgett M. (1991). Search for a male contraceptive: The effect of gossypol on sexual motivation and epididymal sperm. J Med 22:29–44
- Teng CS. (1995). Gossypol-induced apoptotic DNA fragmentation correlates with inhibited protein kinase C activity in spermatocytes. Contraception 52:389–95
- Tso W-W. (1984). Gossypol inhibits Erhlich ascites tumour cells. Cancer Lett 24:257–61
- Tuszynski GP, Cossu G. (1984). Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 44:768–71
- Ueno H, Sahni MK, Segal SJ, Koide SS. (1988). Interaction of gossypol with sperm macromolecules and enzymes. Contraception 37:333–41
- Vadehra DV, Kalla NR, Saxena M, et al. (1985). Antimicrobial activity of gossypol acetic acid. IRCS Med Sci 13:10–11
- Vainio P, Thuren T, Wichman K, et al. (1985). Hydrolysis of phospholipids monolayers by human spermatozoa: Inhibition by male contraceptive gossypol. Biochim Biophys Acta 814:405–8
- Vander Jagt DL, Deck LM, Royer RE. (2000). Gossypol: Prototype of inhibitors targeted to dinucleotide folds. Curr Med Chem 7:479–98
- Wang X, Beckham T, Morris J, et al. (2008). Bioactivities of gossypol, 6-methoxy gossypol and 6,6′-dimethoxy gossypol. J Agric Food Chem 56:4393–8
- Wang X, Howell CP, Chen F, et al. (2009). Gossypol a polyphenolic compound from cotton plants. Adv Food Nutr Res 58:215–63
- Wang YC, Rao PN. (1984). Effect of gossypol on DNA synthesis and cell cycle progression of mammalian cells in vitro. Cancer Res 44:35–8
- Xu LY, Wang S, Tang W, et al. (2005). (−)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther 4:197–205
- Yu Y, Deck A, Hunsaker LA, et al. (2001). Selective active site inhibitors of human lactate dehydrogenases A4, B4 and C4. Biochem Pharmacol 62:81–9
- Zhai D, Jin C, Satterthwait AC, Reed JC. (2006). Comparison of chemical inhibitors of antiapoptotic Bcl2 family proteins. Cell Death Differ 13:1419–21