Abstract
Context: Oleanolic acid (OA) belongs to the triterpenoid compound group existing widely in food, medicinal herbs and other plants. Its effects on gastric cancer cells and the mechanisms involved have not been investigated.
Objective: This study aimed to substantiate whether OA induces apoptosis of gastric cancer cell line (MKN28) and to elucidate the molecular mechanism involved.
Materials and methods: Cell viability was assessed by MTT assay within the range of 0–160 μg/mL. The effects of OA (5, 10 and 20 μg/mL) on apoptosis of MKN28 cells were evaluated by flow cytometry, DNA fragmentation and mitochondrial membrane potential assays. Western blot and FQRT-PCR assays were used to investigate the mechanism of cell apoptosis induced by OA (5 and 10 μg/mL).
Results: OA evidently inhibited cell viability with IC50 of 44.8 and 15.9 μg/mL at 12 and 24 h, respectively. Furthermore, OA increased JNK phosphorylation, decreased AKT phosphorylation, but did not affect p38 and ERK phosphorylation in MKN28 cells. In contrast, OA also significantly enhanced the mRNA expression levels of caspase 3, caspase 9 and Apaf-1 in MKN28 cells.
Conclusion: OA induces apoptosis of MKN28 cells via the mitochondrial pathway regulated by AKT and JNK signaling pathways.
Introduction
Gastric cancer is the second leading cause of cancer-related deaths, and approximately 760 000 cases of stomach cancer are diagnosed worldwide, and more than 24 000 cases are diagnosed in the United States each year (Liu et al., Citation2009). Surgery is the most effective treatment for gastric cancer, and good survival can be achieved if the tumor is resectable. However, most gastric cancer is either diagnosed at an advanced stage or relapses after apparently curative surgery. For these patients, systemic chemotherapy is the only treatment option available besides supportive care (Akagi et al., Citation2013; Middleton & Cunningham, Citation1995).
Oleanolic acid (3b-hydroxyolean-12-en-28-oic acid, OA) is a triterpenoid compound that exists extensively in food products (vegetable oils) and the leaves and roots of Olea europaea L., Viscum album L., Aralia chinensis L. and other more than 120 plant species (Fai & Tao, Citation2009; Li et al., Citation2002; Wang et al., Citation2013). OA is prevalent in Oleaceae family, especially in olive (O. europaea), the plant species after which the compound was named and that still serves as the main source of commercial OA (Pollier & Goossens, Citation2012; Sporn et al., Citation2011). Many pharmacological activities of OA have been reported such as antifungal (Tang et al., Citation2000), insecticidal (Marquina et al., Citation2001), anti-HIV (Ma et al., Citation2000), diuretic (Alvarez et al., Citation2002), complement inhibitory (Assefa et al., Citation2001), liver-protection (Yim et al., Citation2001), anti-inflammatory (Ismaili et al., Citation2001) and gastrointestinal transit modulating activities (Li et al., Citation1999). In recent years, it was found that OA exhibited the significant antitumor effect and cytotoxic activity toward many cancer cell lines such as liver cancer cells, colon carcinoma cells and fibrosarcoma cells (Li et al., Citation2002; Yan et al., Citation2010). However, little work has done to investigate the effects of OA on gastric cancer cells, and the underlying mechanisms involved. The present study was aimed to determine the OA-induced apoptosis of gastric cancer cell line MKN28 and to elucidate the molecular mechanism involved.
Materials and methods
Reagents
Purified OA (98.79%) was procured from Nanjing Zelang Medical Technological Co., Ltd. (Nanjing, China). Antibodies for P38, P-P38, JNK, P- JNK, ERK, P-ERK, AKT and P-AKT were purchased from Cell Signaling Technology, Inc. (Beverley, MA) and GAPDH from Fermentas (Vilnius, LT). Antibodies for caspase 3, caspase 9 and Apaf-1 were purchased from Abcam (Cambridge, MA) and Apoptotic Cell Hoechst 33258 Staining Kit from JRDUN Biotechnology Co., Ltd (Shanghai, China)
Cell culture
Human MKN-28 cell line was obtained from Shanghai Institute of Cell Biology (Shanghai, China). Cells were cultured in RPMI-1640 medium with 10% FBS (Gibco BRL, Rockville, MD), 100 U/mL penicillin G and 100 μg/mL streptomycin in an incubator (37 °C, 100% humidity and 5% CO2).
Cell viability
The MTT assay was used to assess the effect of OA on cell viability (Hansen et al., Citation1989). In brief, the cells were seeded in 96-well culture plates and treated without or with OA (5, 10, 20, 40, 80 and 160 μg/mL) for 12 and 24 h. Subsequently, the cell viability was evaluated by MTT assay. The absorbance was measured at 490 nm test wavelength and 570 nm reference wavelength with an automated Bio-Rad 550 microtiter plate reader (Rome, Italy).
Measurement of apoptotic cells by flow cytometry
MKN28 cells were collected after treatment with OA (5, 10 and 20 μg/mL) for 24 h and fixed with 75% ethanol overnight at 4 °C, and stained with Annexin V and propidium iodide (PI). Cell apoptosis was evaluated using FAC flow cytometry (San Jose, CA).
DNA fragmentation assay
Following treatment with OA (5, 10 and 20 μg/mL) for 24 h, the cells were harvested and fixed for 5 min in 3% para-formaldehyde in phosphate-buffered saline. After air dying, cells were stained for 10 min with Hoechst 33258 (10 mL), mounted in 50% glycerol containing 20 mmol/L citric acid and 50 mmol/L orthophosphate, and stored at −20 °C before analysis. Nuclear morphology was evaluated using a fluorescence microscope (DMI3000B, Leica, Wetzlar, Germany).
Mitochondrial membrane potential (Δψm) analysis
Mitochondrial Δψ was analyzed using JC-1 mitochondrial membrane potential (Δψm) assay kit (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer’s instructions. In brief, MKN28 cells were incubated with 5, 10 and 20 μg/mL OA for 12 h, then centrifuged at 1200 g for 5 min, and resuspended in RPMI 1640 medium. Following the addition of JC-1 to each well, the cells were incubated for an additional 30 min at 37 °C. After washing with PBS, the stained cells were assayed using a flow cytometer. For image analysis of mitochondrial Δψ, the stained cells were mounted onto microscope slides in mounting medium. Microscopic images were collected using the Laser Scanning Microscope 5 PASCAL program on a confocal microscope.
Western blot
Following treatment with OA at the desired concentrations and time, MKN28 cells were harvested. The expression of apoptosis-related proteins was detected by Western blot hybridization according to the manufacturer’s instructions and previous reports (Zhao et al., Citation2004). The experiment was repeated three times independently.
Fluorescent quantitative reverse transcription-PCR
Total mRNA was isolated from MKN-28 cells using TRIzol Reagent (Gibco-BRL, Gaithersburg, MD) and the approach for fluorescent quantitative reverse transcription-PCR (FQRT-PCR) was described by our previous report (Zhang et al., Citation2012) ().
Table 1. Primers used in RT-PCR analysis.
Statistics
Values were expressed as means ± SD. A one-way analysis of variance (ANOVA) followed by Dunnett’s test was used for statistical analysis. Probability (p) values less than 0.05 were considered significant.
Results
OA inhibits cell viability
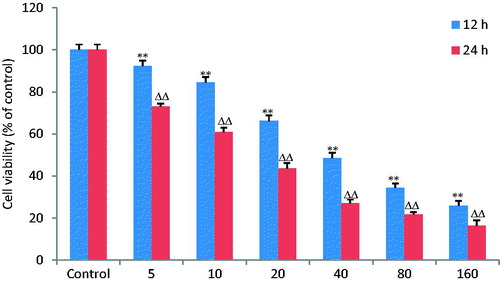
MTT assay was done to determine the cell proliferation of MKN28 cells under the influence of OA. OA (5, 10, 20, 40, 80 and 160 μg/mL) was added to the culture medium for 12 and 24 h. The result showed that OA inhibited MKN28 cell viability in a time- and dose-dependent manner with IC50 of 44.8 and 15.9 μg/mL, respectively ().
Figure 1. OA inhibits viability of gastric cancer cells. MKN28 cells were pretreated with OA at the doses of 5, 10, 20, 40, 80 and 160 μg/mL for 12 and 24 h. The results displayed that OA significantly inhibited MKN28 cell viability in a time- and dose-dependent manner with IC50 of 44.8 and 15.9 μg/mL, respectively. **, ΔΔ p < 0.01 compared with the control group. Data are expressed as the mean ± SD. n = 8.
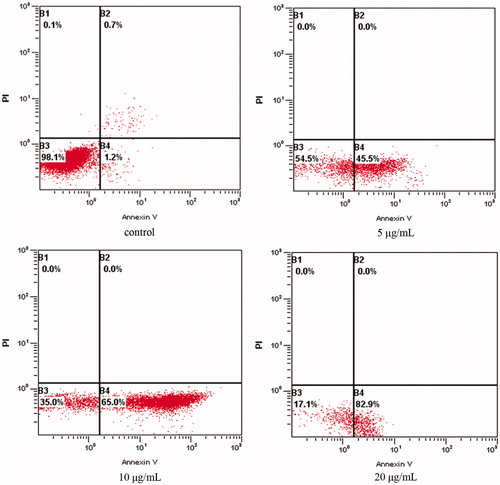
Measurement of apoptotic cells by flow cytometry
The flow cytometry assay was carried out to substantiate cell apoptosis induced by OA treatment under various doses. The number of apoptotic cells was counted as late apoptotic cells shown in the upper right (B2) quadrant and early apoptotic cells as shown in lower right (B4) quadrant of the histograms. As shown in , treatment of OA at the dose of 5, 10 and 20 μg/mL for 24 h significantly increased the number of early apoptotic cells (B4), respectively, from 45.5 ± 2.2% to 82.9 ± 3.2% in a dose-dependent manner in comparison with control cells with that of 1.2 ± 0.1%. The significant induction of apoptosis indicated the anticancer effect of OA against MKN28 cells.
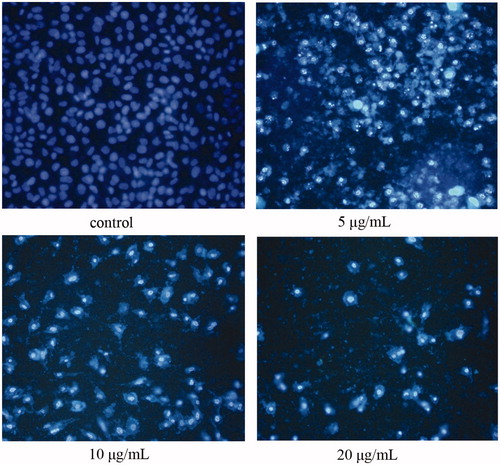
DNA fragmentation assay
OA-induced apoptosis in MKN28 cells was morphologically identified using fluorescence staining with Hoechst 33258, as shown in . Cells were treated with OA () and without OA () for 24 h. Fluorescence microscopy data indicated that OA treatment dose dependently induced apoptosis in MKN28 cells. In these observations, chromosomal condensation and nuclear fragmentation were observed in OA treated cells, as evident in .
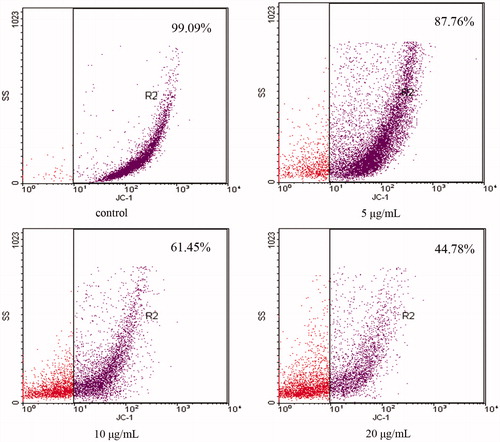
Mitochondrial membrane potential (Δψm) analysis
During the apoptotic process, mitochondrial membrane pores are opened and Δψm was disrupted (Zamzami et al., Citation1996). As shown in , JC-1 staining data showed that the OA-treated cells exhibited a significant decrease in the mitochondrial membrane potential (in right pane) from 87.76 ± 1.65% to 44.78 ± 2.27% (n = 3) in a dose-dependent manner when compared with the control cells with that of 99.09 ± 0.51%.
Figure 4. OA disrupts mitochondrial membrane potential (Δψm). MKN28 cells were incubated with 5, 10 and 20 μg/mL OA for 12 h. For image analysis of Δψm, the JC-1-stained cells were mounted onto microscope slide in mounting medium. OA-treated cells exhibited a significant and dose-dependent decrease in the mitochondrial membrane potential in the right pane when compared with the control group.
Western blot
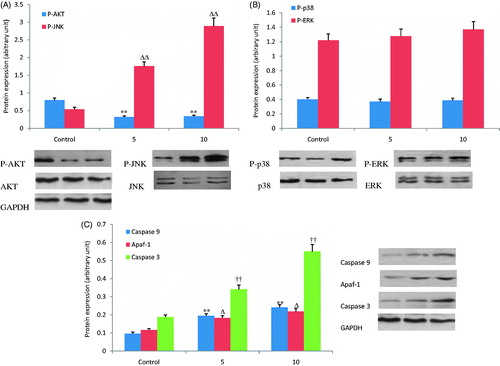
The expression of apoptosis-related proteins and the phosphorylation of kinases was detected by western blot hybridization to clarify the mechanism of MKN28 cell apoptosis induced by OA. As shown in , treatment with OA (5 and 10 μg/mL) for 1 h increased JNK phosphorylation dose dependently and decreased AKT phosphorylation. However, OA treatment did not significantly affect phosphorylation of p38 and ERK proteins (). Treatment with OA (5 and 10 μg/mL) for 12 h also evidently increased the protein expression of caspase 3, caspase 9 and Apaf-1 in a dose-dependent manner compared to the control group ().
Figure 5. Effects of OA on expression of key proteins involved in cell apoptosis. (A) Treatment with OA (5 and 10 μg/mL) for 1 h, JNK phosphorylation increased and AKT phosphorylation decreased dose dependently. (B) The effects of OA on phosphorylation of P38 and ERK were not observed. (C) Treatment with OA (5 and 10 μg/mL) for 12 h increased the expression of caspase 3, caspase 9 and Apaf-1 dose dependently. Δp < 0.05. **, ΔΔ, †† p < 0.01 compared with the control group. Data are expressed as the mean ± SD. n = 3.
Fluorescent quantitative reverse transcription-PCR
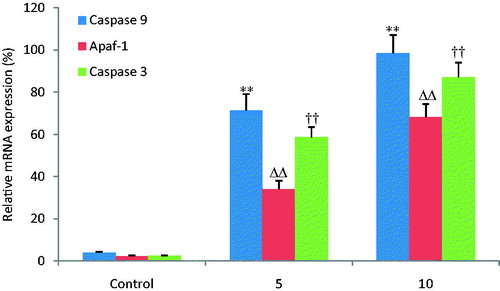
illustrates the gene expression results of caspase 3, caspase 9 and Apaf-1 determined by FQRT-PCR. Activation of caspase 3, caspase 9 and Apaf-1 can evidently induce apoptosis of MKN28 cells. In this study, their mRNA expression levels increased dose dependently in OA treatment groups compared with the control group.
Figure 6. OA increases the relative mRNA expression of caspase -3, -9 and Apaf-1. The mRNA expression of caspase 3, caspase 9 and Apaf-1 was detected by FQRT-PCR. OA increased the mRNA expression levels of caspase 3, caspase 9 and Apaf-1 dose dependently when compared with the control group. **, ΔΔ, †† p < 0.01 compared with the control group. Data are expressed as the mean ± SD. n = 6.
Discussion
OA is a triterpenoid natural product found in a variety of plants, especially abundant in Oleaceae family. OA and its isomer ursolic acid have similar biological activities. In recent years, many reports have suggested that OA and its derivatives exhibit potent antitumor activity against many tumor cell lines such as liver cancer cells, colon carcinoma cells, pancreatic cancer cells and lung cancer cells (Li et al., Citation2002; Lucio et al., Citation2011; Shyu et al., Citation2010; Wei et al., Citation2012). In the present study, we investigated the inhibitory effect of OA on gastric cancer cells MKN28 and elucidated the possible molecular mechanism involved. The results showed that OA inhibited MKN-28 cell proliferation in a time- and dose-dependent manner. The result of flow cytometry analysis by Annexin V/PI staining showed that OA treatment from 5 to 20 μg/mL dose-dependently induced apoptosis and increased DNA fragmentation of MKN28 cells. Our data regarding cell viability, cell apoptosis and DNA fragmentation suggested that OA could penetrate MKN28 cells and destroy the mitochondria membrane integrity, which consequently caused cell apoptosis.
Mitochondria play a crucial role in the complex process of apoptosis (Wang, Citation2001). During this process, mitochondrial membrane pores are opened, resulting in the loss of mitochondrial membrane potential (MMP) (Zamzami et al., Citation1996). The loss of MMP causes an increase in the permeability of the mitochondrial membrane, followed by the release of pro-apoptotic molecules such as cytochrome c. Cytochrome c releasing from mitochondrial interacts with the ATP, Apaf-1 and caspase 9, and subsequently activates caspase 3, which consequently elicits caspase-dependent apoptotic cell death (Yan et al., Citation2010). The western blot and FQRT-PCR analysis results suggest that the protein and mRNA expression levels of caspase 3, caspase 9 and Apaf-1 were increased after treatment with OA.
Accumulating evidence indicates that activation of mitogen activated protein kinases (MAPK) is associated with cell cycle arrest and apoptosis induction. There are three main parallel pathways of MAPK family discovered: ERK, JNK and p38 (Roux & Blenis, Citation2004). In the present study, the phosphorylation levels of ERK, JNK and p38 were detected by western blot; however, only JNK phosphorylation was increased by OA. JNK plays an essential role in activation of the intrinsic apoptotic pathway mediated by mitochondria in response to cellular stress, which facilitates translocation of proapoptotic protein Bax to mitochondria when activated (Davis, Citation2000). AKT is a master regulator involved in transcriptional regulation of the anti-apoptotic protein Bcl-2 which plays a crucial role in prevention of cell death (Ghosh et al., Citation2013). In the present study, AKT phosphorylation was decreased by OA. The data suggested that OA elicited apoptosis of MKN28 cells by regulating phosphorylation levels of JNK and AKT proteins.
In conclusion, OA elicits significant apoptosis in MKN28 cells by the activation of the mitochondrial-dependent apoptosis pathway. Activation of JNK phosphorylation promotes Bax translocation to mitochondria, while inhibition of AKT phosphorylation decreases the combination of Bcl-2 with Bax, resulting in MMP dissipation and cytochrome C release from mitochondria to cytosol. Subsequently, caspase 3 was activated by the apoptosis formed by releasing cytochrome c in combination with Apaf-1, ATP and caspase 9, followed by the death of the target cell.
Declaration of interest
The authors have declared that there is no conflict of interest.
Acknowledgements
The work was financially supported by the Youth Foundation of Shanghai Health Bureau (No. 2008Y038).
References
- Akagi H, Higuchi H, Sumimoto H, et al. (2013). Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric Cancer 16:100–10
- Alvarez ME, Maria AO, Saad JR. (2002). Diuretic activity of Fabiana patagonica in rats. Phytother Res 16:71–3
- Assefa H, Nimrod A, Walker L, Sindelar R. (2001). Enantioselective synthesis and complement inhibitory assay of A/B-ring partial analogues of oleanolic acid. Bioorg Med Chem Lett 11:1619–23
- Davis RJ. (2000). Signal transduction by the JNK group of MAP kinases. Cell 103:239–52
- Fai YM, Tao CC. (2009). A review of presence of oleanolic acid in natural products. Nat Prod Med 2:77–290
- Hansen MB, Nielsen SE, Berg K. (1989). Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119:203–10
- Ismaili H, Tortora S, Sosa S, et al. (2001). Topical anti-inflammatory activity of Thymus willdenowii. J Pharm Pharmacol 53:1645–52
- Li J, Guo WJ, Yang QY. (2002). Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J Gastroenterol 8:493–5
- Li Y, Matsuda H, Yoshikawa M. (1999). Effects of oleanolic acid glycosides on gastrointestinal transit and ileus in mice. Bioorg Med Chem 7:1201–5
- Liu R, Li Z, Bai S, et al. (2009). Mechanism of cancer cell adaptation to metabolic stress: Proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteomics 8:70–85
- Lucio KA, Rocha Gda G, Moncao-Ribeiro LC, et al. (2011). Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS One 6:e28596
- Ma C, Nakamura N, Hattori M, et al. (2000). Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. J Nat Prod 63:238–42
- Marquina S, Maldonado N, Luisa Garduño-RamíRez MA, et al. (2001). Bioactive oleanolic acid saponins and other constituents from the roots of Viguiera decurrens. Phytochemistry 56:93–7
- Middleton G, Cunningham D. (1995). Current options in the management of gastrointestinal cancer. Ann Oncol 6:S17–26
- Pollier J, Goossens A. (2012). Oleanolic acid. Phytochemistry 77:10–15
- Ghosh S, Bishayee K, Paul A, et al. (2013). Homeopathic mother tincture of Phytolacca decandra induces apoptosis in skin melanoma cells by activating caspase-mediated signaling via reactive oxygen species elevation. J Integr Med 11:116–24
- Roux PP, Blenis J. (2004). ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–44
- Shyu MH, Kao TC, Yen GC. (2010). Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J Agric Food Chem 58:6110–18
- Sporn MB, Liby KT, Yore MM, et al. (2011). New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 74:537–45
- Tang HQ, Hu J, Yang L, Tan RX. (2000). Terpenoids and flavonoids from Artemisia species. Planta Med 66:391–3
- Wang X. (2001). The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–33
- Wang X, Bai H, Zhang X, et al. (2013). Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 34:1323–30
- Wei J, Liu H, Liu M, et al. (2012). Oleanolic acid potentiates the antitumor activity of 5-fluorouracil in pancreatic cancer cells. Oncol Rep 28:1339–45
- Yan SL, Huang CY, Wu ST, Yin MC. (2010). Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol In Vitro 24:842–8
- Yim T, Wu W, Pak W, Ko K. (2001). Hepatoprotective action of an oleanolic acid enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother Res 15:589–92
- Zamzami N, Marchetti P, Castedo M, et al. (1996). Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett 384:53–7
- Zhang H, Zhang Y, Jiang YP, et al. (2012). Curative effects of oleanolic acid on formed hypertrophic scars in the rabbit ear model. Evid Based Complement Alternat Med 2012:837581
- Zhao Y, Yang LF, Ye M, et al. (2004). Induction of apoptosis by epigallocatechin-3-gallate via mitochondrial signal transduction pathway. Prev Med 39:1172–9