Abstract
Context: Astragali Radix (Huangqi; Astragalus mongholicus BUNGE, Fabaceae) is used in herbal medicinal products as well as in many food supplements. In traditional Chinese medicine, the roots are used for its Qi tonifying, immunostimulant, cardioprotective, hepatoprotective and hypoglycemic effects.
Objective: Astragaloside IV (AGS-IV), a cycloartane-type triterpene glycoside is used as a marker compound for the quality control of Astragali Radix in various pharmacopoeias.
Materials and methods: In this study, we analyzed the content of AGS-IV and other astragalosides in various commercial samples of Huangqi by reversed-phase HPLC using evaporative light scattering detection.
Results: The analyses revealed that AGS-IV is formed during sample preparation from acylated astragalosides like astragaloside I and astragaloside II, when using the assay method of the European Pharmacopoeia.
Discussion and conclusion: For consistent assay results, the extraction methods of the pharmacopoeias should be re-evaluated and optimized. Alternatively, the hydrolysis by ammonia could be omitted and the genuine compounds like astragaloside I, II and malonyl-AGS-I could be considered for the quality control of Astragali Radix.
Introduction
Astragali Radix, Huangqi, the roots of Astragalus mongholicus var. mongholicus BUNGE [syn. Astragalus membranaceus BUNGE var. mongholicus (BUNGE) PK HSIAO] (Fabaceae) have been used for more than 2000 years in traditional Chinese medicine and represents one of the most important so-called Qi tonifying herbs (Braun & Cohen, Citation2007). The plant is native to China, Korea, Mongolia and Japan and is mainly cultivated in the provinces of Shanxi, Gansu and Heilongjiang and inner Mongolia (Kuhn & Winston, Citation2001; Wu, Citation2005). Pharmacological studies have shown immunostimulatory, cardioprotective, hepatoprotective and hypoglycemic effects (Chu et al., Citation1988; Hikino et al., Citation1976; Rios & Waterman, Citation1997; Zhang et al., Citation2006). The major active constituents in Astragalus are polysaccharides and triterpene saponins (Huang et al., Citation1982; Tang & Eisenbrand, Citation1992). Minor compounds, like flavonoids, act as radical scavengers (Lin et al., Citation2000; Shirataki et al., Citation1997). The saponins in Astragali Radix, known as astragalosides, are derived from the aglycones cycloastragenol (9,19-cyclolanostan type) and astragenol (lanost-9 (11)-ene type) (Kitagawa et al., Citation1983a). In addition to astragalosides I–VIII, isoastragaloside I and II, acetylastragaloside I and a soya saponin have been described (Kitagawa et al., Citation1983b,c). Astragalosides I–VII, isoastragaloside I and II and acetylastragaloside I are derived from cycloastragenol. Selected structures are shown in .
Figure 1. Structures of astragaloside I, II, IV, malonylastragaloside I from A. mongohlicus and methyl-malonylastragaloside I.
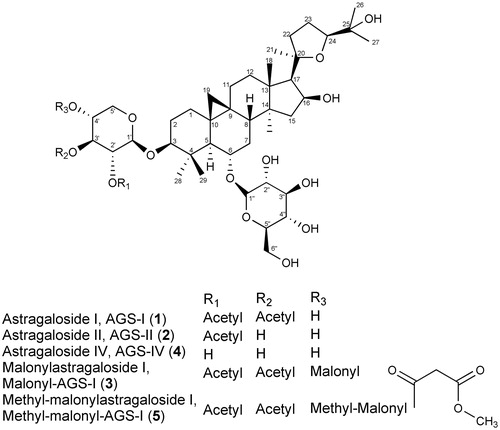
Astragenol is the aglycone of astragaloside VIII, which is of the oleanane-type. Cycloastragenol, in contrast, is of the dammaran type (Kitagawa et al., Citation1983d). Astragaloside IV (AGS-IV, 4, C41H68O14; MW 784.97) is used as an active marker for the quality control of Astragali Radix in the Chinese Pharmacopoeia (Anonymous, Citation2010), and also the European Pharmacopoeia (Ph. Eur. 7.0; Anonymous, Citation2011) considers specifically AGS-IV for the assay. According to the Chinese and European Pharmacopoeia, the content of AGS-IV should be at least 0.04%. Recently, when astragaloside malonates have been identified, it has already been argued, that qualitative and quantitative analysis of AGS-IV may not give the correct information about the genuine saponin profile of Astragalus roots (Chu et al., Citation2010). In this study we analyzed the content of astragalosides and AGS-IV in samples cultivated in Germany and evaluated the assay.
Materials and methods
Plant material and chemicals
The samples of Astragali Radix were supplied from field cultivation at the experimental stations Baumannshof and Puch of the Bavarian State Research Center for Agriculture (Freising, Germany). The roots were harvested in autumn after one season of cultivation, washed, coarsely cut and dried at 42 °C in a drying oven. The dried roots (<10% water content) were stored for 3 years in the dark at room temperature before analysis.
Astragaloside I and II were obtained from PhytoLab (AGS-I: Batch Nr. 1843, AGS-II: Batch Nr. 1303).
Methyl-malonylastragaloside I was obtained from the dried roots of a commercial sample of A. mongholicus var. mongholicus obtained from Asam-Apotheke (Munich, Germany) as follows:
Powdered (SM1 Retsch, Haan, Germany) Astragali Radix (890 g) was extracted three times with 6 l acetonitrile/water 84/16 (v/v) using an Ultraturrax T45N (IKA, Staufen i. Breisgau, Germany) for 10 min and sonicated in an ultrasonic bath (Sonorex RK106, Bandelin, Berlin, Germany) for 15 min. Extracts were filtered and carefully evaporated (<40 °C, 10 mbar). The residue (65.5 g) was dissolved in a mixture of ethyl acetate/methanol/water 100/20/10 (v/v/v) and separated in an open column (Allihn tube, 50 mm inner diameter) on 185 g Silica (MP Silica 32-63, 60 Å, MP Biomedical, Eschwege, Hesse, Germany) by vacuum chromatography. Six fractions, each with a volume of 250 ml, were collected by eluting with ethyl acetate/methanol/water 100/20/10 (fractions 1–3) and methanol (fractions 4–7). Fraction 3 (7.14 g) was further separated on two solid phase extraction (SPE) columns (10 g, 60 ml, C18-E, Phenomenex, Aschaffenburg, Germany) starting with 10% acetonitrile in water, followed by a step gradient with 30, 50, 70 and 100% acetonitrile in water (50 ml each). The combined fraction of the 70% elution step (560 mg) contained the target substance at Rf = 0.17 as indicated by thin layer chromatography (Silica gel 60 F254, Merck, Darmstadt, Germany; mobile phase: ethyl acetate/methanol/water 100/20/10; detection: purple color with anisaldehyde after heating the plate at 144 °C). This crude fraction was used for further purification and derivatization experiments. Methylation was performed as reported by Kobayashi et al. (Citation2003). About 200 mg of the crude fraction were dissolved in a glass vial in benzene/methanol (1600/400 µl) with a stirring rod and 2 ml of (trimethylsilyl)-diazomethane (2.0 M in diethyl ether, Aldrich, Steinheim, Germany) was added. After 1 h of stirring at room temperature, solvents were removed using a stream of nitrogen. The reaction mixture was dissolved in 2–3 ml of a mixture of dichloromethane/methanol 95/5 and subjected to a silica SPE column (2 g, 12 ml, SI-1, Phenomenex, Aschaffenburg, Germany). Five fractions were obtained by eluting with dichloromethane/methanol 95/5, 93/7, 9/1, 8/2, 0/100 (10 ml each). Fractions containing the target molecule (93/7 and 9/1) were pooled and evaporated. The residue was suspended in 500 µl 30% acetonitrile in water and 400 µl methanol was added. A clear solution was obtained by warming to 60 °C in a water bath. The solution was separated with reversed-phase high performance liquid chromatography (RP-HPLC), yielding about 20 mg of the compound. Final purification was achieved by repeated RP18-HPLC.
Identification of methyl-malonylastragaloside I was performed using nuclear magnetic resonance (NMR) analysis ().
Table 1. Comparison of 1H and 13C NMR data (600 and 150 MHz, respectively) of methyl-malonyl-AGS-I (pyridine d5, 23 °C, 5) with published data of malonyl-AGS-I (3).
Astragaloside IV CRS was from Chinás National Institute for Control of Pharmaceutical and Biological Products.
Solvents used for HPLC analysis were of HPLC grade; solvents used for extraction and isolation were p.a. grade.
Sample preparation for analysis of AGS IV
Sample preparation 1 according to the European Pharmacopoeia (Ph. Eur. 7.0, 2010)
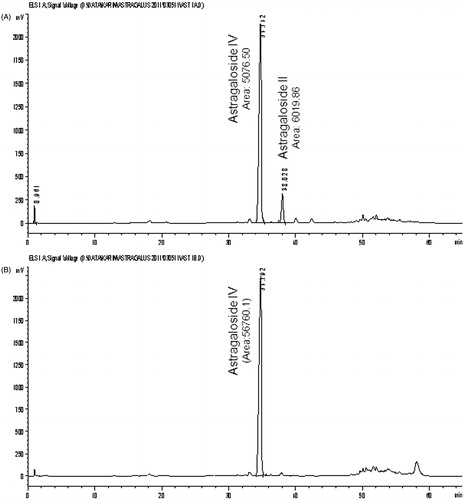
Powdered Astragali Radix (4 g) was macerated in a Soxhlet extractor with 40 ml of methanol overnight. After adding further 80 ml methanol, the roots were extracted under reflux for 4 h. After evaporation of the solvent (CentriVap® Centrifugal Labconco Concentrators: Envirotech, Düsseldorf, Germany; 40 °C, 0 mbar) the residue was suspended in 10 ml water and washed four times with 40 ml n-butanol saturated with water. The combined butanol layers were washed two-times with 40 ml of ammonia R. The ammonia layers were discarded. The obtained butanol layer was evaporated to dryness. The residue was re-dissolved in 5 ml water, cooled and applied to a solid phase column with 1 g of octadecylsilyl silica gel previously washed with 5 ml of methanol and 5 ml of water. After washing with 20 ml of water and 20 ml of 25% ethanol, it was eluted with 25 ml of 70% ethanol and evaporated to dryness. The residue was dissolved in 2.0 ml (Ph.Eur.: 5.0 ml; for comparison reason we dissolved the residue in 2.0 ml) of methanol and used for HPLC analysis (see chromatogram A in ).
New, accelerated sample preparation 2
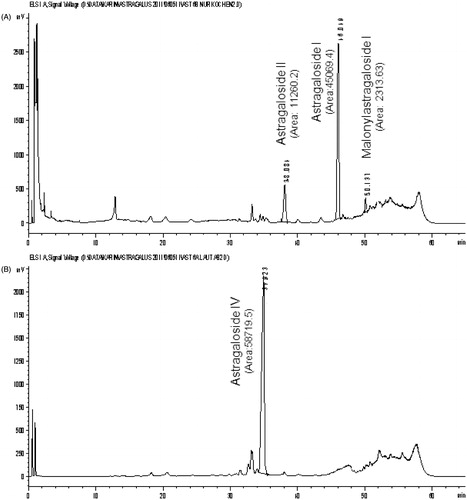
Powdered Astragali Radix (4 g) was macerated with 40 ml methanol overnight. After adding further 80 ml methanol, the roots were extracted under reflux for 4 h. Regarding the first two steps, we used the same extraction procedure as indicated in the European Pharmacopoeia. After evaporation of the solvent (Büchi Syncore, 40 °C, 200 rpm) the residue was suspended in 3.00 ml water and applied to an Extrelut Column (EXtrelut® NT 3 prepacked glass column, 1–3 ml sample solution, Merck KGaA, Darmstadt, Germany). After 10 min exposure the aqueous solution was eluted three-times with 5 ml butanol. The eluted butanol extract was washed 3–4 times with 10 ml ammonia R [72% ammonia (≥25%) in water] in a 100 ml separation funnel until the hydrolysis is completed and visible in the clear ammonia layer. The obtained butanol layer was evaporated to dryness in a 50 ml centrifuge tube in a ZentriVap Concentrator (CentriVap® Centrifugal Labconco Concentrators: Envirotech, Düsseldorf, Germany; 40 °C, 0 mbar). The residue was re-dissolved in 2.0 ml methanol (see chromatogram B in ).
Sample preparation for hydrolysis experiments
The same extraction procedure as indicated for the new, accelerated sample preparation 2 was used, though the eluted butanol extract was evaporated to dryness and re-dissolved in 2.0 ml methanol (see chromatogram A in ), or washed four times with 10 ml ammonia R [72% ammonia (≥25%) in water] in a 100 ml separatory funnel (see chromatogram B in ) before evaporation.
Solution of reference compounds for hydrolysis experiments
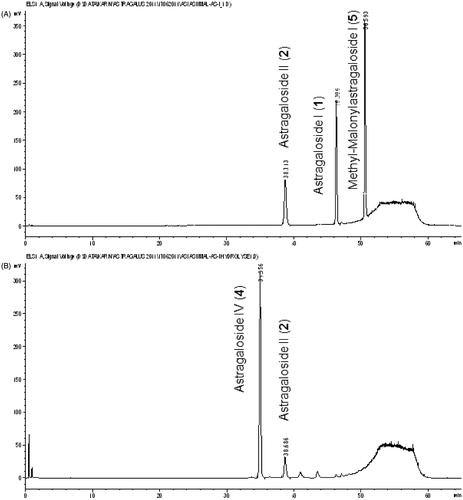
Stock solutions of reference compounds 1, 2 and 5 were prepared and diluted with methanol to 200 µg/ml each. Twenty microliters of these solutions were injected directly (see chromatogram A in ), whereas 500 µl were evaporated to dryness and re-dissolved in 15 ml of a mixture of water and butanol (1:5). This solution was washed two-times with 10 ml ammonia R [72% ammonia (≥25%) in water] in a 100 ml separation funnel. The obtained butanol layer was evaporated to dryness (40 °C, 0 mbar; Laborota 4000-efficient, Heidolph Instruments, Schwabach, Germany). The residue was re-dissolved in 1.0 ml methanol p.a. and used for HPLC analysis (see chromatogram B in ).
Chromatographic conditions
HPLC analysis
The qualitative analysis of A. mongholicus saponins was performed using HPLC–evaporative light scattering detector (ELSD) (HPLC: Agilent 1100 Series; ELSD: Altech 3300 E). A RP-18 analytical column (Phenomenex, S/No. 423108-5, Synergi Fusion-RP; 150 × 2.0 mm, 4.0 µm particle size) was used for the chromatographic separations. The mobile phase consisted of water (solvent A) and MeCN (solvent B) using gradient elution as published in Ph. Eur. 7.0 (2010) starting with 10% B (0–5 min), 10–20% B (5–10 min), 20–25% B (10–20 min), 25–33% B (20–30 min), 33–35% B (30–40 min), 35–60% B (40–50 min) and ended up at 60% B (50–55 min), followed by an equilibration time of 3 min. The flow rate was set to 0.5 ml/min. ELSD was performed with air as carrier gas at a flow rate of 1.5 l/min. The evaporation temperature was 50 °C and the nebulizer was set to room temperature. The injection volume of the extracts and reference solutions was 20 µl.
Results and discussion
Although the extraction method described in the European Pharmacopoeia (Ph. Eur.) yields very clear HPLC chromatogram (A in ), the sample preparation is very time consuming. Huge amounts of solvents are needed and the probability of the formation of emulsions is high. Therefore, we tried to optimize and to accelerate the extraction method described in the European Pharmacopoeia. When analyzing the sample preparation method it became obvious that the washing step with ammonia R is essential for the detection of AGS-IV and the separation of the flavonoids.
We noticed samples with AGS-IV as a major peak and some minor peaks eluting after AGS-IV (see chromatogram A in ), whereas in some other samples there was only the major peak of AGS-IV. Based on these differences in the chromatograms, we investigated the sample preparation and prepared one extract with and one extract without the ammonia washing step (see chromatogram A and B in ). Without ammonia, compounds 1 and 2 seem to be the major constituents in the extract and AGS-IV was only found as a minor compound. This was also shown in chromatograms published by Yu et al. (Citation2007). Treatment of the butanol extract with ammonia as suggested by Ph. Eur. leads to a complete degradation of 1, 2 and 5. Summing up the peak areas of 1 (Rt = 46), 2 (Rt = 38) and 5 (Rt = 50) in results almost in the area of 4 (Rt = 34) in .
Therefore, it was suspected that AGS-IV is formed during sample preparation from acylated astragalosides, like 1, 2 and 3, by hydrolysis with ammonia. This could be confirmed with a reference solution of pure 1, 2 and 5. Treatment with ammonia resulted in an almost quantitative formation of 4 ().
It seems that AGS-IV is not a genuine constituent of Astragalus roots, but it is formed from acylated astragalosides, like 1, 2 and 3 by hydrolysis. The treatment with ammonia is therefore an essential step in the sample preparation of the assay of Astragali Radix and the content of AGS-IV depends finally on the effectiveness of this hydrolytic step.
As the current pharmacopoeia assay does not reveal the genuine content of AGS-IV it must be guaranteed that hydrolysis is performed quantitatively.
Conclusion
For the quality control of Astragali Radix, it should be evaluated whether hydrolysis with ammonia is appropriate or not. As this method does not reveal the genuine content of AGS-IV, a quantitative hydrolysis of acylated astragalosides is necessary for comparison of different samples. Thus, the assays of the pharmacopoeias should be re-evaluated and optimized. The Pharmacopoeias may alternatively develop assays based on the genuine profile of astragalosides for the quality control of Astragali Radix. AGS-I is the major genuine constituent in a genuine extract. Without the hydrolytic step, the method of the European Pharmacopoeia could be more straight-forward and constituents like AGS-I, AGS-II and malonyl-AGS-I could be considered as new markers for the quality control of Astragali Radix.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Anonymous. (2010). Astragali radix. Huangqi Pharmacopoeia of the People’s Republic of China. Chi Med Sci Tec. Chinese Pharmacopoeia Commision Beijing, China
- Anonymous. (2011). European Pharmacopoeia Ph.Eur. 7.0 Chinesischer-Tragant-Wurzel, Astragali mongholici radix, EDQM, Strasbourg
- Braun L, Cohen M. (2007). Herbs and Natural Supplements: An Evidence Based Guide. Sydney: Churchill Livingstone
- Chu DT, Wong WL, Mavligit GM. (1988). Immunotherapy with Chinese medicinal herbs I. Immune restoration of local xenogeneic graft-versus-host reactions in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol 25:119–23
- Chu C, Cai H-X, Ren M-T, et al. (2010). Characterization of novel astragaloside malonates from Astragali Radix by HPLC with ESI quadropole TOF MS. J Sep Sci 33:570–81
- Hikino H, Funayama S, Endo K. (1976). Hypotensive principle of Astragalus and Hedysarum roots. Planta Med 30:297–302
- Huang QS, Lu GB, Li YC, et al. (1982). Studies on the polysaccharides of “Huang Qi” (Astragalus mongholicus Bunge). Acta Pharmaceut Sin 17:200–6
- Kitagawa I, Wang HK, Takagi A, et al. (1983a). Saponin and Sapogenol, XXXIV. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (1). Cycloastragenol, the 9,19-cyclo-lanostantype aglycone of astragalosides, and the artifact aglycone astragenol. Chem Pharm Bull 31:689–97
- Kitagawa I, Wang HK, Saito M, et al. (1983b). Saponin and sapogenol, XXXV. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (2). Astragalosides I, II and IV, acetylastragaloside I and isoastragalosid I and II. Chem Pharm Bull 31:698–708
- Kitagawa I, Wang H, Saito M, Yoshikawa M. (1983c). Saponin and sapogenol XXXVI. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (3). Astragalosides III, V, and VI. Chem Pharm Bull 31:709–15
- Kitagawa I, Wang HK, Yoshikawa M. (1983d). Saponin and sapogenol XXXVII. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (4). Astragalosides VII and VIII. Chem Pharm Bull 31:716–22
- Kobayashi H, Meguro S, Yoshimoto T, Namikoshi M. (2003). Absolute structure, biosynthesis, and anti-microtubule activity of phomopsidin, isolated from a marine-derived fungus Phomopsis sp. Tetrahedron 59:455–9
- Kuhn MA, Winston D. (2001). Herbal Therapy and Supplements: A Scientific and Traditional Approach. Philadelphia: Lippincott
- Lin LZ, He XG, Lindenmaier M, et al. (2000). Liquid chromatography-electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A. membranaceus. J Chromatogr A 876:87–95
- Rios JL, Waterman PG. (1997). A review of the parmacology and toxicology of Astragalus. Phytother Res 11:411–18
- Shirataki Y, Takao M, Yoshida S, Toda S. (1997). Antioxidative components isolated from the roots of Astragalus membranaceus Bunge (Astragali radix). Phytother Res 11:603–5
- Tang W, Eisenbrand G. (1992). Chinese Drugs of Plant Origin. Berlin: Springer
- Wu J-N. (2005) Chinese Materia Medica. New York: Oxford University Press
- Yu Q-T, Qi L-W, Li P, et al. (2007). Determination of seventeen main flavonoids and saponins in the medicinal plant Huang-qi (Radix Astragali) by HPLC-DAD-ELSD. J Sep Sci 30:1292–9
- Zhang WD, Chen H, Zhang C, et al. (2006). Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med 72:4–8