Abstract
Context: Therapy for leukemia has a limited efficacy. There is a need to search for alternative anti-leukemia therapies. Persea americana Mill var. Hass (Lauraceae) is a tropical fruit (avocado) that might be used against cancer.
Objective: To investigate whether P. americana induces death in Jurkat lymphoblastic leukemia cells.
Materials and methods: Four ethanol extracts (0.1, 0.5, 1, 2 and 5 mg/mL) from avocado fruit (endocarp, whole seed, seed and leaves) were analyzed against Jurkat cells. Hydrogen peroxide generation by oxidation of 2′,7′-dichlorodihydrofluorescein diacetate to the fluorescent compound 2′,7′-dichlorfluorescein assay, acridine orange/ethidium bromide staining, flow cytometry analysis of annexin-V/7-amino-actinomycin, mitochondrial membrane potential and immunocytochemistry detection of transcription factor p53, caspase-3 and apoptosis-inducing factor (AIF) were evaluated.
Results: Endocarp, seed, whole seed, and leaf (0.1 mg/mL) extracts induced significant apoptosis in Jurkat cells (p < 0.001) in an oxidative stress-dependent fashion via mitochondrial membrane depolarization (52–87%), activation of transcription factor p53 (6.3–25.4%), protease caspase-3 (8.3–20%) and predominance of AIF reactivity (20.6–36%) in all extracts. Similar results were obtained with 0.5 mg/mL extracts. However, extract ≥1 mg/mL concentration induced necrosis (100%).
Conclusions: P. americana extracts function as a pro-apoptotic compound. Leukemic cells are eliminated through an oxidative stress mechanism. This study contributes to the understanding of the molecular mechanism of the avocado and its therapeutic action on leukemia.
Introduction
Leukemia is a heterogeneous hematologic disease affecting people worldwide (Ferlay et al., Citation2010). This disorder might originate from increased and unregulated growth of predominantly myeloid or lymphoid cells in the bone marrow, and depending on the untreated course, classified as acute or chronic (e.g., acute/chronic lymphocytic leukemia, ALL/CLL). Despite significant improvements in the treatment of leukemia, as many as 30% of pediatric and ∼50% of adult patients develop treatment-resistant disease (August et al., Citation2013; Mathisen et al., Citation2012). Indeed, secondary therapy-related ALL/CLL might emerge following chemotherapy and/or radiotherapy for this primary malignancy (Chen et al., Citation2010), probably due to apoptosis resistance (Hanahan & Weinberg, Citation2011; Tennant et al., Citation2009). Apoptosis is a type of programmed cell death specifically characterized by morphologic (Kroemer et al., Citation2009) and biochemical (Galluzzi et al., Citation2012) features. Reactivation of apoptotic cell death thus appears as a major goal to eliminate cancer cells. Therefore, other therapeutic alternatives should be pursued to eliminate cancer cells.
Medicinal plants represent a vital source of novel bioactive compounds that might be used as cancer chemotherapeutic agents (Martin-Cordero et al., Citation2012; Siu, Citation2011). Accordingly, Persea americana Mill var. Hass (Lauraceae) has been shown to inhibit growth and/or to induce apoptosis in prostate and oral epithelial cancer cell lines (D’Ambrosio et al., Citation2011; Ding et al., Citation2009). Despite this information, no data are available to establish whether P. americana var. Hass, one of the most consumed fruits in the Americas, can induce apoptosis in leukemic cells, especially in ALL.
The aim of this investigation was to evaluate the cytotoxic effect of endocarp (En), seed without endocarp (Se), whole seed [i.e. Ws (seed plus endocarp)] and leaf (Le) ethanol extracts from P. americana var. Hass on Jurkat cells (clone E6–1), used as a model of human T cell ALL. Because Jurkat cells are considered a model to study in vitro pharmacologic activity of diverse chemical compounds against T-ALL (Maioral et al., Citation2013; Takeiri et al., Citation2012), this cell line represents a useful tool to investigate the potential therapeutic effect of P. americana on leukemia.
Materials and methods
Materials
3,3′-Dihexyloxacarbocyanine iodide [DiOC6(3), Catalog No. D-273], ammonium pyrrolidinedithiocarbamate (PDTC, Catalog No. 548000) and 1,9-pyrazoloanthrone (SP600125, Catalog No. 420119) were acquired from Calbiochem (Darmstadt, Germany). Propidium iodide and 7AAD BD Via-Probe™ cell viability were purchased from BD Biosciences (San Jose, CA). Annexin-V-Phycoerythrin (PE) Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA). Dichlorofluorescein diacetate (DCFH2-DA) was obtained from Invitrogen (Carlsbad, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Plant extracts and Jurkat leukemia T cell culture
P. americana extracts
The leaves and seeds of P. americana from a 3.5-m high shrub were generously obtained from “Aguacates de Colombia, Ltda” (Antioquia, Northwestern Colombia). The leaves and seeds were collected from La Creencia Farm in the rural district of La Mulona in the town of Guarne at 2200 m above sea level, 6° 17′ 36.59′′ N, 75° 26′ 21.92′′ W by D. M. Benjumea (collector). Four extracts were obtained: En, Se, Ws and Le. After drying and crushing, individual samples were percolated with 96% ethanol for 2 days. Ethanol was used as solvent for extraction procedures mainly because of its physicochemical properties (e.g., dielectric constant 24, polar protic solvent), ethanol extracts are currently used in in vivo pharmacologic studies (Bakre et al., Citation2013; Saha et al., Citation2013), and finally, ethanol extracts typically dissolve in water or aqueous physiologic solutions (e.g., 0.9% normal saline used to buffer IV drugs) that facilitate intravenous injection for future pre-clinical/clinical assays both in mice or in patients with leukemia. Extracts were concentrated to a solid paste using a rotavapor, lyophilized and stored at −20°C until used. Lyophilized extracts were dissolved in ethanol (0.01 M).
Cell line
Jurkat clone E6–1 (ATCC® Catalog No. TIB-152™) was cultured according to supplier’s guidelines. Cells at 1 × 106 cells/mL (passage 5–10) were exposed to avocado extracts and other products of interest for different time intervals up to 24 h.
Experiments with Jurkat leukemia cell line
Assessment of cell death by fluorescence microscopy using acridine orange/ethidium bromide double staining, flow cytometry analysis of annexin-V/7-amino-actinomycin
Jurkat cells (1 × 106 cells/mL) were incubated in the presence or absence of En, Le, Ws and Se extracts dissolved in ethanol (0.01 M) or ethanol (0.01 M) alone (as control) and other products of interest at 37°C for 24 h. Jurkat cells were treated with (5 μM) zinc chelator N,N,N,N-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) as a positive control of apoptosis. For fluorescent microscopy analysis, cells were mixed with 1 μL acridine orange (AO) (100 μg/mL final concentration) and ethidium bromide (EB) (100 μg/mL final concentration). Cells were evaluated according to Bonilla-Porras et al. (Citation2011). The apoptotic index was assessed three times in independent experiments, where experimenters were blinded. For flow cytometry analysis, 1 × 105 untreated or treated cells were diluted in 1× binding buffer (0.1 M HEPES, pH 7.4, 1.4 M NaCl, 25 mM CaCl2), and incubated for 20 min at room temperature in the dark with (5 μL) annexin-V (AV) antibody and (5 μl) 7-amino-actinomycin (7AAD) dye (BD Pharmingen, San Diego, CA). Fluorescence intensity (FI) was determined in 2 × 104 cells with a FACS Canto TM II Flow Cytometer, Becton Dickinson (San José CA). Cells were classified according to mean fluorescent intensity as normal cells (quadrant 3, Q3), cells undergoing early apoptosis (Q4), late stage of apoptosis (Q2) and cellular debris/necrosis (Q1). Apoptosis was evaluated three times in independent experiments by flow cytometry.
Analysis of mitochondrial membrane potential (Δψm) by flow cytometry
The Jurkat cell line was treated as described above. Then, cells (1 × 105) were incubated for 20 min at room temperature in the dark with cationic lipophilic dye DiOC6(3) (10 nM, final concentration) and intercalating agent propidium iodide (12.5 ng/mL, final concentration). The combination of these two markers allowed us to divide the cells into four different populations: live cells (Q4), early apoptotic (Q3), late apoptotic cells (Q1) and non-apoptotic dead cells (Q2). Δψm analysis was performed according to Mendivil-Perez et al. (Citation2013).
Immunocytochemistry detection of transcription factor p53, caspase-3 and apoptosis-inducing factor
The immunocytochemistry assay was performed according to supplier’s protocol (Santa Cruz Biotechnology, Santa Cruz, CA Goat ABC Staining System, Catalog No. sc-2023) using primary goat polyclonal antibodies p53 (FL-393) (Catalog No. sc-6243-G), caspase-3 (Catalog No. sc-22171) and apoptosis-inducing factor (AIF) (Catalog No. sc-9417). The slides were then stained and quantified according to Bonilla-Porras et al. (Citation2011). The assessment was repeated three times in independent experiments.
Assessment of hydrogen peroxide
H2O2 was evaluated as described by Bonilla-Porras et al. (Citation2011). Green fluorescent cells (GFC, reflecting H2O2 production) were quantified under a fluorescence microscope by counting a minimum of 1000 total cells as follows: % GFC+ cells = 100 × (number of GFC+ cells)/total number of cells (GFC+ cells + GFC− cells).
Statistical analysis
A one-way analysis of variance (ANOVA) was performed among the groups and p < 0.001 was considered statistically significant. Bonferroni post hoc analysis for DCF analysis and Games–Howell post hoc comparison for AO/EB were performed, and p < 0.0001 was considered statistically significant.
Results
P. americana Hass extracts induce apoptosis in the Jurkat T cell line
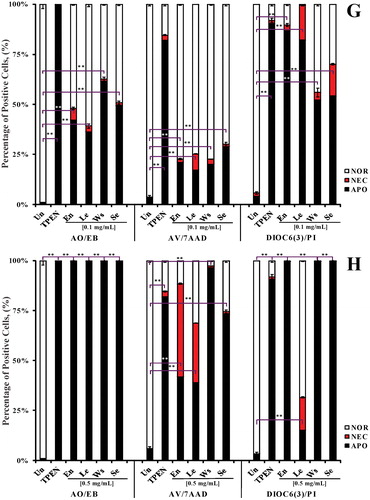
To characterize the avocado extract effect on cells, human Jurkat cells were exposed to increasing concentrations of avocado extracts (0.1, 0.5, 1, 2 and 5 mg/mL) for 24 h. We found that all extracts induced cell death in Jurkat cells, albeit with different cytotoxic effects. Indeed, 0.1–0.5 mg/mL extracts mostly induced apoptosis ( and ), according to AO/EB ( and ), AV/7AAD ( and ) and DiOC6(3) ( and ) staining techniques, but extract ≥1 mg/mL concentration induced necrosis (100%; ) and cell debris (data not shown). Noticeably, no necrosis death was detected when cells were treated with 0.1 mg/mL extracts and examined under fluorescent microscopy ( and ); however, a small percentage of debris (∼9–11%) was detected by AV/7AAD (). Cells exposed to ethanol (0.01 M) alone showed normal nuclei morphology, and displayed comparable AV/7AAD and DiOC6(3) staining parameters to untreated cells (data not shown). Because 0.1 mg/mL produced unambiguous nuclei condensation/fragmentation typical of apoptosis (), we selected this extract concentration for further experiments.
Figure 1. Avocado extract induces nuclei fragmentation, exposure of phosphatidylserine on the outside surface of plasma membrane and mitochondrial membrane depolarization in leukemic cells as indicative of apoptosis process. Jurkat cells were either left untreated, treated with TPEN (5 μM, as positive control) or incubated with two concentrations (0.1–0.5 mg/mL) of avocado extract: En, Se, Ws and Le for 24 h. Illustration shows (A) normal, (B and E) early, (C and F) late apoptotic and (D) necrotic nuclei morphologic changes indicative of apoptosis (B, C, E and F) and necrosis (D) in Jurkat cells exposed to avocado extract (B, C and D) or TPEN (E and F), as evaluated by AO/EB staining. Figure (G and H) represents percentage of cells with normal, apoptotic and necrotic nuclei (AO/EB staining), percentage of exposure of phosphatidylserine from the inside to the outside surface of the plasma membrane, as evaluated by AV/7AAD assay, and Δψm depolarization percentage, as evaluated using DiOC6(3). The ANOVA showed significant differences among the groups, p < 0.001. The Bonferroni comparison test showed significant differences in a percentage of induction of apoptosis between each treatment versus untreated, **p < 0.0001. NOR = normal, NEC = necrosis, APO = apoptosis.
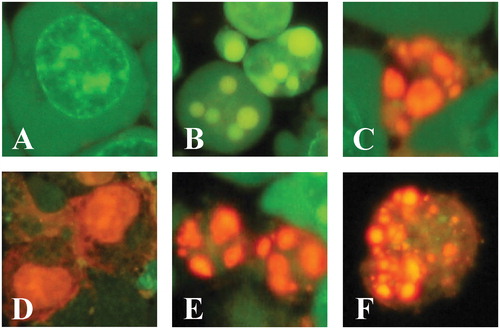
Figure 2. Comparison of apoptosis-induced avocado extract (0.1 mg/mL) in Jurkat cells evaluated by three different techniques: AO/EB (A–F), AV/7AAD (A′–F′) and DiOC6(3) (A″–F″). The circles represent the percentages of total apoptosis (early and late apoptosis) produced by each treatment. Insets in A, B, C, D, E and F showed nuclear fragmentation typical of apoptosis.
P. americana Hass extracts induce apoptosis in Jurkat T cell line dependent on p53, caspase-3 and AIF
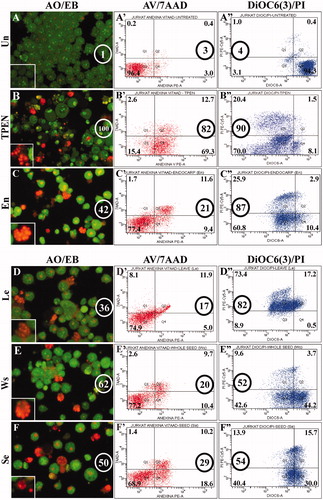
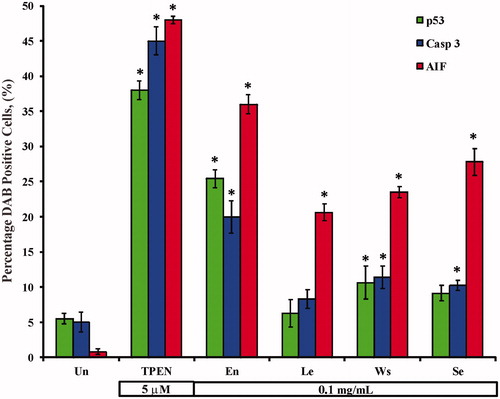
We wanted to further evaluate whether AIF was involved in the extract-induced apoptosis. As shown in , Jurkat cells exposed to 0.1 mg/mL avocado extracts induced activation of p53 (), caspase-3 (), and AIF (), indicative of mitochondrial damage (). Noticeably, the diaminobenzidine positive (DAB+) nuclei corresponded to AO/EB apoptotic morphology [e.g., (inset) versus (inset)]. Interestingly, quantification of p53, caspase-3 and AIF DAB+ nuclei was significantly different compared to control (); however, AIF DAB+ nuclei were significantly higher in Jurkat cells treated with En > Se > Ws than with Le. No differences were found between p53 and caspase-3 among the extracts.
Figure 3. En, Se, Ws and Le extracts induce simultaneous activation of the transcription factor p53, protease caspase-3 and AIF in Jurkat cells. Jurkat cells were either left untreated (A, B and C), treated with TPEN (5 μM; D, E and F) or exposed to 0.1 mg/mL En (G, H and I); Le (J, K and L); Ws (M, N and O) and Se (P, Q and R), at 37 °C for 24 h. After this time of incubation, cells were stained with anti-p53 (A, D, G, J, M and P), anti-caspase-3 (B, E, H, K, N and Q) and anti-AIF (C, F, I, L, O and R) antibodies. Notice that p53, caspase-3 (CAS-3) and AIF+ nuclei (dark brown color in the online version, black color in printed version) reflect their nuclear translocation/activation and appear to correlate with the apoptotic nuclear morphology, i.e. condensed/fragmented nuclei compared with untreated cells (A, B and C) or cytoplasmic activation (brown color in the online version, black color in printed version). Magnification 660× (A–R).
Figure 4. Avocado extracts induce activation of the transcription factor p53, protease caspase-3 and AIF in Jurkat cells. Jurkat cells were either left untreated, treated with TPEN (5 μM) or incubated with 0.1 mg/mL of avocado extract: En, Se, Ws and Le at 37 °C for 24 h. The ANOVA showed significant differences among the groups, p < 0.001 in each strata (e.g., AO/EB). The Bonferroni comparison test showed significant differences in a percentage of DAB+ cells. A *p < 0.0001 showed differences between treated and untreated cells.
P. americana Hass extracts induce reactive oxygen species-dependent apoptosis in Jurkat T cell line
We then evaluated the generation of H2O2 and apoptosis in Jurkat cells treated with extracts in the absence or presence of N-acetyl-cysteine (NAC, 0.5 mM), a well-known antioxidant compound. As shown in , Jurkat cells treated with 0.1 mg/mL Ws, Le, Se and En extracts and NAC, significantly reduced the extract-induced apoptosis and H2O2 generation compared to cells treated with extracts alone or untreated.
Table 1. Hydrogen peroxide (H2O2) is involved in avocado toxic effect of ethanol extracts on Jurkat leukemia cells.
Discussion
In the present investigation, we demonstrate for the first time that En, Se, Ws and Le extracts from avocado P. americana var. Haas induce apoptosis in leukemia Jurkat cells. Remarkably, we observed (data not shown) and others have shown no noxious effect of avocado’s extracts in normal human lymphocyte cells (Paul et al., Citation2011). These observations suggest that avocado extracts might display selective toxicity to leukemic cells. Because ALL patients present >95% lymphoblast counts in blood, we therefore focused our investigation to elucidate the molecular mechanism of P. americana-induced apoptosis in Jurkat cells. The apoptosis phenomenon involves the activation of transcription factor p53, protease caspase-3 and AIF; impairment of mitochondrial potential and phosphatidylserine exposure indicative of cytoplasm membrane damage. These data suggest that avocados induce intrinsic mechanisms of cell death involving two-complementary sub-pathways: a caspase-3-dependent and AIF-dependent mechanism. In agreement with Ding et al. (Citation2009), this cell death process might be ignited by H2O2. Therefore, the cellular response to the damaging agents contained in the extracts [e.g., polyphenols, steroids, tripertenoids, tannins (Ding et al., Citation2007)] appears to involve a sequence of events that leads to cell apoptosis. Several observations support these assumptions. First, we showed that 0.1–0.5 mg/mL avocado extracts induce apoptosis (as assessed by AO/EB staining, one of the most reliable techniques to evaluate condensation/fragmentation of apoptotic nuclei and necrosis) (Leite et al., Citation1999) through oxidative stress. Indeed, it appears that avocado extracts induce mostly nuclei fragmentation in Jurkat cells (Stage II). Secondly, p53 is a crucial tumor suppressor, long-recognized to suppress cancer through the induction of cell-cycle arrest or apoptosis program in response to several different cellular stress signals. We found that avocado extracts induce the activation of transcription factor p53. This observation implies its participation in the cell death process, though moderate when compared to AIF. Indeed, upon activation and stabilization of p53, it transcribes >150 genes (Riley et al., Citation2008), which are negatively (e.g., anti-apoptotic Bcl-2, Bcl-xL and IAP gene) or positively (e.g., pro-apoptotic Bax, Noxa, Puma and Apaf-1 genes) regulated (Vaseva & Moll, Citation2009). Alternatively, p53 might directly mediate mitochondrial death (Wolff et al., Citation2008). These data suggest that by transcription-dependent or transcription-independent regulation, p53 is capable of influencing mitochondria functionality (Vaseva & Moll, Citation2009). As expected, we found that avocado extracts induced Δψm failure and apoptosis. Thirdly, as a result of mitochondrial damage, several apoptogenic proteins (e.g., cytochrome c and AIF) exit from the internal mitochondria membrane, which in turn serve as co-activators of down-stream signaling. Interestingly, we found that avocado extracts induce caspase-3 activation. Indeed, caspase-3 has been found to be responsible for the typical nuclei fragmentation morphology (Mazumder et al., Citation2008). Of note, though caspase-3 activation was activated to a similar extent as p53, its detection was lower than AIF. A possible explanation for this outcome is that caspase-3 cleaves a plethora of cellular substrates including cytoplasmic (e.g., spectrin, gelsolin) and nuclear [e.g., poly(ADP)-ribosylation protein (PARP)-, DNA-dependent protein kinase] proteins, whereas AIF might directly (or even be assisted to) target the nuclei (Sevrioukova, Citation2011). Due to its multi-target sites, caspase-3 is susceptible to negative control. Therefore, caspase-3 detection may be under-rated. Moreover, p53 can up-regulate AIF (Stambolsky et al., Citation2006); DAB+ stained AIF nuclei are therefore higher than caspase-3 and p53. Finally, for the first time we report that avocado extracts induce apoptosis in Jurkat leukemia cells via AIF. This is a flavoprotein that complies with a redox activity in the mitochondria and a pro-apoptotic function in the nucleus (Hangen et al., Citation2010). Recently, it has been recognized that the contribution of AIF to apoptosis depends upon the cell type and apoptotic stimuli. In the present study, we show that avocado extracts induce translocation of AIF to the nuclei. Remarkably, the morphology of DAB+ AIF nuclei resemble the morphology of DAB+ p53/caspase-3 nuclei and nuclei under fluorescent microscopy [e.g., (and inset) versus ]. These data imply that avocado extracts induce apoptosis in Jurkat cells not only via activation of mitochondria mediated by caspase-3 dependent cascade, but also via AIF. Since the NAC antioxidant inhibits apoptosis in cells exposed to avocado extracts (), it is suggested that both pathways are related with oxidative stress. Based on this assumption, we proposed that AIF should be routinely assessed along with p53 and caspase-3 as apoptosis markers. Consequently, understanding the intracellular molecular mechanisms involved in avocado-induced apoptosis may lead to specific targeted therapeutic interventions (Brooke et al., Citation2011). Since constituents isolated from the avocado (e.g., chloroform-soluble extract D003) have been shown to induce apoptosis via a reactive oxygen species-mediated mechanism (Ding et al., Citation2009) and to inhibit proliferation of human oral cancer cell lines by targeting the EGFR/RAS/RAF/MEK/ERK1-2 cancer pathway (D’Ambrosio et al., Citation2011), further investigation on apoptosis induced by avocados in Jurkat cells is warranted.
Conclusions
In conclusion, we demonstrated that ethanol extracts from the avocado fruit P. americana induce apoptosis in Jurkat cells mediated by oxidative stress involving two mitochondria-dependent pathways: caspase-3 and AIF-dependent mechanism. The avocado extract showed the presence of a wide variety of secondary metabolites, which might be used as anticancer compounds. Indeed, the pro-apoptotic activity of avocado extracts all showed moderate or high apoptosis activity. Further in vivo investigations are required to confirm the in vitro results described above. Moreover, the isolation and identification of molecules from the extracts responsible for pro-apoptotic activity on Jurkat cells are our next goals.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
A.R.B.-P. is at the Master Program in Biomedical Science from the University of Antioquia, Colombia. Photomicrographs were taken by C.V.-P. This work was supported by Colciencias (Grant No. 1115-408-20525 to C.V.-P. and M.J.-D.-R.), and the “Small Research Project” program from the Committee for Development Research (CODI), University of Antioquia, Colombia. A.R.B.-P. was supported by Programa Jóvenes Investigadores e Innovadores “Virginia Gutiérrez de Pineda” Colciencias (Grant No. JIC-018-2010).
References
- August KJ, Narendran A, Neville KA. (2013). Pediatric relapsed or refractory leukemia: New pharmacotherapeutic developments and future directions. Drugs 73:439–61
- Bakre AG, Aderibigbe AO, Ademowo OG. (2013). Studies on neuropharmacological profile of ethanol extract of Moringa oleifera leaves in mice. J Ethnopharmacol 149:783--9
- Bonilla-Porras AR, Jimenez-Del-Rio M, Velez-Pardo C. (2011). Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signaling mechanism. Cancer Cell Int 11:19
- Brooke DG, Shelley EJ, Roberts CG, et al. (2011). Synthesis and in vitro evaluation of analogues of avocado-produced toxin (+)-(R)-persin in human breast cancer cells. Bioorg Med Chem 19:7033–43
- Chen W, Wang E, Lu Y, et al. (2010). Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: Report of six cases and a literature review. Am J Clin Pathol 133:75–82
- D’Ambrosio SM, Han C, Pan L, et al. (2011). Aliphatic acetogenin constituents of avocado fruits inhibit human oral cancer cell proliferation by targeting the EGFR/RAS/RAF/MEK/ERK1/2 pathway. Biochem Biophys Res Commun 409:465–9
- Ding H, Chin YW, Kinghorn AD, D’Ambrosio SM. (2007). Chemo-preventive characteristics of avocado fruit. Semin Cancer Biol 17:386–94
- Ding H, Han C, Guo D, et al. (2009). Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer 61:348–56
- Ferlay J, Shin HR, Bray F, et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–917
- Galluzzi L, Vitale I, Abrams JM, et al. (2012). Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–20
- Hanahan D, Weinberg RA. (2011). The hallmarks of cancer: The next generation. Cell 144:646–74
- Hangen E, Blomgren K, Bénit P, et al. (2010). Life with or without AIF. Trends Biochem Sci 35:278–87
- Kroemer G, Galluzzi L, Vandenabeele P, et al. (2009). Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
- Leite M, Quinta-Costa M, Leite PS, Guimaraes JE. (1999). Critical valuation of techniques to detect and measure cell death -- study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol 19:139–51
- Maioral MF, Gaspar PC, Rosa Souza GR, et al. (2013). Apoptotic events induced by synthetic naphthylchalcones in human acute leukemia cell lines. Biochimie 95:866–74
- Martin-Cordero C, Leon-Gonzalez AJ, Calderon-Montano JM, et al. (2012). Pro-oxidant natural products as anticancer agents. Curr Drug Targets 13:1006–28
- Mathisen MS, Jabbour E, Kantarjian HM. (2012). Treatment of adult acute lymphoblastic leukemia (ALL) with a focus on emerging investigational and targeted therapies. Oncology 26:851–9
- Mazumder S, Plesca D, Almasan A. (2008). Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 414:13–21
- Mendivil-Perez M, Jimenez-Del-Rio M, Velez-Pardo C. (2013). Glucose starvation induces apoptosis in a model of acute T leukemia dependent on caspase-3 and apoptosis-inducing factor: A therapeutic strategy. Nutr Cancer 65:99–109
- Paul R, Kulkarni P, Ganesh N. (2011). Avocado fruit (Persea americana Mill) exhibits chemo-protective potentiality against cyclophosphamide induced genotoxicity in human lymphocyte culture. J Exp Ther Oncol 9:221–30
- Riley T, Sontag E, Chen P, Levine A. (2008). Transcriptional control of human p53-regulated genes. Nature Rev Mol Cell Biol 9:402–12
- Saha S, Hossain F, Anisuzzman M, Islam MK. (2013). Pharmacological evaluation of Musa seminifera Lour. fruit. J Integr Med 11:253–61
- Sevrioukova IF. (2011). Apoptosis-inducing factor: Structure, function, and redox regulation. Antioxid Redox Signal 14:2545–79
- Siu D. (2011). Natural products and their role in cancer therapy. Med Oncol 28:888–900
- Stambolsky P, Weisz L, Shats I, et al. (2006). Regulation of AIF expression by p53. Cell Death Differ 13:2140–9
- Takeiri M, Ota E, Nishiyama S, et al. (2012). Structure-activity relationship of 9-methylstreptimidone, a compound that induces apoptosis selectively in adult T-cell leukemia cells. Oncol Res 20:7–14
- Tennant DA, Durán RV, Boulahbel H, Gottlieb E. (2009). Metabolic transformation in cancer. Carcinogenesis 30:1269–80
- Vaseva AV, Moll UM. (2009). The mitochondrial p53 pathway. Biochim Biophys Acta 1787:414–20
- Wolff S, Erster S, Palacios G, Moll UM. (2008). p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res 18:733–44