Abstract
Context: Warburgia (Canellaceae) species have a long history of ethnomedicinal uses in east, central and southern Africa. Due to the popularity of Warburgia as a source of ethnomedicines; all the species are severely over-harvested throughout their distributional ranges.
Objective: This review documents fragmented information on traditional uses and pharmacological evidence of the genus Warburgia.
Methods: Information on Warburgia species was collected from scientific journals, books, theses and reports via library and electronic search using Medline, Pubmed, Google Scholar, ScienceDirect and Web of Science.
Results: Ethnomedicinal uses of Warburgia species have been recorded from east, central and southern Africa for 30 human and 7 animal ailments. Warburgia species are used to treat gastro-intestinal disorders, cold, cough and sore throat; fever or malaria, respiratory and odontological ailments. Warburgia species are rich in drimane and colorotane sesquiterpenoides, and other compounds. The extracts of Warburgia, particularly those from stem bark and leaves, exhibited a wide range of pharmacological effects, including antibacterial, antifungal, antimycobacterial, antioxidant, anti-inflammatory, antifeedant, antiplasmodial, antileishmanial, anthelmintic, cytotoxic and molluscicidal activities.
Conclusion: Pharmacological results have validated the use of this genus in traditional medicine. Further investigations are needed to explore the bioactive compounds responsible for the in vitro and in vivo pharmacological effects and their mode of action.
Introduction
The genus Warburgia Engl. is a member of Canellaceae Martius, a dicot family that contains 16 species grouped in 6 genera (Kubitzki, Citation1993). Genus Warburgia was named after Dr. Otto Warburg (1859–1938), who was born in Hamburg, Germany. He was a lecturer in botany at the University of Berlin and author of several botanical papers. In southern Mozambique and South Africa, Warburgia is commonly referred to as Chibaha (Xibaha) and hence Bertolini’s original generic name Chibaca Bertol. f. The genus Warburgia with 4 plant species is the only genus of family Canellaceae that extends into the African mainland (Verdcourt, Citation1956, Citation1990). Warburgia is distributed in Democratic Republic of Congo (DRC), Ethiopia, Kenya, Malawi, Mozambique, South Africa, Swaziland, Tanzania, Uganda, Zambia and Zimbabwe.
Warburgia elongata Verdc. is a small evergreen tree or shrub endemic to the lowland coastal riverine and swamp forests of Uzaramo district, Tanzania (Verdcourt, Citation1954, Citation1956). Warburgia elongata is endangered mainly due to its small area of occupancy, extent of occurrence and the small number of mature individual plants (Lovett & Clarke, Citation1998a). It is also potentially threatened by harvesting for firewood, charcoal, building poles, tool handles, carvings and medicinal purposes (Lovett & Clarke, Citation1998a). Warburgia salutaris (Bertol. f.) Chiov. is known from a few localities in the north-eastern parts of South Africa, Swaziland, south-eastern Zimbabwe, southern Mozambique, Malawi and Zambia (Palgrave, Citation2000). It is found in evergreen montane forests including wooded ravines and along the coasts (Palgrave, Citation2000; Venter & Venter, Citation2002). Warburgia salutaris is categorized as endangered on the IUCN Red List (IUCN, Citation2012), primarily because of reduction in population size based on decline in area of occupancy, extent of occurrence and/or quality of habitat; and actual or potential levels of exploitation. It is categorized as vulnerable in Mozambique (Izidine & Bandeira, Citation2002), endangered in Malawi (Msekandiana & Mlangeni, Citation2002) and South Africa (Williams et al., Citation2008), critically endangered in Swaziland (Dlamini & Dlamini, Citation2002) and extinct in the wild in Zimbabwe (Maroyi, Citation2008, Citation2013). The species is generally slow growing in the wild; and its limited distribution and low abundance makes it vulnerable to human-induced habitat degradation and over-exploitation as a medicinal plant. Warburgia stuhlmannii Engl. is confined to dry lowland forests of Kenya and Tanzania (Verdcourt, Citation1954, Citation1956). Warburgia stuhlmannii is vulnerable mainly due to its small area of occupancy, extent of occurrence and the small number of mature individual plants (Lovett & Clarke, Citation1998b). It is also potentially threatened by harvesting for timber, firewood, building poles, carvings and medicinal purposes (Lovett & Clarke, Citation1998b). Warburgia ugandensis Sprague ssp. longifolia Verdc. is endemic to southern Tanzania (Verdcourt, Citation1956), while W. ugandensis Sprague ssp. ugandensis is more widespread, recorded in lowland rainforest, evergreen and swamp forest of DRC, Ethiopia, Kenya, Malawi, Tanzania, Uganda (Verdcourt, Citation1956, Citation1990). Both taxa are used for timber, firewood, building poles, charcoal, carvings and medicinal purposes (Lovett & Clarke, Citation1998b).
Warburgia species are characterized by aromatic and pungent bark, which is used medicinally. Warburgia salutaris, W. stuhlmannii and W. ugandensis have yielded a series of drimane sesquiterpenoids such as isopolygodial, muzigadial, polygodial, salutarisolide, ugandensidial and warburganal (Brooks & Draffan, Citation1969; Jansen & de Groot, Citation1991; Kioy et al., Citation1990; Kubo et al., Citation1977, Citation1983; Manguro et al., Citation2003; Mashimbye et al., Citation1999a; Rabe & van Staden, Citation2000; Wube et al., Citation2005; Xu et al., Citation2009). The genus certainly has a high use value. The present review compiles the fragmented information on the traditional uses and pharmacology of the Warburgia species. I hope that this information will highlight the importance of the genus and will provide baseline information for future researchers intending to do further work on genus Warburgia.
Vernacular names and traditional uses
Warburgia species are known by various vernacular names in different geographical areas in eastern, central and southern Africa (). Insight into the societal value of Warburgia species in east, central and southern Africa can be gained by examining these vernacular names. People rarely name plant species that they do not use. For example, a Swahili vernacular name of W. stuhlmannii “Pilipili mwitu” translates to “wood pepper” (Verdcourt, Citation1954), is in reference to the peppery taste of the stem bark, wood, leaves, etc; a diagnostic feature of all Warburgia species. Warburgia salutaris, W. stuhlmannii and W. ugandensis are often referred to as the “Pepperbark tree” alluding to the stem bark’s aromatic and peppery smell. The near-panaceal qualities of W. salutaris, for example, was also early recognized, not only by the traditional users but by the taxonomist who awarded it the epithet “salutaris” meaning “health giving” or “wholesome” (Hollmann & Van der Schijff, Citation1996).
Table 1. Vernacular names of Warburgia species.
A survey of literature shows no fewer than 2 vernacular names for W. elongata, 16 for W. salutaris, 5 for W. stuhlmannii, 3 for W. ugandensis ssp. longifolia and 34 for W. ugandensis ssp. ugandensis (). South Africa and Kenya have the highest number of vernacular names for W. salutaris and W. ugandensis ssp. ugandensis, respectively (). This long list of names indicates that local people in these countries have an active interest in Warburgia species. With the exception of W. elongata, all Warburgia species are well-known medicinal plants that have long been in regular demand among local communities and practitioners of traditional medicines in east, central and southern Africa. Warburgia species have been over-exploited by collectors in the wild as ethnomedicines, with the stem bark as the most sought after and traded plant part.
The traditional uses of Warburgia species are referred to in many folkloric and ethnobotanical studies done in east, central and southern Africa, where the species are still used as primary sources of traditional medicine. A total of 30 human and 7 animal ailments are treated with Warburgia species (). Gastro-intestinal disorders, cold, cough and sore throat; fever or malaria, respiratory and odontological () are the most commonly treated human ailments. Gastro-intestinal disorders, particularly cholera, diarrhea and dysentery are a major concern in Africa (Mathabe et al., Citation2006; Semenya & Maroyi, Citation2012), where dysentery and cholera usually result in high mortality rate if not treated promptly (Ribeiro et al., Citation2010). Many similarities can be recognized when the ethnomedicinal uses of Warburgia species are considered in totality over their distributional range in east, central and southern Africa (). This may be ascribed to shared cultural heritage about Warburgia species through exchange of its ethnobotanical information. The relations of people to their indigenous plants and that of other regions near or further away aids in measuring their cultural status and their contacts with each other (Gilmore, Citation1932).
Table 2. Medicinal uses of Warburgia species.
Table 3. Major ailment categories and uses reported.
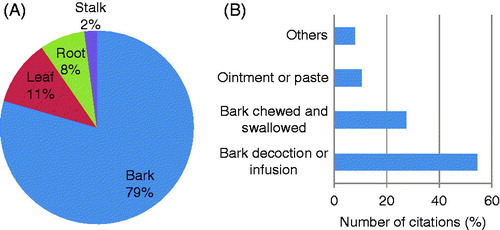
The most frequently used plant parts are bark (79%), leaves (11%), roots (7.5%) and stalks (2.1%) (). It is well recognized by conservationists that medicinal plants primarily valued for their root parts and those which are intensively harvested for their bark often tend to be the most threatened by over-exploitation (Flatie et al., Citation2009). This is the case with all Warburgia species, with its users targeting mainly stem bark as source of ethnomedicine. Warburgia remedies are often utilized in the form of decoction or infusion (54.5%), bark chewed and juice swallowed (27.3%) and ointments or paste (10.4%) (). Other preparation methods include smoking, use of bark fungus, application of bark powder on incision, chewing of bark after it has been boiled and bark powder blown into patient’s anus (). Most (89.9%) of the preparations are prescribed orally (). The majority of the Warburgia remedies (91.3%) are used as monotherapies (). Multitherapies are mostly used in herbal medicines involving W. salutaris in South Africa (). A mixture of W. salutaris bark powder and Erythrophleum lasianthum Corbishley (Fabaceae) is taken as snuff as a remedy for headache (Hutchings et al., Citation1996). Powdered bark of W. salutaris and leaves of Cannabis sativa L. (Cannabaceae) are smoked as herbal remedy for cough (Hutchings et al., Citation1996). Ointments made from a mixture of powdered bark of W. salutaris, leaves of Hibiscus surrattensis L. (Malvaceae) and fat are applied on inflammation of the urethra, irritation and sores on the penis (Hutchings et al., Citation1996). The use of multiple therapies in traditional medicine based on combining plants has recently been shown to increase the efficacy of the herbal medicine (Zonyane et al., Citation2012). Research by Bussmann and Sharon (Citation2006) showed that the use of more than one plant species to prepare a remedy for ailments is attributed to the additive or synergistic effects that they could have during ailment treatment.
Figure 1. Characteristics of Warburgia herbal medicines in east, central and southern Africa. (A) Plant parts used, and (B) herbal preparations. Other herbal preparations in B include smoking, use of bark fungus, application of bark powder on incision and chewing of bark after it has been boiled.
In addition to the medicinal uses, all Warburgia species are used as timber. Warburgia elongata is cultivated for shade and ornamental purposes (Lovett et al., Citation2006). Warburgia salutaris can also be grown around boundaries of homesteads as a barrier, hedge; and ornamental and shade tree (Venter & Venter, Citation2002). Fresh or dried leaves of W. salutaris are used in various dishes to add an aroma and peppery taste (Venter & Venter, Citation2002). Research done by Nichols (Citation2005) in South Africa showed that the leaves of W. salutaris are browsed by hippos. Warburgia stuhlmannii is used in east Africa as a spice or curry and oil from the tree is used as a perfume (Orwa et al., Citation2009). Warburgia ugandensis is used for fodder, food seasoning, insecticide, mulch for soil conservation, ornamental, resin, shade and toothbrush (Maundu & Tengnas, Citation2005; Verdcourt, Citation1954).
Phytochemistry
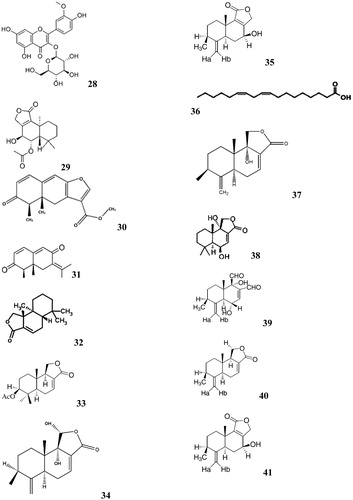
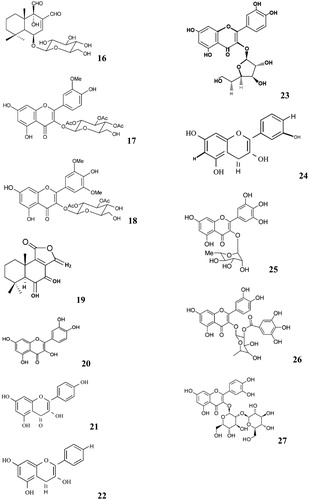
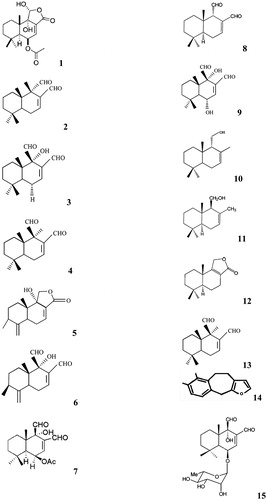
Warburgia species have high pharmaceutical value, both for humans and livestock, this is due to the abundance of drimane and colorotane sesquiterpenoides (Frum et al., Citation2005; Frum & Viljoen, Citation2006; Jansen & de Groot, Citation1991; Kioy et al., Citation1990), tannins and mannitol (Van Wyk & Gericke, Citation2000; Watt & Breyer-Brandwijk, Citation1962). Drimane sesquiterpenes that have been isolated from W. salutaris include: 11α-hydroxycinnamosmolide (1) () (Madikane et al., Citation2007), isopolygodial (isotadeonal) (2), warburganal (3) (Mashimbye et al., Citation1992), polygodial (4) (Mashimbye, Citation1993), salutarisolide (5) (Frum & Viljoen, Citation2006; Frum et al., Citation2005; Mashimbye et al., Citation1999a), muzigadial (cannelal) (6) (Rabe & van Staden, Citation2000), ugandensidial (cinnamodial) (7), isopolygodial (8) (Mashimbye et al., Citation1999a), and mukaadial (9) (Mashimbye et al., Citation1999b). Other drimane type sesquiterpenes isolated from W. salutaris include isodrimenol (10), drimenol (11), confertifolin (12) and a non-sesquiterpene monoaldehyde, polygodial (13) (Mashimbye et al., Citation1999b). Mohanlall & Odhav (Citation2009) isolated a sesquiterpenoid 5, 10-dihydro-6, 7-dimethyl-4H-benzo [5,6] cyclohepta [1, 2-b]-furan (14) from W. salutaris bark.
Figure 2. Drimane, colorotane sesquiterpenoides and other compounds isolated from Wurburgia species.
Phytochemical investigations of W. stuhlmannii leaves showed the presence of mukaadial 6-O-β-d-glucopyranoside (15), mukaadial 6-O-α-l-rhamnopyranoside (16) together with a novel flavonol glycosides 3′,5′-O-dimethylmyricetin 3-O-β-d-2″,3″-diacetylglucopyranoside (17)and 3′-O-methylquercetin 3-O-β-d-2″,3″,4″-triacetylglucopyranoside (18) (Manguro et al., Citation2003). Other compounds isolated from W. stuhlmannii include mukaadial (9), deacetylugandensolide (19), quercetin (20), kaempferol (21), kaempferol 3-O-α-l-rhamnopyranoside (22), quercetin 3-O-β-d-glucopyranoside (23), kaempferol 7-O-β-d-glucopyranoside (24), myricetin 3-O-α-l-rhamnopyranoside (25), quercetin 3-O-α-l-rhamnopyranoside (26), quercetin 3-O-sophoroside (27) and isorhamnetin 3-O-β-d-glucopyranoside (28) (Kioy et al., Citation1990; Manguro et al., Citation2003).
Phytochemical investigations of W. ugandensis showed the presence of ugandensolide (29), ugandesidial (cinnamodial) (7), warburgin (30) and warburgiadione (31) from the heartwood (Brooks & Draffan, Citation1969), cinnamolide (32), cinnamolide-3β-acetate (33), 11α-hydroxymuzigadiolide (34), 7α-hydroxy-8-drimen-11,12-olide (35), deacetylugandensolide (19), linoleic acid (36), mukaadial (9), muzigadiolide (37), ugandensolide (29), ugandensidial (cinnamodial) (7), muzigadial (cannelal) (6), pereniporin B (38), polygodial (4) and waburganal (3) from the stem bark (Kioy et al., Citation1990; Kubo et al., Citation1976; Wube et al., Citation2005); and monoterpenes (Kioy et al., Citation1990) from the leaves. Wube et al. (Citation2005) isolated coloratane sesquiterpenes 6α,9α-dihydroxy-4(13),7-coloratadien-11,12-dial (39), 4(13),7-coloratadien-12,11-olide (40), and 7β-hydroxy-4(13),8-coloratadien-11,12-olide (41) from the stem bark of W. ugandensis.
Pharmacological reports
Antibacterial activity
In a preliminary antibacterial screening of South African medicinal plants used for treating wounds, sores and boils, W. salutaris stem bark methanol extract inhibited the growth of Escherichia coli (Rabe & van Staden, Citation1997). The leaves and bark of W. salutaris have also demonstrated similar antibacterial properties (Zschocke et al., Citation2000). In addition, Zschocke et al. (Citation2000) reported that the TLC-fingerprints of the leaf and bark extracts of W. salutaris were very similar. In further studies on W. salutaris by Rabe & van Staden (Citation2000), fractionation of the ethyl acetate extract of the stem bark by chromatographic techniques yielded muzigadial which was found to be the main antibacterial agent with an MIC of 12.5 μg/ml against both Staphylococcus aureus and Bacillus subtilis; and 50 μg/ml against Micrococcus luteus. In another experiment by Mohanlall and Odhav (Citation2009), W. salutaris heartwood extract in methanol/ethyl acetate and stem bark in methanol/hexane showed activity against Staphylococcus aureus and Bacillus subtilus. Leaf extract in ethyl acetate/dichloromethane showed activity against Escherichia coli, Staphylococcus aureus and Bacillus subtilus (Mohanlall & Odhav, Citation2009). Mbwambo et al. (Citation2009) demonstrated that ethanol extract from the dried leaves of W. ugandensis exhibited antibacterial activity against Staphylococcus aureus, Escherichia coli, Vibrio cholerae, and Bacillus cereus. In another experiment by Kuglerova et al. (Citation2011), W. ugandensis stem bark exhibited antibacterial activity with an MIC of 256 μg/ml against Staphylococcus aureus and 512 μg/ml against Enterococcus faecalis.
Antifungal activity
Experiments by Mohanlall and Odhav (Citation2009) demonstrated that W. salutaris heartwood extract in methanol/ethyl acetate and stem bark in methanol/hexane have antifungal activity against Fusarium moniliforme. Leaf extract of W. salutaris in dichloromethane showed antifungal activity against Fusarium moniliforme (Mohanlall & Odhav, Citation2009). Taniguchi et al. (Citation1983) demonstrated using a two-fold dilution method that warburganal in W. ugandensis exhibited a broad antifungal activity against yeasts and filamentous fungi; and it was highly active against Saccharomyces cerevisiae, Candida utilis and Sclerotinia libertiana. Olila et al. (Citation2001) demonstrated that W. ugandensis ethanol extract of stem bark had antifungal activity against Candida albicans. Mbwambo et al. (Citation2009) demonstrated that ethanolic extract from the dried leaves of W. ugandensis exhibited antifungal activity against Candida albicans and Cryptococcus neoformans. In another experiment by Kuglerova et al. (Citation2011), W. ugandensis stem bark exhibited antifungal activity with an MIC of 256 μg/ml against Candida albicans. Warburgia ugandensis was also found to exhibit antifungal activity against Candida utilis (Kubo, Citation1995; Taniguchi et al., Citation1978).
Antimycobacterial activity
Madikane et al. (Citation2007) show that crude extracts and drimane sesquiterpenoid lactone, 11α-Hydroxycinnamosmolide obtained from W. salutaris exhibited anti-mycobacterial activity against Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG Pasteur. The crude extract and purified compound inhibited pure recombinant arylamine N-acetyltransferase (NAT), an enzyme involved in mycobacterial cell wall lipid synthesis (Madikane et al., Citation2007). Dichloromethane extract of the stem bark of W. ugandensis demonstrated antimycobacterial activity against Mycobacterium aurum, Mycobacterium fortuitum, Mycobacterium phlei and Mycobacterium smegmatis (Wube et al., Citation2005). The active constituents showed MIC values ranging from 4 to 128 μg/ml compared to the antibiotic drugs ethambutol with MIC ranging from 0.5 to 8 μg/ml and isoniazid with MIC ranging from 1 to 4 μg/ml (Wube et al., Citation2005). Therefore, the use of W. ugandensis stem bark to treat tuberculosis in traditional medicine can be attributed to the presence of linoleic acid and the drimane sesquiterpenoids.
Anti-inflammatory and antioxidant properties
The leaves and bark of W. salutaris have been shown to display equipotent activity against the cyclooxygenase-1 enzyme (Zschocke et al., Citation2000). Methanol extracts of W. salutaris leaves displayed 5-lipoxygenase inhibitory activity with an IC50 of approximately 32.11 ppm (Frum, Citation2006). In the same study, warburganal and mukaadial displayed 5-lipoxygenase inhibitory activities with IC50 values of 61.86 ppm and >100 ppm respectively (Frum, Citation2006). These results serve as evidence that both mukaadial and warburganal are contributing to the 5-lipoxygenase inhibitory activity of W. salutaris. In another experiment, Frum (Citation2006), demonstrated that W. salutaris bark has anti-inflammatory activity; and mukadiaal and warburganal were partially responsible for displayed anti-inflammatory activity of the plant species. Due to its antioxidant properties, extracts of W. salutaris showed protective effects against crystalline silica induced inflammatory cytokine expression, activation of nuclear transcription factor-κB, DNA strand breakage and lipid peroxidation (Leshwedi et al., Citation2008). Hence, W. salutaris may be a potential therapeutic agent against the fibrogenic and carcinogenic effects of crystalline silica (Leshwedi et al., Citation2008). Antioxidant activity of the methanol extract of W. salutaris could possibly be due to the presence of flavonoid compounds (Frum, Citation2006). Warburgia ugandensis showed antioxidative properties with IC50 = 6.59 μg/ml, which was very close to the inhibitory effect achieved by reference compound Trolox (IC50 = 3 μg/ml), suggesting strong potent antioxidative properties (Kuglerova et al., Citation2011).
Molluscicidal activity
Aqueous extracts of dried leaf samples of W. salutaris were assessed for in vitro molluscicidal activity against Bulinus africanus and found to be active (IC50 = 2.483 mg/ml) (Clarke & Appleton, Citation1997). Warburganal has shown effective molluscicidal activity (Appleton et al., Citation1992; Nakanishi & Kubo, Citation1978). Warburgia stuhlmannii and W. ugandensis demonstrated molluscicidal activity (Kubo et al., Citation1983), making them potential in the control of schistosomiasis. Drimane sesquiterpenoids are known to have molluscicidal activity (Frum, Citation2006; Fukuyama et al., Citation1982; Taniguchi & Kubo, Citation1993).
Antifeedant activity
The methanol extract of the bark of W. ugandensis was found to exhibit potent insect antifeedant activity against the African armyworm Spodoptera exempta (Kubo, Citation1991, Citation1995). Based on fractionation guided by the assay, three antifeedants were isolated from the bark, leaves and fruit of W. ugandensis (Kubo & Nakanishi, Citation1977; Kubo, Citation1993, Citation1995). Warburganal and muzigadial inhibited the feeding of larvae of two species of African armyworm, the monophagous Spodoptera exempta and the polyphagous Spodoptera littoralis at a concentration of 0.1 ppm in a regular leaf disk method (Kubo & Nakanishi, Citation1977). In another experiment, Kubo et al. (Citation1977) found that polygodial and ugandensidial were also antifeedants for Spodoptera but less active. Antifeedant activity was also observed against Spodoptera frugiperda, Heliothis armigera and Heliothis virescens (Meinwald et al., Citation1978). Sesquiterpene dialdehyde present in W. stuhlmannii and W. ugandensis demonstrated insect antifeedant against the African armyworm Spodoptera exempta (Kubo et al., Citation1977). Drimane sesquiterpenoids are known to have insect-antifeedant activity (Frum, Citation2006; Fukuyama et al., Citation1982; Taniguchi & Kubo, Citation1993).
Antiplasmodial activity
Muthaura et al. (Citation2007b) investigated antiplasmodial activity of W. stuhlmannii showing that the methanol extract was highly active against Plasmodium falciparum clones. The activity of W. stuhlmannii could presumably be ascribed to the presence of the drimane sesquiterpenes in addition to flavonols, which collectively may be involved in the antiplasmodial activity. Anti-plasmodial activity of stem bark of W. ugandensis has also been demonstrated against Plasmodium knowlesi and P. berghei (Were et al., Citation2010). Methanol extracts from various parts of W. ugandensis have shown antiplasmodial activity with an IC50 value of less than 5 mg/ml against both chloroquine-sensitive (D6) and chloroquine resistant (W2) strains of Plasmodium falciparum (Nanyingi et al., Citation2010). Extract of W. ugandensis also showed moderate in vivo antiplasmodial activity in mice infected with P. burghei (Nanyingi et al., Citation2010). The plant extracts offer the potential for the isolation of lead antimalarial compounds, or for the characterization of some active compounds that could be used as markers for standardization of the extracts for use as antimalarials.
Cytotoxic, anthelmintic and antileishmanial activities
Mbwambo et al. (Citation2009) demonstrated that ethanolic leaf extracts of W. ugandensis exhibited cytotoxic activity (95% CI), against brine shrimp larvae with reference to cyclophosphamide, a standard anticancer drug. Research by Xu et al. (Citation2009), showed that the ethyl acetate extract of W. ugandensis bark exhibited potent cytotoxic activity on KB cell line (99% and 64% inhibition at 10 and 1 μg/ml, respectively. Drimane sesquiterpenoids are known to have cytotoxic activities (Frum, Citation2006; Fukuyama et al., Citation1982; Taniguchi & Kubo, Citation1993). Ethanol, dichloromethane and water extracts at 2.5, 5, 10 and 30% concentrations of W. salutaris exhibited anthelmintic effects against Haemonchus contortus (Ahmed et al., Citation2012).
Ngure et al. (Citation2009) demonstrated in vitro antileishmanial activity of W. ugandensis hexane extract against Leishmania major and Leishmania donovani. The hexane extract had the best activity against L. major promastigotes and amastigotes with IC50 value of 9.95 for promastigotes and 8.65 for amastigotes and MIC of 62.5 μg/ml (Ngure et al., Citation2009). The activity of the hexane extract on amastigotes was comparable to that of pentostam and amphotericin B. Similar results were obtained for L. donovani with IC50 values of 8.67 for promastigotes and 100-fold reduction of amastigotes in macrophage cultures (Ngure et al., Citation2009). The water and methanol extracts of the stem bark of W. ugandensis showed anti-leishmanial activity (with IC50 of 1.114 mg/ml against Leishmania major) and immunomodulative effects (Githinji et al., Citation2010). These results confirm that natural products from Warburgia species are potential sources of new and selective agents for the treatment of important tropical diseases caused by protozoans.
Other activities
A sesquiterpene characterized as muzigadial, isolated from W. ugandensis showed trypanocidal activity against Trypanosoma brucei in vitro (Olila et al., Citation2001). A cytotoxic sesquiterpine (characterized as muzigadial) has been isolated from W. ugandensis and used to treat trypanosomiasis (Olila et al., Citation2002) and other parasitic diseases (Kioy et al., Citation1990) in animals. In another experiment by Rugutt et al. (Citation2006) in Kenya, W. ugandensis showed its activity against soil pathogens namely Fusarium oxysporum, Alternaria passiflorae and Aspergillus niger.
Conclusion
Data collected in the present review illustrates that drimane and colorotane sesquiterpenoides are the major constituents in Warburgia genus as they have been detected in three out of four species of the genus. Despite the presence of this phytochemical compound and other phytochemical metabolites, Warburgia species have not been fully explored. Previous research by Heinrich et al. (Citation2005) has shown that phytomedicines are complex mixtures of compounds and they have a more pronounced effect than individual compounds. The role of such complex mixtures and the “ideal” composition of an active extract needs to be investigated first using a combination of in vitro (or in vivo animal) techniques in combination with phytochemical or metabolomic techniques (Jagtap & Bapat, Citation2010; Verpoorte et al., Citation2005). There is need therefore to validate the traditional medicinal applications of Warburgia species through tests in vitro and in vivo as well as clinical trials. Further investigations on phytochemical constituents and subsequent screening are needed for opening new opportunities to develop pharmaceuticals based on Warburgia constituents.
Reports of the uses of Warburgia species for the same ailments in east, central and southern Africa indicate that the species are valuable sources of ethnomedicine. Pharmacological studies carried out on crude extracts and purified compounds of Warburgia species provided support for the documented traditional uses, and have revealed this genus to be a valuable source for medicinally important molecules. With further research, Warburgia species may also prove to be suitable for product development. Significant advances in the utilization of W. salutaris in southern Africa has been made over the years, and also an increasing number of commercial products of the species have appeared in the market (Maroyi, Citation2013). Warburgia salutaris is sold in South Africa in tablet form to treat bronchitis, chest infections and ulcers (Botha et al., Citation2004). Tablets made from the leaves are also used as a natural antibiotic, thought to be effective against oral and esophageal thrush (Botha et al., Citation2004; Van Wyk et al., Citation2009).
Warburgia species are used as fresh and dried plant material. The stem bark is widely used; and leaves and roots are used as well. As all Warburgia species are undergoing considerable decline throughout their natural habitats in east, central and southern Africa due to over-exploitation for medicinal purposes, the presence of biologically active drimane sesquiterpenoides in the leaves provides rational basis for their substitution in ethnomedicine (Drewes et al., Citation2001; Frum, Citation2006; Zschocke et al., Citation2000). Expansion of research materials would provide more chances for discovery of new bioactive principle from the genus Warburgia. Validating the correlations of the ethnomedicinal uses, bioactive substances and pharmacological effects is of special importance, and is still the primary task for future research. Efforts are also needed to investigate the physiological and biochemical functions demonstrated by these species, identify the individual bioactive natural products, and illustrate their mechanism of action. The current results are largely limited to in vitro bioassay, and in vivo studies using laboratory animals. Furthermore, the promising results confirmed by animal models should be further investigated by clinical trials. The pharmacological study of Warburgia species is still in its infancy and a great deal of work needs to be done. For example, with our present knowledge of chemistry, phytochemistry and pharmacological activities of Warburgia species, it is not yet known whether there are significant qualitative and quantitative phytochemical differences between the various plant material forms used in ethnomedicine. Such phytochemical analysis will highlight the chemical constituents in Warburgia plant material and how the limited population numbers of the plant group can be utilized.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Ahmed M, Laing MD, Nsahlai IV. (2012). In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J Helminth [Online]. Available from: http://dx.doi.org/10.1017/S0022149X1200020X [last accessed 9 March 2013]
- Appleton CC, Drewes SE, Mashimbye MJ, Cunningham AB. (1992). Observations on the molluscicidal properties of warburganal on the South African Bulinus africanus (Planorbidae). J Med Appl Malacology 4:37–40
- Botha J, Witkowski ETF, Shackleton CM. (2004). The impact of commercial harvesting on Warburgia salutaris (‘pepper-bark tree’) in Mpumalanga, South Africa. Biod Cons 13:1675–98
- Brooks CJW, Draffan GH. (1969). Sesquiterpenoids of Warburgia species. II. Ugandensolide and ugandensidial. Tetrahedron 25:2887–98
- Bryant AT. (1966). Zulu Medicine and Medicine-men. Cape Town, South Africa: Struik Publisher
- Bussmann RW, Sharon D. (2006). Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J Ethnobiol Ethnomed 2:47
- Clarke TE, Appleton CC. (1997). The molluscicidal activity of Apodytes dimidiata E. Meyer ex Arn (Icacinaceae), Gardenia thunbergia L. F (Rubiaceae) and Warburgia salutaris (Berthol. f.) Chiov. (Canellaceae), three South African plants. J Ethnopharmacol 56:15–30
- Cook FEM. (1995). Economic Botany Data Collection Standard. Kew: Royal Botanic Gardens
- Deutschländer MS, Lall N, van de Venter M. (2009). Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharm Biol 47:348–65
- Dlamini TS, Dlamini GM. (2002). Swaziland. In: Golding JS, ed. Southern African Plant Red Data Lists: Southern African Botanical Diversity Network Report No. 14. Pretoria, South Africa: SABONET, 121–34
- Drewes SE, Crouch NR, Mashimbye MJ, et al. (2001). A phytochemical basis for the potential use of Warburgia salutaris (pepper-bark tree) leaves in place of bark. S Afr J Sci 97:283–6
- Felhaber T, Mayeng I. (1997). South African Traditional Healers Primary Healthcare Handbook. Cape Town, South Africa: Kagiso Publishers
- Flatie T, Gedif T, Asres K, Gebre-Mariam T. (2009). Ethnomedicinal survey of Berta ethnic group Assosa Zone, Benishangul-Gumuz regional state, mid-west Ethiopia. J Ethnobiol Ethnomed 5:14
- Frum Y. (2006). In vitro 5-lipoxygenase and antioxidant activities of South African medicinal plants commonly used topically for skin diseases [MSc dissertation]. Johannesburg: Witwatersrand University
- Frum Y, Viljoen AM. (2006). In vitro 5-lipoxygenase and anti-oxidant activities of South African medicinal plants commonly used topically for skin diseases. Skin Pharmacol Physiol 19:329–35
- Frum Y, Viljoen AM, Drewes SE. (2005). In vitro 5-lipoxygenase and anti-oxidant activities of Warburgia salutaris and drimane sesquiterpenoids. S Afr J Bot 71:447–9
- Fukuyama T, Sato T, Asakawa Y, Takemoto T. (1982). A potent cytotoxic warburganal and related drimane-type sesquiterpenoids from Polygonum hydropiper. Phytochemistry 21:2895–8
- Gelfand M, Drummond RB, Mavi S, Ndemera B. (1985). The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia. Gweru, Zimbabwe: Mambo Press
- Gerstner J. (1939). A preliminary checklist of Zulu names of plants. Bantu Stud 13:307–26
- Gilmore MR. (1932). Importance of ethnobotanical investigation. American Anthropol 34:320–7
- Githinji EK, Irungu LW, Tonui WK, et al. (2010). In vitro effects of Warburgia ugandensis, Psiadia punctulata and Chasmanthera dependens on Leishmania major promastigotes. Afr J Trad Complim Altern Med 7:264–75
- Gordon PM. (1953). The medicinal value of some indigenous trees to the Bantu people. Trees S Afr 5:13–15
- Heinrich M, Heneka B, Ankli A, et al. (2005). Spasmolytic and antidiarrhoeal properties of the Yucatec Mayan medicinal plant Casimiroa tetrameria. J Pharm Pharmacol 57:1081–5
- Henke B. (1994). Kenya Trees: Shrubs and Lianas. Nairobi, Kenya: National museums of Kenya
- Hollmann J, Van der Schijff M. (1996). Portrait of a medicinal tree: Has the publicity of being one of the trees of the year for 1996 helped conserve the pepper bark tree?. Veld Flora 82:115–16
- Hutchings A, Scott AH, Lewis G, Cunningham A. (1996). Zulu Medicinal Plants: An Inventory. Pietermaritzburg, South Africa: University of Natal Press
- IUCN. (2012). IUCN Red List of Threatened Species Version 2012.2 [Online]. Available from: http://www.iucnredlist.org [last accessed 7 Feb 2013]
- Izidine S, Bandeira SO. (2002). Mozambique. In: Golding JS, ed. Southern African Plant Red Data Lists: Southern African Botanical Diversity Network Report Series No. 14. Pretoria, South Africa: SABONET, 43–53
- Jagtap UB, Bapat VA. (2010). Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 129:142–66
- Jansen BMJ, de Groot A. (1991). The occurrence and biological activity of drimane sesquiterpenoids. Nat Prod Reports 8:309–18
- Kayombo EJ, Uiso FC, Mbwambo ZH, et al. (2007). Experience of initiating collaboration of traditional healers in managing HIV and AIDS in Tanzania. J Ethnobiol Ethnomed 3:6
- Kioy D, Gray AL, Waterman PG. (1990). A comparative study of the stem bark drimane sesquiterpenes and leaf volatile oils of Warburgia ugandensis and W. stuhlmanii. Phytochemistry 29:3535–8
- Kiringe JW. (2006). A survey of traditional health remedies used by the Maasai of southern Kajiado District, Kenya. J Ethnobot Res Appl 4:61–73
- Kokwaro JO. (2009). Medicinal Plants of East Africa. Nairobi, Kenya: East African Literature Bureau
- Krog M, Falcão MP, Olsen CS. (2006). Medicinal Plant Markets and Trade in Maputo, Mozambique. Forest and Landscape Working Papers, Danish Centre for Forest. Denmark: Landscape and Planning, KVL
- Kubitzki K. (1993). Canellaceae. In: Kubitzki K, Rohwer JG, Bittrich V, eds. The Families and Genera of Vascular Plants: Flowering Plants Dicotyledon, Magnoliid, Hamamelid and Caryophyllid. Berlin: Springer-Verlag, 200–3
- Kubo I. (1991). Screening techniques for plant-insect interactions. In: Hosttestmann K, ed. Methods in Plant Biotechnology. London: Academic Press, 179–93
- Kubo I. (1993). Insect control agents from tropical plants. In: Downum KR, Romeo JT, Stafford HA, eds. Recent Advances in Phytochemistry: Phytochemical Potential of Tropical Plants. New York: Plenum, 133–51
- Kubo I. (1995). Antifungal sesquterpene dialdehydes from the Warburgia plants and their synergists. In: Atta-ur-Rahman, ed. Studies in Natural Products Chemistry: Structure and Chemistry (part D). Amsterdam: Elsevier Science, 233–49
- Kubo I, Matsumoto T, Kakooko AB, Mubiru NK. (1983). Structure of mukaadial, a molluscicide from Warburgia plants. Chem Soc Japan Chem Lett 1983:979–80
- Kubo I, Miura I, Pettei MJ, et al. (1977). Muzigadial and warbuganal: Potent antifungal, antiyeast and African army worm antifeedant agents. Tetrahedron Lett 1:4553–6
- Kubo I, Nakanishi K. (1977). Insect antifeedants and repellents from African plants. In: Hedin PA, ed. ACS Symposium Series 62: The Chemical Basis for Host Plant Resistance to Pest. Washington: American Chemical Society, 165–78
- Kubo I, Lee YW, Pettei MJ, et al. (1976). Potent army worm antifeedants from the East African Warburgia plants. J Chem Soc Chem Comm 24:1013–14
- Kuglerova M, Tesarova H, Grade JT, et al. (2011). Antimicrobial and antioxidative effects of Ugandan medicinal barks. Afr J Biotech 10:3628–32
- Leshwedi M, Steenkamp V, Dutton M, Gulumian M. (2008). The ability of Warburgia salutaris extracts to protect against crystalline silica-induced cell injury. Hum Exp Toxicol 27:827–35
- Lovett J, Clarke GP. (1998a). Warburgia elongata. In: IUCN 2012, IUCN Red List of Threatened Species Version 2012.2 [Online]. Available from: http://www.iucnredlist.org [last accessed 8 Feb 2013]
- Lovett J, Clarke GP. (1998b). Warburgia stuhlmannii. In: IUCN 2012, IUCN Red List of Threatened Species Version 2012.2 [Online]. Available from: http://www.iucnredlist.org [last accessed 8 Feb 2013]
- Lovett JC, Ruffo CK, Gereau RE, Taplin JRD. (2006). Field Guide to the Moist Forest Trees of Tanzania. York: Centre for Ecology Law and Policy, Environment Department, University of York
- Mabogo DEN. (1990). The ethnobotany of the VhaVenda [MSc dissertation]. Pretoria: University of Pretoria
- Madikane VE, Bhakta S, Russell AJ, et al. (2007). Inhibition of mycobacterial arylamine N-acetyltransferase contributes to anti-mycobacterial activity of Warburgia salutaris. Bioorganic Mol Chem 15:3579–86
- Mander W, Mander J, Crouch N, et al. (1995). Catchment Action: Growing and Knowing Muthi Plants. Scotsville, South Africa: Institute of Natural Resources
- Manguro LOA, Ugi I, Hermann R, Lemmen P. (2003). Flavonol and drimane-type sesquiterpene glycosides of Warburgia stuhlmannii leaves. Phytochemistry 63:497–502
- Maroyi A. (2008). Ethnobotanical study of two threatened medicinal plants in Zimbabwe. Int J Biod Sci Manag 4:1–6
- Maroyi A. (2013). Warburgia salutaris (Bertol. f.) Chiov.: A multi-use ethnomedicinal plant species. J Med Plant Res 7:53–60
- Mashimbye MJ. (1993). Chemical Constituents of plants native to venda [PhD thesis]. Pietermaritzburg, South Afica: University of Natal
- Mashimbye MJ, Drewes SE, Appleton CC, Cunningham AB. (1992). Observations on the molluscicidal properties of (−)-warburganal on the South African Bulinusa fricanus (Planorbidae). J Med Appl Malacology 4:37–40
- Mashimbye MJ, Maumela MC, Drewes SE. (1999a). A drimane sesquiterpinoid lactone from Warburgia salutaris. Phytochemistry 51:435–38
- Mashimbye MJ, Maumela MC, Drewes SE. (1999b). Novel and bioactive metabolites of Warburgia salutaris indigenous to the Northern Province, South Africa. Nigerian J Nat Prod Med 3:28–30
- Mathabe MC, Nikolova RV, Lall N, Nyazema NZ. (2006). Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J Ethnopharmacol 105:286–93
- Maundu P, Tengnas B. (2005). Useful Trees and Shrubs for Kenya. Nairobi, Kenya: World Agroforestry Centre, Eastern and Central Africa Regional Programme
- Mbwambo ZH, Erasto P, Innocent E, Masimba PJ. (2009). Antimicrobial and cytotoxic activities of fresh leaf extracts of Warburgia ugandensis. Tanzania J Health Res 11:75–8
- Meinwald J, Prestwich GD, Nakanishi K, Kubo I. (1978). Chemical ecology: Studies from east Africa. Science 199:1167–73
- Mohanlall V, Odhav B. (2009). Furans and furanones with antimycotoxigenic activity isolated from Warburgia salutaris (Canellaceae). J Med Pl Res 3:231–40
- Msekandiana G, Mlangeni E. (2002). Malawi. In: Golding JS, ed. Southern African Plant Red Data Lists: Southern African Botanical Diversity Network Report No. 14. Pretoria, South Africa: SABONET, 135–56
- Mukamuri B, Kozanayi W. (1999). Socio-economic issues related to Warburgia salutaris: A powerful medicinal plant in Zimbabwe. Institute of Environmental Studies Working Paper No. 17. Harare, Zimbabwe: University of Zimbabwe
- Muthaura CN, Rukunga GM, Chhabra SC, et al. (2007a). Traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J Ethnopharmacol 114:377–86
- Muthaura CN, Rukunga GM, Chhabra SC, et al. (2007b). Antimalarial activity of some plants traditionally used in treatment of malaria in Kwale district of Kenya. J Ethnopharmacol 112:545–51
- Nakanishi K, Kubo I. (1978). Studies on warburganal, muzigadal and related compounds. Israel J Chem 16:28–31
- Nanyingi MO, Kipsengeret KB, Wagate CG, et al. (2010). In vitro and in vivo antiplasmodial activity of Kenyan medicinal plants. In: Midiwo JO, Clough J, eds. Aspects of African Biodiversity: Proceedings of the Pan-Africa Chemistry Network. Cambridge: RCS Publishing, 20–8
- Nanyingi M, Mbaria J, Lanyasunya A, et al. (2008). Ethnopharmacological survey of Samburu district, Kenya. J Ethnobiol Ethnomed 4:14
- Ngure PK, Tonui WK, Ingonga J, et al. (2009). In vitro antileishmanial activity of extracts of Warburgia ugandensis (Canellaceae), a Kenyan medicinal plant. J Med Plant Res 3:61–6
- Nichols G. (2005). Growing rare plants: A practical handbook on propagating the threatened plants of Southern Africa. Southern African Botanical Diversity Network Report No. 36. Pretoria, South Africa: SABONET
- Olila D. (1993). A study of the antimicrobial activities of Zanthoxylum chalybeum and Warburgia ugandensis: Ugandan medicinal plants [MSc dissertation]. Kampala, Uganda: Makerere University
- Olila D, Olwa-Odyek A, Opudo A. (2002). Screening extracts of Zanthoxylum chalybeum and Warburgia ugandensis for activity against measles virus (Swartz and Edmonston strains) in vitro. Afr Health Sci 2:2–10
- Olila D, Opunda-Asibo J, Odyek O. (2001). Bioassay-guided studies on the cytotoxic and in vitro trypanocidal activities of a sesquiterpene (Muzigadial) derived from a Ugandan medicinal plant (Warburgia ugandensis). Afr Health Sci 1:12–15
- Orwa C, Mutua A, Kindt R, et al. (2009). Agroforestree database: A tree reference and selection guide version 4.0. [Online]. Available from: http://www.worldagroforestry.org [last accessed 18 Jan 2013]
- Palgrave KC. (2000). Trees of Southern Africa. Cape Town: Struik Publisher
- Rabe T, van Staden J. (1997). Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol 56:81–7
- Rabe T, van Staden J. (2000). Isolation of an antibacterial sesquiterpenoid from Warburgia salutaris. J Ethnopharmacol 73:171–4
- Ribeiro A, Romeiras MM, Tavares J, Faria MT. (2010). Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J Ethnobiol Ethnomed 6:33
- Rugutt JK, Ngigi AN, Ndalut PK. (2006). Native Kenyan plants as alternatives to methyl bromide in soil fumigation. Phytomedicine 13:576–83
- Scott-Shaw R, Hilton-Taylor C, Kasseepursad B, Church B. (1998). The conservation status of the pepper bark tree. SABONET News 3:73–5
- Semenya SS, Maroyi A. (2012). Medicinal plants used by the Bapedi traditional healers to treat diarrhoea in the Limpopo Province, South Africa. J Ethnopharmacol 144:395–401
- Taniguchi M, Adachi T, Haraguchi H, et al. (1983). Physiological activity of warburganal and its reactivity with sulfhydryl groups. J Bioch 94:149–54
- Taniguchi M, Chapya A, Kubo I, Nakanishi K. (1978). Screening of east African plants for antimicrobial activity 1. Chem Pharm Bull 26:2910–13
- Taniguchi M, Kubo I. (1993). Ethnobotanical drug discovery based on medicine men’s trials in African savanna: Screening of east African plants for antimicrobial activity II. J Nat Prod 56:1539–46
- Van Wyk B-E, Gericke N. (2000). People’s Plants: A Guide to Useful Plants of Southern Africa. Pretoria, South Africa: Briza Publications
- Van Wyk B-E, Van Oudtshoorn B, Gericke N. (2009). Medicinal Plants of South Africa. Pretoria, South Africa: Briza Publications
- Venter F, Venter J. (2002). Making the Most of Indigenous Trees. Cape Town, South Africa: Briza Publications
- Verdcourt B. (1954). Notes on the tropical African Canellaceae. Kew Bull 60:541–4
- Verdcourt B. (1956). Canellaceae: Flora of Tropical East Africa. Kew: Royal Botanic Gardens
- Verdcourt B. (1990). Canellaceae. In: Launert E, Pope GV, eds. Flora Zambesiaca 7. Kew: Royal Botanic Gardens, 1–3
- Verpoorte R, Choi YH, Kim HK. (2005). Ethnopharmacology and systems biology: A perfect holistic match. J Ethnopharmacol 100:53–6
- Wamalwa NL, Oballa P, Gicheru J. (2006). Genetic Variation of Warburgia ugandensis in Kenya and Implications for its Cultivation. Nairobi, Kenya: Kenya Forestry Research Institution (KEFRI)
- Watt JM, Breyer-Brandwijk MG. (1962). The Medicinal and Poisonous Plants of Southern and Eastern Africa. London: Livingstone
- Were PS, Kinyanjui P, Gicheru MM, et al. (2010). Prophylactic and curative activities of extracts from Warburgia ugandensis Sprague (Canellaceae) and Zanthoxylum usambarense (Engl.) Kokwaro (Rutaceae) against Plasmodium knowlesi and Plasmodium berghei. J Ethnopharmacol 130:158–62
- Williams VL, Geldenhuys CJ, Scott-Shaw CR, Victor JE. (2008). Warburgia salutaris (G. Bertol.) Chiov. National Assessment: Red List of South African Plants Version 2012.1. [Online]. Available from: http://redlist.sanbi.org [last accessed 12 Feb 2013]
- Wube AA, Bucar F, Gibbons S, Asres K. (2005). Sesquiteroenes from Warburgia ugandensis and their antimycobacterial activity. Phytochemistry 66:2309–15
- Xu M, Litaudon M, Krief S, et al. (2009). Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis. Molecules 14:3844–50
- Zonyane S, Van Vuuren SF, Makunga NP. (2012). Pharmacological and phytochemical analysis of a medicinal plant mixture that is used as traditional medicine in Western Cape. Paper presented at South Africa Association of Botanist 38th Annual Conference, 15–18 January 2012. Pretoria, South Africa: University of Pretoria
- Zschocke S, Rabe T, Taylor JLS, et al. (2000). Plant part substitution: A way to conserve endangered medicinal plants? J Ethnopharmacol 71:281–92.