Abstract
Context: Formononetin, an isoflavone, can inhibit the proliferation of cancer cells, including those of the prostate. However, its antitumor mechanism remains unclear.
Aim: To investigate whether the insulin-like growth factor 1 (IGF-1)/insulin-like growth factor 1 receptor (IGF-1 R) signaling pathway mediates the formononetin antitumor effect on prostate cancer cells.
Materials and methods: The viability of PC-3 cells was measured by MTT assay 48 h after formononetin treatment (25, 50 and 100 μM). Formononetin-induced cell apoptosis was measured by Hoechst 33258 staining and flow cytometry. Expression of Bax mRNA was detected by real-time PCR, and the expression levels of Bax and IGF-1 R proteins were detected by western blots.
Results: At concentrations >12.5 μM, formononetin significantly inhibited the proliferation of human prostate cancer cells. Formononetin increased Bax mRNA and protein expression levels and decreased the expression levels of pIGF-1 R protein in a dose-dependent manner.
Conclusion: High concentrations of formononetin-induced apoptosis in androgen-independent prostate cancer cells through inhibition of the IGF-1/IGF-1 R pathway.
Introduction
Prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in USA. In 2012, 241,740 men were diagnosed with prostate cancer resulting in 28,170 deaths in USA alone (Siegel et al., Citation2012). In China, however, the estimation of incidence and mortality of prostate cancer were 33,802 and 14,297, respectively, in 2008 (Peng et al., Citation2012). Remarkably, the incidence of prostate cancer increases substantially in Asian men after emigration to USA, partly due to the great change in dietary habits (Shimizu et al., Citation1991). Epidemiologic studies have suggested that soy, one of the most popular foods in Asia, may play an important role in reducing the risk of prostate cancer (Lee et al., Citation2003; Yan & Spitznagel, Citation2009).
Isoflavone, found in many soy products, is classified as a phytoestrogen. By binding to both ERα and ERβ estrogen receptors, isoflavones can act as either estrogen agonists or antagonists (Chen et al., 2011a; Kuiper et al., Citation1998). Moreover, in vitro and in vivo studies have demonstrated that isoflavones have significant effects on cell cycle arrest and induction of apoptosis in prostate cancer (Bemis et al., Citation2004; Jarred et al., Citation2002; Li et al., Citation2008). Formononetin (C16H12O4), one kind of isoflavone, is found in the red clover plant, a traditional Chinese herbal medicine (Trifolium pratense). Red clover contains several isoflavonoids including daidzein, formononetin, biochanin A and genistein that are detected in the root, stem, leaf and bloom of plants (Kašparová et al., Citation2012).
A previous study by our group determined that formononetin inhibited the proliferation of breast cancer cells via an ER-dependent mechanism (Chen & Sun, Citation2012). Others have shown that formononetin also significantly suppressed prostate cancer cell proliferation via the ERK1/2 MAPK pathway (Ye et al., Citation2012). Similarly, formononetin mediated the cell cycle arrest of MCF-7 human breast cancer cells by the inactivation of the insulin-like growth factor 1 (IGF-1)/P13K/Akt pathway (Chen et al., 2011b). As both ERα and ERβ are expressed in PC-3 human prostate cancer cells (Lau et al., Citation2000), it may be possible that isoflavones, such as formononetin, might also have an anti-proliferative effect. However, whether formononetin exhibits this antitumor effect in prostate cancer cells is still unknown.
The function of IGF-1 is primarily mediated by the insulin-like growth factor 1 receptor (IGF-1 R), a transmembrane protein consisting of two α-chains and two β-chains. Following ligand binding to the extracellular α-subunit, the intrinsic tyrosine kinase of the IGF-1 R is activated, resulting in the autophosphorylation of tyrosines on the intracellular portion of the β-subunit (Samani et al., Citation2007). This IGF-1 mediated signaling pathway is believed to be important in promoting cellular proliferation and inhibiting apoptosis, and aberrant IGF-1 expression has been implicated in tumorigenesis (Pollak et al., Citation2004). Additionally, increased plasma IGF-1 levels are associated with a higher risk for several cancer types, including those of the prostate, ovary and breast (Chan et al., Citation1998; Key et al., Citation2010; Peeters et al., Citation2007). Studies have demonstrated that expression of IGF-1 in prostate epithelium successfully induces prostate neoplasia in transgenic mice (DiGiovanni et al., Citation2000). The blockade of IGF-1 R expression by antisense RNA inhibits proliferation and invasion of prostate cancer cells in vitro and in vivo (Burfeind et al., Citation1996; Grzmil et al., Citation2004). These previous findings indicate that the IGF-1/IGF-1 R signaling pathway contributes to the progression of prostate cancer; thus, the inhibition of this signaling pathway may present a potential approach for the treatment of prostate cancer.
In this study, we evaluated the effects of formononetin on the proliferation and apoptosis of human prostate cancer cells. We found that formononetin significantly inhibited cell proliferation and induced apoptosis in the prostate cancer cell line, PC-3, via the downregulation of the IGF-1/IGF-1 R signaling pathway. Our findings support the antitumor effect of formononetin in prostate cancer cells and may provide a potential therapeutic agent for the treatment of prostate cancer.
Materials and methods
Drug preparation
Formononetin (purity >98%, purchased from Phytomarker Ltd., Tianjin, China) was dissolved in dimethyl sulfoxide (DMSO). The stock of formononetin (100 mM) was stored at 4 °C until further use.
Cell culture
The human prostate cancer cell line PC-3 was obtained from Shanghai Institute of Cell Biology (Shanghai, China). The cells were cultured in RPMI-1640 (Gibco-BRL, Grand Island, NY) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and incubated in a 37 °C humidified atmosphere of 5% CO2.
MTT assay
The effect of formononetin on the viability of PC-3 cells was measured by the MTT assay. Cells were plated at a density of 4 × 103 cells/well in 96-well plates and cultured at 37 °C for 24 h. Cells were subsequently treated with formononetin at various concentrations (0, 6.25, 12.5, 25, 50, 100, 150 and 200 μM). After 48 h, 20 μl of [3-(4,5-dimethylthiazol-2-yl)]-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml) was added to each well, followed by incubation for an additional 4 h. For the colorimetric reaction, DMSO was added to each well. The optical density (OD) was measured using a microplate reader (Bio-Rad, Hercules, CA) at a wavelength of 570 nm.
Hoechst 33258 staining
Formononetin-induced apoptosis in PC-3 cells was measured by Hoechst 33258 staining (Apoptosis-Hoechst Staining Kit, Beyotime, Jiangsu, China). Cells were cultured in 6-well plates and treated with formononetin at various concentrations (0, 25, 50 and 100 μM). After culture for 48 h, cells were stained with Hoechst 33258 for 5 min and examined immediately by fluorescence microscopy at a wavelength of 480 nm (Olympus, Tokyo, Japan).
Flow cytometric assay
An Annexin V-FITC Apoptosis Detection Kit (Boster Biological Technology Co., Ltd., Hubei, China) was used to measure formononetin-induced apoptosis in PC-3 cells. Cells were treated with 0, 25, 50 or 100 μM formononetin for 48 h, followed by staining with annexin V-FITC and propidium iodide (PI) according to the manufacturer’s instructions. Early apoptotic cell death, as measured by annexin V positive and PI negative cells, was monitored by FACS Calibur Flow Cytometer (Becton Dickinson, San Jose, CA).
Real-time PCR assay
PC-3 cells treated with formononetin (0, 25, 50 or 100 μM) for 48 h were harvested and total RNA was prepared using TRIzol reagent (Gibco-BRL, Grand Island, NY). cDNA was subsequently synthesized from 5 μg total RNA using RevertAid First Strand cDNA Synthesis Kit (Fermentas Inc., Glen Burnie, MD), as per the manufacturer’s instructions. Expression of Bax mRNA was measured by quantitative real-time PCR using the ABI PRISM 7500 Sequence Detector System (Applied Biosystems, Carlsbad, CA). GAPDH was used to normalize the level of Bax mRNA. The primers used for Bax detection were 5′-ACCAGCTCTGAGCAGATCATG-3′ (forward) and 5′-ATCATCCTCTGCAGCTCCATG-3′ (reverse). The primers used for GAPDH detection were 5′-TCACCCACACTGTGCCCATCTACGA-3′ (forward) and 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (reverse).
Western-blot assay
After treating with formononetin (0, 25, 50 and 100 μM) for 48 h, PC-3 cells were solubilized in ice-cold lysis buffer. The supernatant was collected by centrifugation at 12,000 g for 10 min at 4 °C, and the protein concentrations were analyzed using Bio-Rad assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (40 μg) were loaded onto gels, separated by SDS-PAGE, and transferred onto PVDF membranes (Millipore, Billerica, MA). Membranes were incubated with specific primary antibodies for IGF-1 R (1:1000), phospho-IGF-1 R (1:1000), Bax (1:1000) or β-actin (1:1000), followed by incubation with the appropriate horseradish peroxidase-linked secondary antibody. Signals were visualized with electrochemiluminescence (ECL) using Quantity One software (Bio-Rad, Hercules, CA), and the band intensity of β-actin was used as an internal control.
Statistical analyses
The data are presented as mean ± standard deviation (SD). The statistical analysis was performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL) using one-way analysis of variance followed by Dunnett’s post hoc test. A p value <0.05 was considered statistically significant.
Results
Effect of formononetin on PC-3 cell proliferation
The dose-dependent effect of formononetin on the proliferation of PC-3 cells was detected by MTT assay. Compared with the non-treated, control group (0 µM), formononetin treatment at the low concentration of 6.25 µM resulted in increased cell numbers (p < 0.05). However, at higher concentrations (12.5, 25, 50, 100, 150 and 200 µM), formononetin significantly inhibited the proliferation of PC-3 cells in a dose-dependent manner (p < 0.05), indicating an anti-proliferative effect on PC-3 cells. After 48 h of treatment, the IC50 value of formononetin was 88.3 µM.
Effect of formononetin on PC-3 cell apoptosis
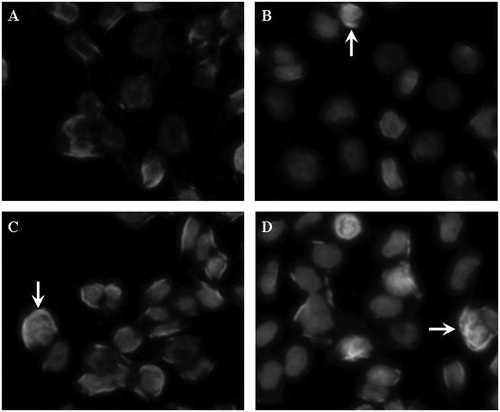
Hoechest 33258 staining was used to detect apoptotic morphologic changes in PC-3 cells after formononetin treatment for 48 h. Apoptotic bodies containing nuclear fragments were observed in formononetin-treated cells (25, 50 or 100 μM) but not in the non-treated, control group (0 μM). The number of apoptotic cells increased in a dose-dependent manner (). Formononetin-induced apoptosis was further confirmed by flow cytometry. Annexin V (positive)/PI (negative) cells were scored as early apoptotic cells. The results show that the early apoptotic rate of the control group was 0.57%. After treatment with formononetin for 48 h, the early apoptotic rates increased to 3.45, 8.34 and 17.78% with 25, 50 and 100 μM formononetin treatment, respectively. These data indicate that formononetin had potent apoptosis-inducing activity on PC-3 human prostate cancer cells in a dose-dependent manner.
Figure 1. Morphologic changes of PC-3 cells treated with various concentrations of formononetin for 48 h. (A) Representative image of cells from the non-treated group. (B–D) Representative images of cells treated with 25, 50 and 100 μM formononetin, respectively. The apoptotic cells showed condensed nuclei or apoptotic bodies (indicated by arrows). The images were taken using an Olympus IX71FL fluorescence microscope (400×).
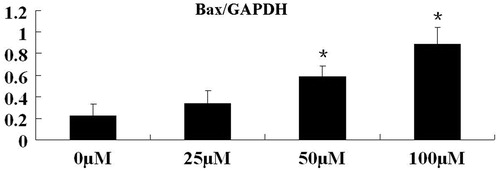
Effect of formononetin on Bax mRNA levels
Using real-time PCR, we assessed the effect of formononetin on Bax mRNA, a pro-apoptotic gene marker. Quantification of Bax mRNA was normalized to the housekeeping gene GAPDH. As shown in , Bax mRNA levels were significantly upregulated in PC-3 cells with treatment of 50 and 100 μM formononetin (p < 0.05).
Effect of formononetin on expression of IGF-1 R, p-IGF-1 R and Bax proteins
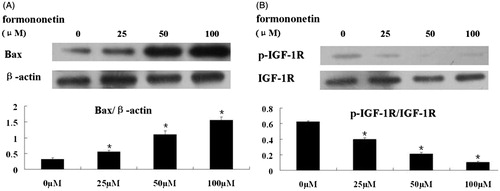
Consistent with the real-time PCR results, western-blot analysis revealed that treatment of formononetin dose-dependently induced the expression of the pro-apoptotic Bax protein (). In order to explore the upstream signaling pathways regulating Bax, we also analyzed the expression levels of IGF-1 R and phosphorylated IGF-1 R proteins. The results demonstrate that formononetin dose-dependently inhibited the expression of p-IGF-1 R (p < 0.5, ).
Figure 3. Western-blot analysis of Bax, IGF-1 R and p-IGF-1 R protein levels with formononetin treatment. (A) Formononetin increased the expression levels of Bax protein in a dose-dependent manner. β-Actin was used as the loading control. (B) Formononetin inhibited the expression of p-IGF-1 R protein in a dose-dependent manner. IGF-1 R protein was used as the loading control. The quantification of the western-blot assays are presented as mean ± SD for triplicate experiments.*p < 0.05 versus non-treated group.
Discussion
Most types of prostate cancer are initially responsive to androgen deprivation therapy. Despite a good initial response, some patients with prostate tumors develop castration resistance, leading to poor responsiveness to any androgen deprivation therapy (Harris et al., Citation2009; Hoimes & Kelly, Citation2010). Fortunately, estrogen shows powerful inhibitory effects on prostate cancer in androgen-depleted environments, suggesting novel mechanisms of estrogen-mediated antitumor activity (Coleman et al., Citation2006). Formononetin, a phytoestrogen, may have therapeutic potential in androgen-independent prostate cancer.
In this report, we examined the effects of various concentrations of formononetin on PC-3, androgen-independent, human prostate cells. The results indicate that high concentrations of formononetin (>12.5 μM) inhibited cell proliferation, while lower concentrations (6.25 μM) stimulated cell growth. Hoechest 33258 staining and flow cytometry verified a dose-dependent increase in apoptosis with formononetin treatment. Furthermore, western blotting detected a dose-dependent increase in Bax and decrease in IGF-1 R protein expression. These results suggest that formononetin may serve as a potential anti-proliferative agent in androgen-independent prostate cancer.
The Bcl-2 family of proteins plays an essential role in regulating cellular apoptosis. Members of the Bcl-2 family can be divided into two groups. Some members, such as Bcl-2 and Bcl-XL, antagonize cell death, whereas others, such as Bax and Bad, exhibit pro-apoptotic activities. Bad has been found to be the first protein that initiates apoptosis and interacts with Bax to aggregate on the mitochondrial membrane. A conformational change in Bax contributes to its oligomerization, followed by the release of cytochrome C from mitochondria into the cytosol. Cytochrome C then activates caspase 9 by binding to Apaf-1 and caspase 9 itself, and the subsequent activation of caspases 3 and 7 ultimately causes cell death (Zong et al., Citation2003). Here, we detected increased expression of Bax mRNA and protein after formononetin treatment, which suggested that formononetin (25, 50 and 100 μM) could induce apoptosis of androgen-independent prostate cancer cells through the mitochondrial pathway.
To better understand the molecular basis of the effect of formononetin on cell apoptosis, we examined the expression levels of IGF-1 R and p-IGF-1 R proteins by western blot. Our results show that formononetin dose-dependently inhibited the phosphorylation of IGF-1 R. Two signaling protein families downstream of IGF-1 R are IR substrates-1 to -4 (IRS-1 to -4) and the Src-homology collagen protein (Shc) isoforms. While IRS triggers an anti-apoptosis cascade via the PI3-K pathway, Shc activates the Ras/mitogen-activated protein kinase (MAPK) signaling pathway that can result in the activation of the pro-apoptosis cascade depending on the stimulus (Gallagher & LeRoith, Citation2010). There are three parallel MAPK signaling cascades, the ERK, JNK and p38 MAPK pathways (Xu et al., Citation2012); both JNK and p38 MAPK have been shown to induce Bax activation and mitochondrial translocation from the cytosol to mitochondria prior to apoptosis (Venkatesan et al., Citation2010). Thus, signals controlled by the IGF-1 R pathway are involved in the regulation of apoptosis. Our results confirmed that decreased activation of IGF-1 R was associated with increased apoptosis via up-regulation of Bax expression.
In summary, we found that high concentrations of formononetin induced the mitochondrial pathway of apoptosis in androgen-independent PC-3 prostate cancer cells through inhibition of the IGF-1/IGF-1 R pathway.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 81260376).
References
- Bemis DL, Capodice JL, Desai M, et al. (2004). A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res 10:5282–92
- Burfeind P, Chernicky CL, Rininsland F, et al. (1996). Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA 93:7263–8
- Chan JM, Stampfer MJ, Giovannucci E, et al. (1998). Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science 279:563–6
- Chen J, Liu L, Hou R, et al. (2011a). Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett 308:144–51
- Chen J, Sun L. (2012). Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm Metab Res 44:943–8
- Chen J, Zeng J, Xin M, et al. (2011b). Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res 43:681–6
- Coleman IM, Kiefer JA, Brown LG, et al. (2006). Inhibition of androgen-independent prostate cancer by estrogenic compounds is associated with increased expression of immune-related genes. Neoplasia 8:862–78
- DiGiovanni J, Kiguchi K, Frijhoff A, et al. (2000). Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA 97:3455–60
- Gallagher EJ, LeRoith D. (2010). The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab 21:610–18
- Grzmil M, Hemmerlein B, Thelen P, et al. (2004). Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Pathol 202:50–9
- Harris WP, Mostaghel EA, Nelson PS, Montgomery B. (2009). Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 6:76–85
- Hoimes CJ, Kelly WK. (2010). Redefining hormone resistance in prostate cancer. Ther Adv Med Oncol 2:107–23
- Jarred RA, Keikha M, Dowling C, et al. (2002). Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol Biomarkers Prev 11:1689–96
- Kašparová M, Siatka T, Klimešová V, Dušek J. (2012). New synthetic pyridine derivate as potential elicitor in production of isoflavonoids and flavonoids in Trifolium pratense L. suspension culture. Scientific World J 2012:746412
- Key TJ, Appleby PN, Reeves GK, Roddam AW. (2010). Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol 11:530–42
- Kuiper GG, Lemmen JG, Carlsson B, et al. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139:4252–63
- Lau KM, LaSpina M, Long J, Ho SM. (2000). Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: Regulation by methylation and involvement in growth regulation. Cancer Res 60:3175–82
- Lee MM, Gomez SL, Chang JS, et al. (2003). Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev 12:665–8
- Li Y, Wang Z, Kong D, et al. (2008). Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem 283:27707–16
- Peeters PH, Lukanova A, Allen N, et al. (2007). Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: The European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 14:81–90
- Peng P, Gong YM, Bao PP, et al. (2012). Estimates and prediction of prostate cancer incidence, mortality and prevalence in China, 2008. Zhonghua Liu Xing Bing Xue Za Zhi 33:1056–9
- Pollak MN, Schernhammer ES, Hankinson SE. (2004). Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–18
- Samani AA, Yakar S, LeRoith D, Brodt P. (2007). The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr Rev 28:20–47
- Shimizu H, Ross RK, Bernstein L, et al. (1991). Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63:963–6
- Siegel R, Naishadham D, Jemal A. (2012). Cancer statistics, 2012. CA Cancer J Clin 62:10–29
- Venkatesan B, Prabhu SD, Venkatachalam K, et al. (2010). WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal 22:809–20
- Xu T, Wang NS, Fu LL, et al. (2012). Celecoxib inhibits growth of human autosomal dominant polycystic kidney cyst-lining epithelial cells through the VEGF/Raf/MAPK/ERK signaling pathway. Mol Biol Rep 39:7743–53
- Yan L, Spitznagel EL. (2009). Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am J Clin Nutr 89:1155–63
- Ye Y, Hou R, Chen J, et al. (2012). Formononetin-induced apoptosis of human prostate cancer cells through ERK1/2 mitogen-activated protein kinase inactivation. Horm Metab Res 44:263–7
- Zong WX, Li C, Hatzivassiliou G, et al. (2003). Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69