Abstract
Context: Aging leads to endothelial dysfunction and vascular stiffness which are the main causes of many cardiovascular diseases. Previous reports have shown that the cell protective effect of silymarin (SM) is dependent on its antioxidant properties.
Objectives: We investigated the effect of SM on vascular functions of aged rats and the involvement of nitric oxide or cyclooxygenase (COX) activity in this effect.
Materials and methods: Isolated rat aortas were obtained from 22-month old rats. Each ring was incubated with SM (50 mg/L), SM/l-nitro-arginine methyl ester (100 μM, l-NAME) or SM/indomethacin (10 μM, INDO) in tissue bath. Three- to four-month-old rats were used as young controls. Endothelium-intact rings were precontracted with α-receptor agonist phenylephrine (0.001–30 µM) or voltage-dependent high potassium (40 mM), endothelium dependent/independent relaxant responses were obtained using acetylcholine (0.001–30 µM) and sodium nitroprusside (0.0001–3 µM), respectively.
Results: Aging increased phenylephrine sensitivity (6.45 ± 0.08; 6.88 ± 0.09) and decreased KCl contraction (882 ± 118.4; 499 ± 80.4). SM treatment decreased the Emax of both agents (548 ± 109; 223 ± 48.9). Aging deteriorated acetylcholine relaxation (93.9 ± 2.09; 72.0 ± 2.56) and SM improved the response (86.3 ± 1.90). l-NAME prevented the SM effect whereas INDO was ineffective.
Discussion and Conclusion: Immediate SM treatment partially restored endothelial dysfunction and vascular tone in aging. The possible mechanism might not be mediated by prostacyclin or the COX pathway in acute administration; the nitric oxide pathway and calcium antagonistic features of SM relate to its action on the vessel.
Introduction
Some of morphologic and pathophysiologic changes such as endothelial dysfunction, inflammation, atherosclerosis, vascular stiffness and increased reactive oxygen species (ROS) contribute to vascular aging and further complicate hypertension and age-related cardiovascular pathologies. Nitric oxide (NO), the main vasodilator, is derived by endothelial nitric oxide synthase (eNOS) from healthy endothelium or the inducible form of NOS (iNOS) in inflammatory conditions. When NO reacts with ROS in oxidative stress, this situation results in a decrease in NO bioavailability and production of harmful substances such as peroxinitrite in aged vasculature (Cau et al., Citation2012; Demirci et al., Citation2005). On the other hand, the activity of cyclooxygenase (COX) also regulates vascular contraction and/or relaxation by generating prostaglandins. The inducible form of COX in inflammation is known as COX-2. Hence, pharmacologic modulation of these vasoactive mediators and enzymes may represent therapeutic benefit to prevent the progression of cardiovascular aging (Cau et al., Citation2012; Wong et al., 2010a).
A standardized extract of milk thistle, silymarin (SM), contains many compounds, mainly silibinin, dehydrosilibinin, isosilibinin, silychristin and silidianin (Chlopcikova et al., Citation2004). SM by itself is a candidate drug against mushroom poisoning and also each SM compound gives promising evidence for the treatment of hepatic, cardiovascular, neurodegenerative diseases and cancer by possessing anti-inflammatory, anti-proliferative, anti-oxidative and phytoestrogenic activity (Feher & Lengyel, Citation2012; Gabrielova et al., Citation2010; Kroll et al., Citation2007; Pliskova et al., Citation2005; Tyagi et al., Citation2012). It has been shown that SM prevents doxorubicin (Chlopcikova et al., Citation2004; El-Shitany et al., Citation2008) and isoproterenol-induced injury (Zhou et al., Citation2006) and dehydrosilybin attenuates ROS production (Gabrielova et al., Citation2010) in rat cardiomyocytes. SM modulates COX-2 and iNOS enzymes in proliferating mesenchymal stem cells (Ahmadi-Ashtiani et al., Citation2012). Similarly, silibinin inhibits tumor-associated macrophages, the production of inflammatory cytokines and regulates mouse lung epithelial cell COX-2 and iNOS expression (Tyagi et al., Citation2012).
Meanwhile, the extract itself is consumed as an alternative self-medication treatment option as an over-the-counter product. Therefore, the aim of this study was first to determine the safety and efficacy of SM extract on the endothelium and smooth muscle functions of the vessel at certain age. Secondly, a possible relationship between its effect and NO and/or COX-mediated pathway activities on vessels were investigated. However, this study did not dissect signaling pathways in detail; it might provide a general concept about SM to enable further research.
Materials and methods
Chemicals and equipment
Phenylephrine hydrochloride (PE), acetylcholine hydrochloride (Ach), sodium nitroprusside (SNP), indomethacin (INDO), l-nitro-arginine methyl ester (l-NAME), SM (S0292), dimethyl sulfoxide (DMSO) and Kreb’s chemical salts were purchased from Sigma Chemicals (Interlab, Izmir, Turkey). Stock solution of INDO and SM were diluted in DMSO just prior to experiments. The maximum concentration of DMSO in the bath was <0.05%.
Measurement of isometric force were recorded with a force transducer (MAY FDT 05, Commat Ltd., Ankara, Turkey) and a data acquisition system (MP 150, Biopac Systems, Inc., Commat Ltd, Ankara, Turkey).
Animals
Three- to four-month-old younger and 22-month-old elder rats were obtained from the Experimental Animal Center of Adnan Menderes University (ADU) and housed under standardized conditions (12 h dark/light cycle, 20 ± 1 °C room temperature) in regular cages and allowed free access to food and water. All experiments were performed in accordance with the principles and guidelines of the ADU Animal Ethical Committee (approval HADYEK 50.04/2011/116).
Experimental design
On the study day, under anesthesia with ketamine and xylasine (50 and 5 mg/kg, respectively), four rings ∼3 mm in length from the thoracic aorta were obtained. The rings were mounted in 20 ml organ baths containing Krebs–Henseleit solution with the following composition (in millimolar): NaCl 118.1, KCl 4.56, CaCl2 1.22, MgSO4 1.22, KH2PO4 1.1, NaHCO3 25, d-glucose 10.1 at 37 °C and constantly oxygenated with carbogen (Demirci et al., Citation2008; Tugrul et al., Citation2011). Under 2 g tension, the rings rested for 60–70 min for the young group, and 50 min for aged groups. Then each ring of the aged group was randomly assigned to a specific subgroup and further pre-treated with the relevant agents for 20 min, before PE pre-construction. The agents remained in the bath throughout the experiments. The experimental groups were as follows. Y: young 3–4-month old to compare the aging progress. O: aged 22-month old. O + SM: aged group incubated in SM (50 mg/L). O + SM + l-NAME: aged group incubated in SM (50 mg/L) and l-NAME (100 μM). O + SM + INDO: aged group incubated in SM (50 mg/L) and INDO (10 μM). O + DMSO: old group incubated in DMSO (0.05%).
Cumulative concentration-response curves were obtained by adding increased PE (0.001–30 µM) or KCl (40 mM) concentrations to the bath. Relaxant responses were determined by using cumulative concentrations of Ach (0.001–30 µM) or SNP (0.0001–3 µM). Co-incubation of l-NAME (a non-selective NOS inhibitor) with SM excluded the possible NO pathway and co-incubation of INDO (a non-selective COX inhibitor) with SM excluded the possible COX pathway on SM action in this setup.
Data presentation and statistics
Concentration-response curves were fitted by non-linear regression with simplex algorithm and the rings maximum responses (Emax) and vascular sensitivity (pD2; −logEC50) values were calculated. Emax values of PE and KCl were normalized to the dry tissue weight of each ring. Relaxant responses were given as the percentages of PE precontraction. Emax and pD2 were assessed by using Mann–Whitney U-test. Variance analysis was used to determine significance among the cumulative concentration curves. Data were presented as mean ± SEM, p values <0.05 were considered significant.
Results
Contractile responses
Although the comparison of concentration-response curves with PE did not show any significance and the maximal response to PE did not significantly change (), the sensitivity of the tissue to PE was significantly increased in the old group (p < 0.01, ). SM incubation tended to shift concentration-response curves to the right, decrease Emax and the sensitivity of the rings in old aortas (, ). These declines were not significant from the youngsters and co-existance of INDO did not prevent the effects of SM on vessels. l-NAME completely inhibited SM effects, the concentration-response curves shifted to the left, and Emax and the sensitivity of the rings increased significantly (p < 0.001; , ).
Figure 1. Concentration-response curves for phenylephrine in intact endothelium aortic rings of control rats (young) and of old rats. Some old animal rings were incubated with SM (50 mg/L), SM + l-NAME (100 µM) or SM + INDO (10 µM). Values are mean ± SEM of eight or nine rings. #p < 0.001 versus young group.
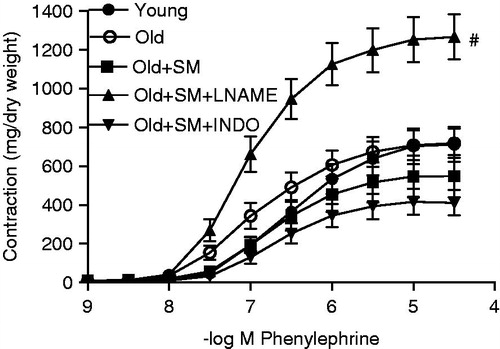
Table 1. Contractile responses to phenylephrine and KCl (milligram/dry tissue weight) and relaxant responses to acetylcholine and SNP (% phenylephrine precontraction) in intact endothelium aortic rings of control rats (young) and old rats.
Contractility induced by high KCl was lower in aged than the young control values (p < 0.05). SM further decreased the response (p < 0.01) and the presence of INDO did not change SM’s effect, but l-NAME increased the contractility ().
Relaxant responses
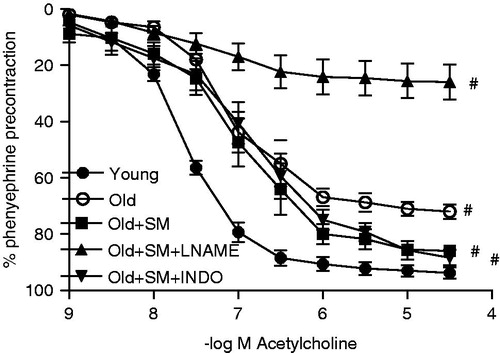
Aging shifted the Ach concentration-response curve to the right (p < 0.001, ), resulting in decreased maximum relaxation and vascular sensitivity to Ach (both p < 0.001, ). Although the concentration-response curve and the sensitivity of the rings to Ach remained unchanged, SM treatment improved the Emax response (p < 0.05) and this improvement was not prevented by INDO. Giving l-NAME completely blocked the beneficial effect of SM on endothelium (, ).
Figure 2. Concentration-response curves for acetylcholine in intact endothelium aortic rings of control rats (young) and old rats. Some old animal rings were incubated with SM (50 mg/L), SM + l-NAME (100 µM) or SM + INDO (10 µM). Values are mean ± SEM of eight or nine rings. #p < 0.001 versus young group.
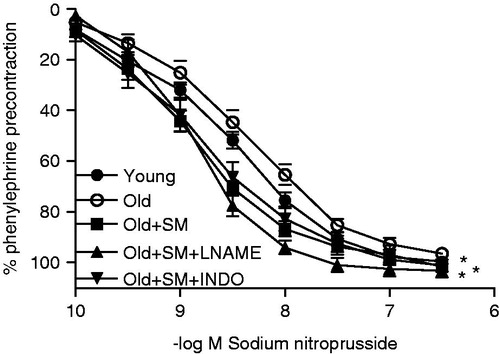
Smooth muscle sensitivity of the ring decreased (p < 0.05) and relaxation to SNP tended to deteriorate by aging. With the concentration-response curve, the SM group showed a better relaxant effect to SNP than the young controls (p < 0.05) and decreased vessel sensitivity (p < 0.001). INDO and l-NAME did not influence the SM responses (, ).
Figure 3. Concentration-response curves for SNP in intact endothelium aortic rings of control rats (young) and old rats. Some old animal rings were incubated with SM (50 mg/L), SM + l-NAME (100 µM) or SM + INDO (10 µM). Values are mean ± SEM of eight or nine rings. *p < 0.05 versus young group.
Incubation of the rings with the vehicle (DMSO) did not affect the contractile or relaxant responses in aortic rings (data not shown).
Discussion
Many different aspects of SM and its compounds have drawn scientific attention. It has not only been used traditionally, but also clinical studies have been established for the prevention and treatment of liver diseases. It has been accepted as a liver tonic. It is used as a conventional dietary supplement and under the investigation of cancer researches as well (Cheung et al., Citation2010; Feher & Lengyel, Citation2012; Kroll et al., Citation2007). For instance, large doses of silibinin–phosphatidylcholine were given to 13 men with prostate carcinoma (mean age of 70 years) in apple sauce (Kroll et al., Citation2007). In spite of the numerous studies carried out to understand the mechanism of SM hepatoprotective and anticancer effects, studies establishing the effect of SM on the cardiovascular system are limited.
Healthy vascular endothelium modulates the delicate balance on vascular functions by synthesizing and secreting vasoactive agents, mainly NO and prostaglandins (Wong et al., 2010b). Endothelial dysfunction is the main event in cardiovascular pathology. For research purposes, mostly two agents are used as a valuable tool for vascular relaxation studies, Ach and SNP. Ach stimulates NO release from the endothelium and it diffuses to underlying smooth muscle of the vessel; SNP works as a direct NO donor for vascular smooth muscle to understand the vascular integrity. Our finding about the impaired Ach vasodilatation response is consistent with previous reports. The senescence of endothelial cells impairs endothelium-dependant vasodilatation which is associated with an elevated activity of arginase, decreased eNOS expression and increased oxidative stress. Apart from NO, endothelial cells synthesis and liberate prostacyclin (PGI2) to reduce vascular tone and also endothelium-derived constricting factors such as prostaglandin H2, thromboxane A2 for increasing the tonus. COX is the key enzyme for conversion of arachidonic acid into different kinds of prostaglandins. Both NOS and COX enzymes have isoforms (constituve or inducible), their activity can be changed depending on the conditions (Demirci et al., Citation2005; Wong et al., 2010b).
The important findings of this study are SM partially restored endothelial dysfunction and blocked NOS enzyme preventing SM action. On the other hand, when synthesis and liberation of prostaglandins from arachidonic acid are inhibited by COX enzyme inhibitor, SM could exert its effect on the vessels. It is clear that the effect SM has on vasculature is not associated with prostaglandins and its action could be mediated on the NO pathway. ROS play a critical role in initiating the process of cellular senescence and limiting the lifespan of cells (Cau et al., Citation2012). If ROS are elevated, they react with existing NO, decrease NO functions and participate in the inflammatory process of aging vasculature (Ungvari et al., Citation2010). It results in increased contractility of the vessel. A large number of studies have established that SM and its compounds possess cell-protective antioxidant properties in vitro and in vivo. Silybin has been found to be protective on human umbilical vein endothelial cell (HUVEC) against H2O2 injury through its antioxidant activity, by increasing the NO content and the activity of glutathione peroxidase and inhibiting the caspase-3 apoptotic pathway (Wang et al., Citation2005). Additionally, SM has been found to be effective in improving Ach responses in obese diabetic mice vessel by reducing in both circulating and vascular asymmetric nG,nG-dimethyl-l-arginine (ADMA) level which is an inhibitor of all forms of NOS activity (Volti et al., Citation2011).
We have found that the impairment of smooth muscle function has started on 22-month-old rats. In the presence of prostaglandin inhibition and l-NAME treatment, SM’s relaxant effect could be achieved on smooth muscle of aged rats. In addition to endothelial dysfunction, low-grade vascular inflammation and vascular stiffness can occur, even in healthy aging it is known that there is up regulation of inflammatory cyokines, adhesion molecules and iNOS (Cau et al., Citation2012; Ungvari et al., Citation2010). It has been suggested that SM is an anti-atherogenic by inhibiting adhesion molecules on HUVEC (Kang et al., Citation2003). SM has been also shown to protect cardiomyocytes death on myocardial infarction in rats by protecting endogenous antioxidant enzymes, such as superoxide dismutase, catalase, reduced glutathione and glutathione S-transferase (Rao & Viswanath, Citation2007), and it increased ATP level of the cell against toxicity of doxorubicin (Chlopcikova et al., Citation2004). Its component, silibinin, decreased iNOS and increased superoxide dismutase against isoproterenol toxicity (Zhou et al., Citation2006) and dehydrosilybin attenuated ROS production by 80% to rotenone (Gabrielova et al., Citation2010) in cardiomyocytes.
Vascular aging is also characterized by increased tonus by vasoconstrictor mediators or ROS (Cau et al., Citation2012). PE-agonist activation of the membrane receptors and high K+-induced membrane depolarization can be used to understand calcium regulation of the vessel (Karaki et al., Citation1997). In this situation, the tendency to increase PE contractility and sensitivity showed that the receptor mechanism of calcium signaling was affected. Also, disruption of NO synthesis or reduced bioavailability in aging due to dysfunctional endothelium can be the reason for the increased trend in PE contractility. Meanwhile, the voltage-regulated calcium signaling was lower than in healthy rats, supporting that smooth muscle contractile function was deteriorated due to vascular stiffness. Giving that SM by itself restored α-agonist responses, but further decreased voltage-operated calcium signaling. These findings suggest that SM and constituents have a calcium blocker effect on smooth muscle. The calcium antagonistic feature of SM should be of clinical benefit for diseases which have increased vessel contractility, but it might be unfavorable for drug interactions in the elderly. The presence of COX inhibitor and blocking the generation of prostaglandins did not influence the results, but NOS inhibition had a large impact and SM could not exert its effect, therefore it suggests that the SM effect might be mostly mediated by NO.
SM given to cardiomyocytes at 19.5, 39 and 78 mg/L for 9 h and protected them against doxorubicin toxicity (Chlopcikova et al., Citation2004). We preferred to keep the dose in a similar range and incubated the vessels with 50 mg/L SM in the tissue chamber. We consider that whole extract is used as traditionally; therefore, we have chosen to use a standardized extract of SM, instead of one of the constituents. Its potential utility in protection against cardiovascular dysfunction should be further studied with different interventions, dose regimes and chronic in vivo administration by considering the NO pathway perspective.
Conclusion
The results of this study indicate that SM is a potential candidate for preventing and treating oxidative stress-induced damage of endothelial cells in aging. The possible immediate mechanisms for the reversal of endothelial dysfunction by SM in aging might be associated with its effect on the NO pathway, calcium antagonistic and antioxidant features. Moreover, this study will be helpful for researchers who consider SM’s potential value and safety for future chemotherapeutic or liver protectant trials.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. The research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgements
We wish to thank to Dr. Emre Cecen for his generously providing us with silymarin.
References
- Ahmadi-Ashtiani H, Allameh A, Rastegar H, et al. (2012). Inhibition of cyclooxygenase-2 and inducible nitric oxide synthase by silymarin in proliferating mesenchymal stem cells: Comparison with glutathione modifiers. J Nat Med 66:85–94
- Cau SB, Carneiro FS, Tostes RC. (2012). Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Front Physiol 3:218 . doi: 10.3389/fphys.2012.00218
- Cheung CW, Gibbons N, Johnson DW, Nicol DL. (2010). Silibinin – a promising new treatment for cancer. Anticancer Agents Med Chem 10:186–95
- Chlopcikova S, Psotova J, Miketova P, Simanek V. (2004). Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part I. Silymarin and its flavonolignans. Phytother Res 18:107–10
- Demirci B, McKeown PP, Bayraktutan U. (2005). Blockade of angiotensin II provides additional benefits in hypertension- and ageing-related cardiac and vascular dysfunctions beyond its blood pressure-lowering effects. J Hypertens 23:2219–27
- Demirci B, McKeown PP, Bayraktutan U. (2008). The bimodal regulation of vascular function by superoxide anion: Role of endothelium. BMB Rep 41:223–9
- El-Shitany NA, El-Haggar S, El-desoky K. (2008). Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol 46:2422–8
- Feher J, Lengyel G. (2012). Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol 13:210–17
- Gabrielova E, Jaburek M, Gazak R, et al. (2010). Dehydrosilybin attenuates the production of ROS in rat cardiomyocyte mitochondria with an uncoupler-like mechanism. J Bioenerg Biomembr 42:499–509
- Kang JS, Park SK, Yang KH, Kim HM. (2003). Silymarin inhibits TNF-alpha-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett 550:89–93
- Karaki H, Ozaki H, Hori M, et al. (1997). Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49:157–230
- Kroll DJ, Shaw HS, Oberlies NH. (2007). Milk thistle nomenclature: Why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6:110–19
- Pliskova M, Vondracek J, Kren V, et al. (2005). Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology 215:80–9
- Rao PR, Viswanath RK. (2007). Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol 12:179–87
- Tugrul I, Demirci B, Demir O, et al. (2011). The effect of Hypericum perforatum on isolated rat aorta. Pharm Biol 49:879–83
- Tyagi A, Agarwal C, Dwyer-Nield LD, et al. (2012). Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog 51:832–42
- Ungvari Z, Kaley G, de CR, et al. (2010). Mechanisms of vascular aging: New perspectives. J Gerontol A Biol Sci Med Sci 65:1028–41
- Volti GL, Salomone S, Sorrenti V, et al. (2011). Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovascular Diabetol 10:1–8
- Wang YK, Hong YJ, Huang ZQ. (2005). Protective effects of silybin on human umbilical vein endothelial cell injury induced by H2O2 in vitro. Vascul Pharmacol 43:198–206
- Wong SL, Wong WT, Tian XY, et al. (2010a). Prostaglandins in action indispensable roles of cyclooxygenase-1 and -2 in endothelium-dependent contractions. Adv Pharmacol 60:61–83
- Wong WT, Wong SL, Tian XY, Huang Y. (2010b). Endothelial dysfunction: The common consequence in diabetes and hypertension. J Cardiovasc Pharmacol 55:300–7
- Zhou B, Wu LJ, Tashiro S, et al. (2006). Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation of cell cycle. Biol Pharm Bull 29:1900–5

