Abstract
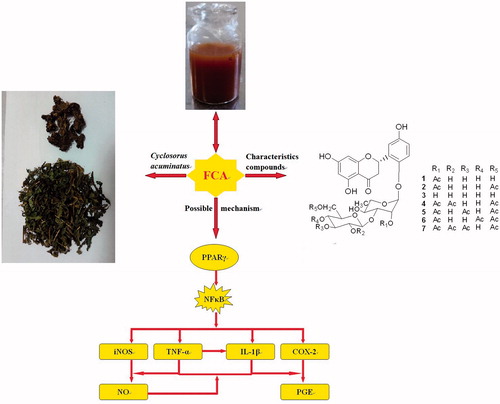
Context: Cyclosorus acuminatus (Houtt.) Nakai (Thelypteridaceae) is used in Chinese traditional medicine for inflammation and pyretic stranguria.
Objective: This study investigates the prostatic protective potential of the flavonoid-rich [(2S)-5,7,5′-trihydroxyflavanone glycosides] fraction from C. acuminatus (FCA).
Materials and methods: Chronic non-bacterial prostatitis (CNBP) was induced by injecting 20 μl of 1% carrageenan into the rat prostate. Subsequently, FCA (150 or 300 mg/kg/d) was orally given once a day for 4 weeks. Finally, the levels of proinflammatory cytokines and the prostatic expression of peroxisome proliferator activated receptor-γ (PPAR-γ) were evaluated.
Results: Treatment with 300 mg/kg/d FCA ameliorated the carrageenan-induced higher prostatic index (PI) state and proinflammatory cytokines levels (NFκB from 2602 ± 588 to 1348 ± 300 pg/ml, TNF-α from 151.6 ± 10.4 to 126.0 ± 3.52 pg/ml, IL-1β from 153.7 ± 14.8 to 63.9 ± 6.7 pg/ml, COX-2 from 313.3 ± 16.5 to 263.1 ± 15.1 pg/ml, PGE from 1532 ± 130 to 864 ± 126 pg/ml, NOS from 33.7 ± 3.0 to 23.6 ± 1.6 U/mg protein, and NO from 40.3 ± 2.9 to 27.1 ± 2.9 μmol/g protein) as well as regulated the prostatic expression of PPAR-γ (increased about 3.50-fold) when compared to the rat model of prostatitis.
Discussion and conclusion: FCA could exert a prostatic protective response via modulating the prostatic expression of PPAR-γ and eventually alleviating the NFκB dependent inflammatory response.
Introduction
Chronic prostatitis (CP) is a very common syndrome of adult men. However, all of the etiology, diagnosis and the appropriate therapy of CP have remained elusive (Lu et al., Citation2011). During the past years, some treatment means have been developed, including antibiotics, α-blockers, and anti-inflammatories (Suh & Lowe, Citation2011). Unfortunately, although these means have certain clinical efficacy, there is still no universally effective treatment or standardized therapy that can provide significant lasting benefits for CP (Luzzi, Citation2002).
In recent years, natural plants have attracted more attention from researchers because of their diverse biological activities. The traditionally oriental herbals and natural flavonoids comprise a huge source of potential drugs that have anti-inflammatory and prostatic protective properties (Suh & Lowe, Citation2011). In fact, some drugs (such as the Saw palmetto) developed from natural plants, have already been used for CP and benign prostatic hyperplasia (BPH) and are available in the market (Agbabiaka et al., Citation2009).
Cyclosorus acuminatus (Houtt.) Nakai (Thelypteridaceae) is widely distributed in the Yangtze River and the southern area of China. The rhizome of this plant is used in Chinese traditional medicine for the treatment of blood circulation stasis, edema, inflammation and pyretic stranguria (CP, BPH, cystitis, pyelonephritis and urethritis) (Hu et al., Citation1998). Many novel (2S)-5,7,5′-trihydroxyflavanone glycosides (the structures showed in ) were first separated from the chloroform and ethyl acetate fractions of the methanol extract from C. acuminatus (Fang et al., Citation2006). In the previous study, we investigated the effects of this flavonoid-rich fraction from C. acuminatus (FCA) on metabolic syndrome and diabetes in mice. The whole results indicated that FCA was able to up-regulate the expression of peroxisome proliferator-activated receptor γ (PPAR-γ), and subsequently attenuated the nuclear factor κB (NFκB)-dependent inflammatory responses (J.L. Chen et al., 2011). Additionally, evidence shows that systemic metabolic disease including diabetes and obesity may lead to prostate disorders. On one hand, diabetes and obesity will alter the metabolism of sex steroid hormone. On the other hand, a chronic inflammatory environment will be induced. Subsequently, the prostatic inflammation contributes a higher risk of BPH. PPAR-γ seems to be the nexus of metabolic syndrome and prostatic disorders via regulating inflammation and insulin resistance (Jiang et al., Citation2011; Joseph et al., Citation2003).
Therefore, this study was undertaken to assess the potential of FCA to protect against carrageenan-induced chronic non-bacterial prostatitis (CNBP) in rat model and the possible mechanisms.
Materials and methods
Reagents
Carrageenan was purchased from Dingguo Changsheng Biotechnology, Co, Ltd. (Wuhan, Hubei, China). Commercial kits for the analysis of prostatic acid phosphatase (PACP), inducible nitric synthase (iNOS, one subtype of NOS that is expressed little in normal status) and nitric oxide (NO) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The commercial enzyme-linked immunosorbent assay (ELISA) kits used for the determination of NFκB, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), prostaglandin E (PGE), and cyclo-oxygenase-2 (COX-2) were purchased from R&D Systems, Inc. (Minneapolis, MN). The total protein extraction kit was purchased from ProMab Biotechnologies, Inc. (Richmond, CA). The bicinchoninic acid (BCA) kit, used for the determination of protein content, was purchased from Beyotime Institute of Biotechnology (Shanghai, China). The antibodies of peroxisome proliferator activated receptorγ (PPAR-γ) used for Western blot was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Cernilton (Prostat Tablet) were purchased from Meirui Pharmacy, Co, Ltd. (Nanjing, Jiangsu, China). All other solvents and chemicals used in the study were of analytical grade and purchased from Sinopharm Chemical Reagent, Co, Ltd. (Shanghai, China).
Plant material
Cyclosorus acuminatus was collected on August 2011 in the Jiangxi province, China and identified by Prof. Ceming Tan at the Jiujiang Forest Plants Specimen Mansion, China. A specimen was deposited in the College of Pharmacy, Tongji Medical Center, Huazhong University of Science and Technology. The voucher number was No. JX1108. The FCA fraction was prepared according to a previous method (R.Z. Chen et al., 2011). The content of total flavonoid in FCA was 32.96 ± 3.22%.
Animals and administration
Sprague-Dawley (S.D.) rats, weighing 200–220 g, were obtained from the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China. The animals were housed at a controlled room (temperature 22 ± 3 °C and humidity 50 ± 10%) and were kept on a 12 h light:12 h dark cycle. The rats were fed standard diet and water ad libitum and acclimated 7 days before they were used for the study. After acclimatization to the laboratory conditions, the rats were randomly divided into five groups (n = 7): the vehicle control group (C), the model group (M), the high dose FCA treated group (HF), the low dose FCA treated group (LF), and the positive group (P). All the animals were given an operation to make the prostatic tissue visualized. The M, P, HF and LF groups were injected 20 μl of 1% carrageenan into the prostate. The C group was injected with 20 μl physiological saline (sham-operated) (R.Z. Chen et al., 2011; Lu et al., Citation2011). All the drugs were dissolved in physiological saline with 0.5% CMC-Na. From the next week (7 days later), the rats of HF and LF were treated with 300 and 150 mg/kg/d FCA for 4 weeks, respectively. The rats of P were treated with 200 mg/kg/d Cernilton for 4 weeks while those of C and M groups were treated with the same volume of physiological saline with 0.5% CMC-Na.
At the end of the 4 week treatment period, blood samples were collected. Plasma was prepared and stored at −80 °C until the analysis of plasma biochemical criterion. The prostate samples were rapidly removed, perfused with PBS to remove residual blood, blotted dry and weighted for the analysis of prostatic index (PI). PI was calculated by the following equation: PI = weight of prostate/body weight.
Then, the prostate tissue was excised. One section was stored at −80 °C for the subsequent Western blot assay for the prostatic expression of PPAR-γ. Another section of prostate was homogenized in 10 volumes of ice-cold physiological saline and the homogenate was spun at 15 000 rpm for 20 min at 4 °C. The supernatant was then stored at −80 °C for the subsequent assay of iNOS, NO, NFκB, TNF-α, IL-1β, PGE, and COX-2.
All experiments were performed in compliance with the Chinese legislation on the use and care of laboratory animals and were approved by the Huazhong University of Science and Technology Committee on Animal Care and Use, the approval number was IACUC Number: S276.
Biochemical analysis
The prostatic levels of NFκB, TNF-α, IL-1β, PGE, and COX-2 were measured by specific ELISA using commercial kits according to the manufacturer’s instructions. The results of NFκB, TNF-α, IL-1β, PGE, and COX-2 were expressed as pg/ml. The levels of PACP, iNOS and NO were assayed by using commercial available kits (Milsom et al., Citation2010). All the procedures were performed according to the manufacturer’s instructions. The activity of plasma PACP was expressed as U/l. The levels of iNOS and NO were expressed as U/mg protein and μmol/g protein, respectively. Protein concentration of each prostate sample was determined based on the BCA method (Jeong et al., Citation2005). Bovine serum albumin was the standard.
Western blot analysis
The prostatic expression of PPAR-γ was evaluated by Western blot analysis according to the previous method (Lei et al., Citation2011). Briefly, the tissue samples were ground in liquid nitrogen and the total protein was extracted using a protein extraction kit. Protein concentrations were determined using the BCA protein assay kit. Protein samples (50 μg) were separated by 12% SDS-polyacrylamide gel electrophoresis and then transferred to a PVDF membrane (Roche Diagnostics Corporation, Indianapolis, IN) by electrophoretic transfer (Bio-Rad Laboratories, Inc., Hercules, CA). Transferred membranes were blocked for 1 h at room temperature with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST), and then incubated overnight at 4 °C with primary antibodies [anti-PPAR-γ (1:500)]. After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in TBST with 3% nonfat milk for 1 h at room temperature. Immunoblots were developed on films using the enhanced chemiluminescence technique (Super Signal West Pico; Pierce Biotechnology, Rockford, IL). Quantification of bands was determined by densitometric analysis using Bio-Rad Quantity One. The data were normalized using GAPDH (1:1000) as an internal control.
Statistical analysis
The values were presented as mean ± SD. Results were analyzed statistically by one-way ANOVA followed by Tukey’s multiple comparison using SPSS 11.5 Software for Windows (Chicago, IL). Differences were considered as significant at p < 0.05. Figures were made using Origin 6.0 Software for Windows.
Results
Biochemical analysis
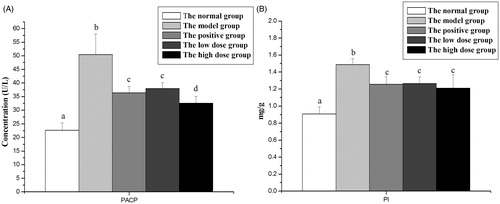
Effects of FCA on the level of PI, and the plasma activity of PACP on carrageenan-induced non-bacterial prostatitis in rat are shown in . Carrageenan resulted in a significant enhancement in the level of PI and the activity of PACP when compared to vehicle control. FCA-treated groups produced marked (p < 0.05) effects on decreasing the activity of PACP () and the level of PI (). There was no significance (p > 0. 05) among the two FCA-treated groups and positive control in the PI state.
Figure 1. Effects of the flavonoid-rich fraction from Cyclosorus acuminatus (FCA) on the levels of prostatic acid phosphatase (PACP, ) and prostatic index (PI, ) in the experimental non-bacterial prostatitis rat induced by carrageenan. Values are given as means ± SD from each group (n = 7). Values marked a common (a, b, c, d) in each row do not differ significantly at p < 0.05 (SPSS). 200 mg/kg/d Cernilton was used as the positive control. The high dose group and the low dose group are treated with 150 and 300 mg/kg/d FCA for 4 weeks, respectively.
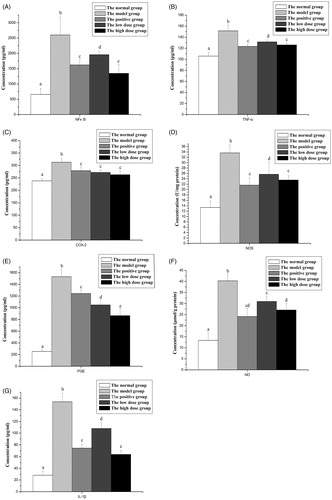
The effects of FCA on modulating the levels of inflammatory factors are described in . The model group expressed an obvious chronic inflammation performance. All the levels of iNOS, NO, NFκB, TNF-α, IL-1β, PGE, and COX-2 in CNBP model group were significantly higher than the vehicle control (p < 0.05). As can be seen from , 4 week treatment of FCA (150 or 300 mg/kg/d) alleviated this change. Compared to those in the untreated model group, all the levels of NFκB (), TNF-α (), COX-2 (), iNOS (), PGE (), NO () and IL-1β () in two dose FCA treated groups were noticeable decreased (p < 0.05). On the other hand, the FCA at highest concentration exerted similar (p > 0.05) activity to that of the positive control on regulation the levels of TNF-α, COX-2, iNOS and NO. Treated with 300 mg/kd/d FCA even resulted in lower (p < 0.05) levels of NFκB, PGE and IL-1β when compared to the positive control.
Figure 2. Effects of the flavonoid-rich fraction from Cyclosorus acuminatus (FCA) on the levels of prostatic nuclear factor κB (NFκB, ), tumor necrosis factor-α (TNF-α, ), cyclo-oxygenase-2 (COX-2, ), inducible nitric synthase (iNOS, ), prostaglandin E (PGE, ), nitric oxide (NO, ) and interleukin-1β (IL-1β, ) in the experimental non-bacterial prostatitis rat induced by carrageenan. Values are given as means ± SD from each group (n = 7). Values marked a common (a, b, c, d, e) in each row do not differ significantly at p < 0.05 (SPSS). Both c and d do not differ significantly with cd. 200 mg/kg/d Cernilton was used as the positive control. The high dose group and the low dose group are treated with 150 and 300 mg/kg/d FCA for 4 weeks, respectively.
Western blot analysis
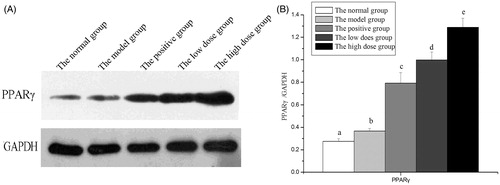
represents the effects of FCA on regulating the prostatic expression of PPAR-γ in experimental CNBP rats induced by carrageenan. Carrageenan obviously enhanced the prostatic expression of PPAR-γ when compared to the vehicle control. Along with the treatment of FCA for 4 weeks, further up-regulated expression of PPAR-γ was observed. The expression of PPAR-γ in the positive control, low dose FCA and high dose FCA were incrementally increased.
Figure 3. Effects of the flavonoid-rich fraction from Cyclosorus acuminatus (FCA) on the prostatic expression of peroxisome proliferators activated receptor-γ (PPAR-γ) in the experimental non-bacterial prostatitis rat induced by carrageenan. Values are given as means ± SD from each group (n = 7). Values marked a common (a, b, c, d) in each row do not differ significantly at p < 0.05 (SPSS). 200 mg/kg/d Cernilton was the positive control. The high dose group and the low dose group are treated with 150 and 300 mg/kg/d FCA for 4 weeks, respectively. (A) Representative Western blot images. (B) The PPAR-γ /GAPDH ratios in rats prostate.
Discussion
In the present study, carrageenan-induced CNBP in rat model was used to evaluate the prostatic protective nature of FCA and the possible mechanisms. The major findings were tha four continuous weeks administration of FCA (150 or 300 mg/kg/d) significantly attenuated the carrageenan-induced CNBP. As compared to the untreated model group, there was markedly decrease in all levels of iNOS, NO, NFκB, TNF-α, IL-1β, PGE, and COX-2. Additionally, an increased prostatic expression of PPAR-γ was observed in FCA treated group.
An obviously increased incidence of CP is observed these years. Furthermore, both the etiological agent and pathological mechanisms have remained unclear. In general, CNBP usually results in prostatic complex inflammatory conditions. Cytokines are important mediators in the process of prostate damage (Lu et al., Citation2011; Suh & Lowe, Citation2011). The signaling molecule NFκB is a major proinflammatory switch that can regulate the efflux of many cytokines including iNOS, COX-2, TNF-α and IL-1β, and is commonly suggested to contribute to the inflammatory related pathologic process (Han et al., Citation2004; Lee et al., Citation2012). Therefore, regulating NFκB signaling and NFκB-dependent proinflammatory mediators is a potential therapeutic approach for anti-inflammatory activity, and has attracted an increasing attention from the scientific community and interested individuals (Zheng et al., Citation2009). Moreover, NFκB plays an important role in the development of prostatic tumors from chronic prostatic infection. Controlling the activation of NFκB signaling is one key step in inhibiting the progress of prostatitis-induced prostate cancer (Pei et al., Citation2010).
NOS can be classified as at least three subtypes: the Ca2+-dependent NOS-I and NOS-III (both also be called as eNOS), and the Ca2+-independent NOS-II (be known as iNOS). NO comes from eNOS at a low level is necessary for many physiological processes. However, a much higher level of NO will emerge when iNOS is activated by proinflammatory cytokines (including IFNγ) (Chan et al., Citation2000). Excessive NO will participate in inflammatory responses in vivo, and exert negative effects on nearby healthy tissues (Sareila et al., Citation2008). Moreover, there is a general agreement that NO will interact with free radicals and aggravate the tissue injury. Antioxidant can suppress NO via inhibiting the activation of NFκB. However, peroxynitrite formation will be enhanced when high NO levels along with decreased SOD activity occur. Furthermore, NO will consume GSH resource in vivo and interfere with the degradation of H2O2 (Park et al., Citation2005; Suzuki et al., Citation2000). PGE2, a COX-2-derived main proinflammatory bioactive lipid, can stimulate cell proliferation and invasion (Mann et al., Citation2005). In fact, selective COX-2 inhibitors have already been used clinically as anti-inflammatory agents (Liang et al., Citation2009). In addition, biochemical reactions catalyzed by COX-2 involved in the formation of ROS and contribute to the oxidation of DNA. COX-2 inhibition results in the lower levels of ROS and cell proliferation. Marked up-regulated expression of COX-2 gene has also been observed when prostate cancer occurs. Therefore, COX-2 inhibitors could be used in prevention and treatment of prostate carcinoma (Lin & Nelson, Citation2003; Nishimura et al., Citation2006). Reports indicates that both the two biochemical reactions of NO and PEG2 production are catalyzed by IL-1β in combination with (or without) TNF-α, and inhibiting the secretion of TNF-α will lead to the suppression of IL-1β (Chow et al., Citation2012; Kim et al., Citation2007). Furthermore, it is known that NO could stimulate the generation of various inflammatory mediators including TNF-α and IL-1β (Chow et al., Citation2012). TNF-α will induce the expression of adhesion molecules and the production of chemokines, and subsequently mediate the aggregation of inflammatory cells. Finally, the tissue injury and inflammatory response happen (Dayer et al., Citation1985). Moreover, it is demonstrated that the synthesis and secretion of NO from iNOS will be stimulated by TNF-α (Suzuki et al., Citation2000). Most important, the levels of TNF-α and IL-1β in prostatitis patients are significantly higher than in healthy men, and will increase in accordance with the degree of CP (Nadler et al., Citation2000).
It can be summarized that NFκB-dependent inflammatory responses is a network system, and play an important part in the development and progression of CAP (). In this study, the levels of NFκB and NFκB-dependent proinflammatory mediators were significantly enhanced in the rat with carrageenan-induced CNBP. Daily treatment of FCA (150 or 300 mg/kg/d for 4 weeks) significantly decreased all the levels of NFκB, TNF-α, IL-1β, iNOS, COX-2, NO and PEG2. The results suggested that FCA possessed significant anti-inflammatory properties and could inhibit the inflammatory responses through suppressing the NFκB signaling pathway.
PPARs are members of the nuclear receptor superfamily and consist of PPARα, PPARγ and PPARβ/δ. They are able to regulate the expression of genes related to the control of lipid and lipoprotein metabolism, glucose homeostasis and inflammatory processes (Yoshimura et al., Citation2004). Additional evidence suggests that PPAR is an upstream target involved in the NFκB signaling pathway (Kersten et al., Citation2000). PPAR-γ activation will result in NFκB signaling repression via inhibition of IκB protein inhibitory degradation, and inhibit the production of NFκB-dependent inflammatory cytokines (Ji et al., Citation2001; Ricote et al., Citation1998). Subsequently, the cytokine-induced expressions of iNOS and COX-2 will be attenuated, as well as inhibition of the secretion of TNF-α, IL-1β and NO (Kim et al., Citation2007; Sánchez-Hidalgo et al., Citation2005; Zhang et al., Citation2009). PPAR-γ agonist can inhibit cell proliferation and ameliorate the development of tumor including prostate cancer (Matsuyama & Yoshimura, Citation2008). PPAR-γ ligands, thiazolidinediones, can inhibit the growth of prostate cancer cell (Segawa et al., Citation2002). This is in line with the phenomena in our present study. To sum up, FCA could modulate the prostatic expression of PPAR-γ and alleviate NFκB-dependent inflammatory responses in experimental CNBP rats induced by carrageenan ().
Conclusion
The overall results indicated that FCA was able to exert prostatic protective response in experimental CNBP rats via modulating the prostatic expression of PPAR-γ and eventually alleviating the NFκB dependent inflammatory responses ().
Declaration of interest
This research was supported by the National Natural Science Foundation of China (81173065) and the Research Foundation of Tongji Hospital (2013B003). The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Agbabiaka TB, Pittler MH, Wider B, Ernst E. (2009). Serenoa repens (Saw Palmetto): A systematic review of adverse events. Drug Saf 32:637–47
- Chan MM, Mattiacci JA, Hwang HS, et al. (2000). Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol 60:1539–48
- Chen JL, Lei YF, Liu YJ, et al. (2011). Extract of Cyclosorus acuminatus attenuates diabetic nephropathy in mice via modifying peroxisome proliferaors activated receptor signaling pathway. Food Chem 128:659–66
- Chen RZ, Cui L, Guo YJ, et al. (2011). In vivo study of four preparative extracts of Clematis terniflora DC. for antiociceptive activity and anti-inflammatory activity in rat model of carrageenan-induced chronic non-bacterial prostatitis. J Ethnopharmacol 134:1018–23
- Chow YL, Lee KH, Vidyadaran S, et al. (2012). Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int Immunopharmacol 12:657–65
- Dayer JM, Beutler B, Cerami A. (1985). Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med 162:2163–8
- Fang W, Ruan J, Wang Z, et al. (2006). Acetylated flavanone glycosides from the rhizomes of Cyclosorus acuminatus. J Nat Prod 69:1641–4
- Han M, Wen JK, Zheng B, Zhang DQ. (2004). Acetylbritannilatone suppresses NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci 75:675–84
- Hu XM, Zhang WK, Zhu QS, et al. (1998). Editorial Board of China Herb of State Administration of Traditional Chinese Medicine. China herbal. Shanghai, China: Shanghai Scientific and Technical Publishers, 0551–4
- Jeong JC, Yoon CH, Lee WH, et al. (2005). Effects of Bambusae concretio Salicea (Chunchukhwang) on amyloid beta-induced cell toxicity and antioxidative enzymes in cultured rat neuronal astrocytes. J Ethnopharmacol 98:259–66
- Ji JD, Cheon H, Jun JB, et al. (2001). Effects of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) on the expression of inflammatory cytokines and apoptosis induction in rheumatoid synovial fibroblasts and monocytes. J Autoimmun 17:215–21
- Jiang M, Strand DW, Franco OE, et al. (2011). PPARγ: A molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation 82:220–36
- Joseph MA, Harlow SD, Wei JT, et al. (2003). Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol 157:906–14
- Kersten S, Desvergne B, Wahli W. (2000). Roles of PPARs in health and disease. Nature 405:421–4
- Kim EK, Kwon KB, Koo BS, et al. (2007). Activation of peroxisome proliferator-activated receptor-γ protects pancreatic β-cells from cytokine-induced cytotoxicity via NFκB pathway. Int J Biochem Cell Biol 39:1260–75
- Lee HS, Kwon SH, Ham JE, et al. (2012). Zaprinast activates MAPKs, NFκB, and Akt and induces the expressions of inflammatory genes in microglia. Int Immunopharmacol 13:232–41
- Lei Y, Fu W, Chen J, et al. (2011). Neuroprotective effects of abacopterin E from Abacopteris penanagiana against oxidative stress stress-induced neurotoxicity. J Ethnopharmacol 134:275–80
- Liang M, Yang H, Fu J. (2009). Nimesulide inhibits IFN-γ programmed death-1-ligand 1 surface expression in breast cancer cells by COX-2 and PGE2 independent mechanisms. Cancer Lett 276:47–52
- Lin DW, Nelson PS. (2003). The role of cyclooxygenase-2 inhibition for the prevention and treatment of prostate carcinoma. Clin Prostate Cancer 2:119–26
- Lu B, Cai H, Huang W, et al. (2011). Protective effect of bamboo shoot oil on experimental nonbacterial prostatitis in rats. Food Chem 124:1017–23
- Luzzi GA. (2002). Chronic prostatitis and chronic pelvic pain in men: Aetiology, diagnosis and management. J Eur Acad Dermatol Venereol 16:253–6
- Mann JR, Backlund MG, DuBois RN. (2005). Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2:202–10
- Matsuyama M, Yoshimura R. (2008). Peroxisome proliferators-activated receptor-γ is a potent target for prevention and treatment in human prostate and testicular cancer. PPAR Res 2008:249849 (1--12)
- Milsom AB, Patel NS, Mazzon E, et al. (2010). Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric Oxide 22:141–8
- Nadler RB, Koch AE, Calhoun EA, et al. (2000). IL-1 beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol 164:214–18
- Nishimura K, Takayama H, Nakayama M, et al. (2006). Molecular-targeted therapy for hormone-refractory prostate cancer. Hinyokika Kiyo 52:487–90
- Park JY, Cho HY, Kim JK, et al. (2005). Chlorella dichloromethane extract ameliorates NO production and iNOS expression through the down-regulation of NFκB activity mediated by suppressed oxidative stress in RAW 264.7 macrophages. Clin Chim Acta 351:185–96
- Pei Z, Li H, Guo Y, et al. (2010). Sodium selenite inhibits the expression of VEGF, TGFβ1 and IL-6 induced by LPS in human PC3 via TLR4-NFκB signaling blockage. Int Immunopharmacol 10:50–6
- Ricote M, Li AC, Willson TM, et al. (1998). The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79–82
- Sánchez-Hidalgo M, Martín AR, et al. (2005). Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol 69:1733–44
- Sareila O, Korhonen R, Kärpänniemi O, et al. (2008). Janus kinase 3 inhibitor WHI-P154 in macrophages activated by bacterial endotoxin: Differential effects on the expression of iNOS, COX-2 and THF-α. Int Immunopharmacol 8:100–8
- Segawa Y, Yoshimura R, Hase T, et al. (2002). Expression of peroxisome proliferator-activated receptor (PPAR) in human prostate cancer. Prostate 51:108–16
- Suh LK, Lowe FC. (2011). Alternative therapies for the treatment of chronic prostatitis. Curr Urol Rep 12:284–7
- Suzuki Y, Deitch EA, Mishima S, et al. (2000). Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia reperfusion injury. Crit Care Med 28:3692–6
- Yoshimura R, Matsuyama M, Segawa Y, et al. (2004). Study of peroxisome proliferator-activated receptor (PPAR)-gamma in renal ischemia-reperfusion injury. Transplant Proc 36:1946–8
- Zhang H, Matsuda H, Yamashita C, et al. (2009). Hydrangeic acid from the processed leaves of Hydrangea macrophylla var. thunbergii as a new type of anti-diabetic compound. Eur J Pharmacol 606:255–61
- Zheng M, Ye S, Zhai Z, et al. (2009). Rosiglitazone protects diabetic rats against kidney disease through the suppression of renal moncyte chemoattractant protein-1 expression. J Diabetes Complications 23:124–9