Abstract
Context: Although the antitumor, immunomodulatory activities, and other effects of shikonin have been studied for decades, its systemic toxicity in vivo remains unclear.
Objective: To estimate the long-term systemic toxicity of shikonin derivatives (ShD) in a rat model.
Materials and methods: The roots of Arnebia euchroma (Royle) Johnst. (Boraginaceae) were extracted in ethanol, passed through a molecular sieve, and dried. A microemulsion solution in water was subsequently prepared. Adult Wistar rats were treated with ShD by gavage at concentrations of 200, 400, and 800 mg/kg per day for 90 days or 180 days. Hematological and biochemical examinations were performed, and the vital organs were subjected to pathological analyses.
Results: We did not observe hematological or non-hematological toxicity of ShD at a dose as high as 800 mg/kg per day for 6 months.
Discussion and conclusion: Our findings may offer some beneficial information for the practical application and research of Arnebia euchroma. We demonstrated in an animal model that ShD may be safe for usage.
Introduction
Chinese medical herbs designated as “Zicao” are actually dried roots of the borage plants belonging to the Boraginaceae family, Lithospermum erythrorhizon Sieb. et Zucc. and Arnebia euchroma (Royle) Johnst., which have been used for the treatment of macular eruption, measles, and sore throat for thousands of years in China (Chen et al., Citation2002). Naphthoquinone pigments, shikonin, and alkannin have been found to be the major components in both the plants. An early study that used an extract from Boraginaceae reported that it could inhibit the propagation of pulmonary carcinomas and improve immune functions (Guo et al., Citation1991). We also found that shikonin derivatives could protect the immune organs from damage and promote immune responses in tumor-bearing mice (Su et al., Citation2012). Furthermore, the shikonin extract of Lithospermum erythrorhizon induced apoptosis when added to cultures of human colorectal carcinoma cells (Hsu et al., Citation2004), HL60 cells (Gao et al., Citation2002; Hsu et al., Citation2004), and Hela cells (Wu et al., Citation2004). The underlying mechanisms of tumor suppression have been identified to induce of apoptosis via signaling pathways that possibly involve reactive oxygen species (Chang et al., Citation2010), p53, p27, Bcl-2, caspases (Fujii et al., Citation1992), and inhibition of telomerase and DNA topoisomerase I/II (Hsu et al., Citation2004).
In addition to antitumor effects, Sasaki et al. (Citation2000) reported that extracts from either Lithospermum erythrorhizon or Arnebia euchroma had antifungal activity against Candida albicans in vitro. Bactericidal research by Shen et al. (Citation2002) demonstrated the activities of this agent against Gram-positive cocci, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Ho et al. (Citation2003) showed that an extract of Arnebia euchroma diminished Vero cell infection by hepatitis C virus (HCV) in an in vitro inoculation model. Additionally, Wang et al. (Citation2009) reported that shikonin had therapeutic effects on lupus nephritis in NZB/W F1 mice. However, no study has evaluated the long-term systemic toxicity of shikonin. Thus, this study was designed to estimate the long-term systemic toxicity of shikonin derivatives in a rat model.
Materials and methods
Animals
Male and female in-bred Wistar rats were purchased from Changchun Experimental Animal Center and were maintained in a conventional animal housing facility throughout the experiment. The rats were 4-weeks-old, with a qualified permission No. SCXK-2008-0004. All experiments were performed according to Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China and were approved by the Ethics Committee of Jilin University (No. JLU-20080128002).
Shikonin derivatives
Shikonin derivatives (ShD) were prepared as described previously in our laboratory (Su et al., Citation2012). In brief, the crude Arnebia euchroma drug (“Xinjiang Zicao” in Chinese) was purchased from the Changchun Pharmaceutical Group, Co., which is authorized by the State Administration of Traditional Chinese Medicine of the People’s Republic of China to collect (October, verified by Chinese Academy of Medical Sciences), store, and sell Chinese medicinal herbs. The crude Arnebia euchroma drug was lixiviated with absolute ethanol, and the majority of the solvent was removed using a rotary evaporator. Subsequently, the solution was filterted, passed through a molecular sieve (Macrosep 1 K; Omega Centrifugal Devices, Port Washington, NY), and dried at 60 °C to obtain the ShD powder. Finally, ShD was collected as a dry crystallized powder. A microemulsion was used to prepare a water-soluble reagent (Shikonin derivative concentration: 1 mg/mL) using 1.08% of polyvinylpyrrolidone (PVP), and the drug was autoclaved and preserved at 4 °C. Acetylshikonin was found to be the main component using the extract characterization procedure described in detail in our previous study (Su et al., Citation2012).
Acute toxicity study
Forty Kunming mice were used to evaluate acute toxicity of ShD with maximal tolerated dose (MTD) test. After overnight fasting (approximately 10 h), 20 mice comprising an equal number of males and females were treated with ShD at dose of 10 g/kg (3 times per day for 3 days). The remaining 20 mice (equal number of males and females) treated with normal saline were set as control group. After a 2 week observation period, the animals were sacrificed for analyses.
Chronic toxicity study
We used 100 Wistar rats for the chronic toxicological investigation. The rats were randomly divided into 5 groups (20 animals per group) for each of the following treatments: normal control (without treatment), solvent control (PVP only without ShD), and ShD at 200, 400, and 800 mg/kg per day. The solvent or ShD was gavaged once a day for up to 90 days or 180 days (from March 2008 to August 2008), and the body weight of each of the rats was measured every 10 days during this period. Blood was collected for hematological and biochemistry examinations on days 90 and 180. Blood samples were collected from the rats by heart puncture and were added to 2 bottles; one with anticoagulant and one without (2 mL per bottle). The blood cells were examined with a hemocyte counter (Coulter STKS, Hialeah, FL). Serum samples were separated after overnight coagulation of the blood and were analyzed automatically with the Coulter LX2D (Beckman, Brea, CA). The animals were sacrificed after the blood samples were collected by heart puncture. The organs were removed and fixed in ethanol, and the paraffin sections were stained with hematoxylin--eosin. Pathological analyses were conducted on these organs by two independent investigators blinded to group assignment.
Statistics
Data are expressed as mean ± standard error of the mean (SEM). The data were analyzed by analysis of variance (ANOVA) using the Statistics Package for Social Science (SPSS) software (Version 16.0) (SPSS Inc., Chicago, IL). The Student--Newman--Keuls (SNK) test was used to assess significant differences between the control and treated groups. p Values of <0.05 were considered significant.
Results
Acute toxicity study in mice
No deaths occurred during the 2 week observation period. There was no significant differences in body weights, food consumption, clinical signs, and tissue morphology between ShD treated and control group (data not shown).
General conditions of the rats treated with ShD
Toxicological investigations were performed in an officially authorized national laboratory. The rats were given ShD for up to 90 days or 180 days, and food and water consumption in each group was observed daily during this period. In the first 10 days, the rats ingested an average of 18 g of food and 20 ml of water per animal in each of the 5 groups, with no significant differences between the groups. The food and water consumption increased an average of 1 g and 6 ml, respectively, per 10 days and increased to approximately 25 g and 60 ml, respectively, per day at 3 months after ShD administration. Thereafter, dietary consumption did not change and no significant differences were found among the five groups. The body weight of each rat was measured every 10 days: this value was approximately 100 g before ShD administration, increased to nearly 200 g in 50–60 days and further increased to approximately 300 g on day 100. When treatment ended on day 180, the average body weight of the rats in the 5 groups had increased to approximately 370 g, without an obvious difference among the groups (). Additionally, the glossiness of the coats and the activities of the rats were not obviously different among the 5 groups.
Table 1. Body weight changes of the rats in different groups.
Evaluation of non-hematological and hematological toxicities of ShD in Wistar rats
No rats died during the first 3 months, and 50 rats (10 from each group) comprising an equal number of females and males were analyzed on day 90. During the subsequent 90 days, no rats died as well, and the remaining 50 rats were sacrificed for analysis at the end of the study.
Blood biochemistry was assessed to monitor the functions of the vital organs of the rats, and no obvious abnormality was observed in the rats treated with ShD compared with the control groups ().
Table 2. Blood biochemistry examinations of the rats treated with ShD.
The influence of ShD on the hemogram of the rats was evaluated at 90 day and 180 day after ShD administration. Long-term ShD administration did not affect the peripheral blood cell counts or the functional parameters of these cells ().
Table 3. Values of blood cell counts of rats treated with ShD.
The hematopoietic status of the bone marrow was observed in a histological sample of the sternum. The bone marrow exhibited a normal structure in the ShD-treated groups comparable to those observed in the normal and solvent controls ( and ). The morphology and percentages of different cell lines were almost identical in the different groups.
Figure 1. Pathological analyses of the vital organs of Wistar rats in different groups. Wistar rats of both ShD treated groups and control groups were kept for 90 or 180 days, then they were sacrificed and the organs were removed for pathological analyses. A to D: sternum, heart, liver and kidney of rats without treatment. E to H: sternum, heart, liver and kidney of rats treated with solvent. I to L: sternum, heart, liver and kidney of rats treated with ShD at the concentration of 800 mg/kg. No significant abnormality and difference were found among the different groups. ☆: bone marrow; ⋆: bone; ▴:myocardial cell; ♦: hepatocyte; •: glomerulus; Δ: renal tubule.
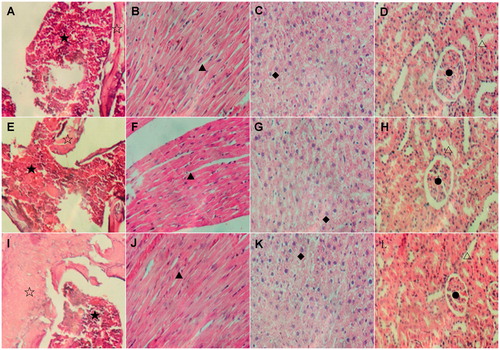
Histological examination of the heart, liver, and kidney of the rats was also conducted in this study (). Compared with the normal and solvent groups, no significant aberrations were observed in these organs in the ShD-treated rats at different concentrations. Interestingly, the absence of the so-called “cat-eye” structure of neurons was the only abnormality observed in the brain slices approximately 90 days after ShD administration. However, this feature appeared to be reversible because the typical morphology of neuronal cells was observed thereafter (at 180th day).
Discussion
In this study, shikonin derivatives were extracted by ethanol, and waxy contaminants were deleted using a molecular ultrafiltration sieve rather than ether treatment. Industrially, it may be replaced by binding to a macroporous resin. Hence, the traditional dewaxing procedure that uses a dangerous chemical reagent such as ether should no longer be required. Several protocols are available for facilitating ShD solubilization into water solution. In the present study, the dried shikonin derivative powder was dispersed into a solvent heated at a constant temperature of 60 °C, leading to formation of a stable microencapsulation reagent. The shikonin solution was maintained at 4 °C for over 1 year without water–oil dissociation, and it was stored as a form of dried, bright violet crystal for a long period. PVP is a microencapsulating solvent with a high molecular weight, and its water solution can adequately dissolve the shikonin crystals. The profile of this preparation did not differ from those of the preparations from the original ShD of Arnebia euchroma in thin-layer silicon G chromatography, indicating that the solubility of shikonin was improved without changes in the other physical properties of the shikonin fraction.
The systemic toxicity of shikonin in vivo remains unclear although Arnebia euchroma has been studied for decades. Thus, we performed this investigation to evaluate the long-term toxicity of ShD. In chronic toxicity studies, the top dose should not exceed 1000 mg/kg body weight/day, and 2- to 4-fold intervals are frequently optimal for setting the descending dose levels. Therefore, we set 3 dose levels: 800, 400, and 200 mg/kg. The smallest daily dosage of the agent was 200 mg/kg, which was estimated to be 20-fold of that in the pharmacokinetic observation. For chronic toxicity studies of biotechnology-derived pharmaceuticals, duration of 6 months has generally been accepted for regulatory approval (Clarke et al., Citation2008). As a result, we chose 6 months in the present study. Quality of life, hematological examinations, clinical biochemistry assessment, and organ pathology were investigated in this study.
Both hematological examinations and clinical biochemistry determinations are routinely performed in clinical practice because they are closely related to the selection, dose, and duration of the drug. Hence, it is very important to monitor hematological and biochemical changes during the development of a new drug, and measurement of these parameters is required for chronic toxicity studies, as instructed in the OECD guideline for testing of chemicals.
We observed no acute toxicity of ShD to mice in this study. In the chronic toxicity study, ShD did not affect either peripheral blood cells or bone marrow at a concentration as high as 800 mg/kg during the 6-month administration period. Additionally, ShD administration did not influence the functions of vital organs (e.g., the liver and kidney), as demonstrated by blood biochemistry and organ pathological analyses. These results suggest that ShD did not induce either hematological or non-hematological toxicity in Wistar rats.
Chemotherapy is one of the main therapeutic strategies for cancer, but it usually causes severe toxic effects such as leukopenia and other systemic discomforts. In previous studies, we demonstrated in vitro that ShD significantly inhibits proliferation of tumor cell lines such as SMMC-7721, Hela, and HepA22 at concentrations of 2.5–10 μg/mL,, whereas it does not interfere with the growth of normal cell lines such as HEK93 and NIH3T3 at the same concentrations (Wang et al., Citation2007; Yan et al., Citation2007). Further, we found that ShD inhibits the growth of transplantable tumors in vivo as effectively as chemotherapy with improved life quality in Swiss mice (Su et al., Citation2012), which suggests that the mechanisms of shikonin differ from those of chemotherapy. Shikonin may inhibit the production of monokines such as tumor necrosis factor α (Staniforth et al., Citation2004) as well as down-regulate the expression of chemokine receptors (Chen et al., Citation2003). In conclusion, our results in an animal model indicate that ShD may be safe for usage in vivo.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We thank the First Hospital, Bethune Medical College of Jilin University and Jilin Provincial Institute of Tumor Prevention and Therapy, for their help to this work.
References
- Chang IC, Huang YJ, Chiang TI, et al. (2010). Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol Pharm Bull 33:816–24
- Chen X, Yang L, Oppenheim JJ, Howard MZ. (2002). Cellular pharmacology studies of shikonin derivatives. Phytother Res 16:199–209
- Chen X, Yang L, Zhang N, et al. (2003). Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother 47:2810–16
- Clarke J, Hurst C, Martin P, et al. (2008). Duration of chronic toxicity studies for biotechnology-derived pharmaceuticals: Is 6 months still appropriate? Regul Toxicol Pharmacol 50:2–22.
- Fujii N, Yamashita Y, Arima Y, et al. (1992). Induction of topoisomerase II-mediated DNA cleavage by the plant naphthoquinones plumbagin and shikonin. Antimicrob Agents Chemother 36:2589–94
- Gao D, Hiromura M, Yasui H, Sakurai H. (2002). Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol Pharm Bull 25:827–32
- Guo XP, Zhang XY, Zhang SD. (1991). Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi 11:598–9
- Ho TY, Wu SL, Lai IL, et al. (2003). An in vitro system combined with an in-house quantitation assay for screening hepatitis C virus inhibitors. Antiviral Res 58:199–208
- Hsu PC, Huang YT, Tsai ML, et al. (2004). Induction of apoptosis by shikonin through coordi native modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem 52:6330–7
- Sasaki K, Yoshizaki F, Abe H. (2000). The anti-Candida activity of shikonin. Yakugaku Zasshi 120:5897–9
- Shen CC, Syu WJ, Li SY, et al. (2002). Antimicrobial activities of naphthazarins from Arnebia euchroma. J Nat Prod 65:1857–62
- Staniforth V, Wang SY, Shyur LF, Yang NS. (2004). Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human TNF-a promoter in vivo. J Biol Chem 279:5877–85
- Su L, Yan GZ, Guan BJ, et al. (2012). Shikonin derivatives protect immune organs from damage and promote immune responses in vivo in tumour-bearing mice. Phytother Res 26:26–33
- Yan GZ, Suo J, Liu LH. (2007). Experimental study of hepatoma carcinoma cell proliferation inhibited by lithospermi naphthoquinol extracts. Chinese J Gerontol 27:507–8
- Wang XC, Feng J, Huang F, et al. (2009). Effects of shikonin isolated from Zicao on lupus nephritis in NZB/W F1 mice. Biol Pharm Bull 32:1565–70
- Wang YL, Zhang Yang, Liu LH. (2007). Shikonins induces apoptosis of the carcinoma of genital system. Matern Child Health Care of China 22:3585–7
- Wu Z, Wu LJ, Li LH, et al. (2004). Shikonin regulates HeLa cell death via caspase-3 activation and blockage of DNA synthesis. J Asian Nat Prod Res 6:155–66

