Abstract
Context: Bergenia ciliata Haw. (Saxifragaceae) is widely used in traditional medicines for renal disorders including kidney stones, inflammation and also well known for its antioxidant activity. Use of traditional herbs proved to be an important strategy for the management of kidney stones by modulating the oxidative stress imposed by calcium oxalate nephrolithiasis.
Objectives: To evaluate the antinephrolithiatic and antioxidative activity of B. ciliata rhizomes as a preventive agent on ethylene glycol (EG)-induced nephrolithiasis.
Materials and methods: The hydro-methanol extract (30:70, v/v) of B. ciliata rhizomes was orally administrated simultaneously at a dose of 150 and 300 mg/kg body weight/day, to adult female Wistar rats for 28 d along with EG (0.75%, v/v) in drinking water. The results were compared to a parallel study conducted with marketed polyherbal drug cystone under identical dosage conditions. The biochemical parameters were measured in urine, serum and kidney followed by histochemistry. A validated HPLC method was used for standardization using gallic acid as a marker.
Results: EG caused a significant increase in calcium, oxalate and phosphate levels in urine and kidney and concurrent decrease in calcium, sodium and magnesium in serum (p < 0.001). EG also caused an increase in lipid peroxidation and a decrease in activities of antioxidative enzymes in kidney. Co-treatment with B. ciliata rhizomes extract caused restoration of all these parameters (p < 0.001). Histochemical studies showed reduced calcifications with extract treatment.
Conclusion: B. ciliata has a significant prophylactic effect in preventing the nephrolithiasis, which might be mediated through antioxidant activity of these active compounds.
Introduction
The prevalence and incidence of urolithiasis is reported to be increasing across the world and calcium oxalate (CaC2O4) is the predominant component of most stones followed by uric acid, struvite, cystine and other stones (Trinchieri, Citation2006). The prevalence rate of kidney stones was 8.8% in the United States (Scales et al., Citation2012), while in Brazil, nearly 7% of hospitalizations were associated with urolithiasis in 2004 (Barros et al., Citation2006). In India, Pendse and Singh (Citation1986) had reported a high and progressively increasing incidence of urolithiasis in Udaipur and some other parts of Rajasthan in the western part of India, Gujarat, and in Manipur also.
The medical management of urolithiasis, today, includes lithotripsy and surgical procedures and that too depends on the size and location of stones. Unfortunately, the propensity for stone recurrence is not altered by removal of stones with extracorporeal shock wave lithotripsy (ESWL) and its recurrence is still about 50% (Butterweck & Khan, Citation2009). In addition, ESWL might show some significant side effects such as renal damage, induced hypertension or renal impairment. The underlying risk factors are unfortunately not corrected by these techniques; there is a need to continue the medical supervision and therapy to prevent stone recurrence.
In this regard, herbal sources have become a vital area in the search for new drugs, because their parts as such, extract and compounds isolated from them have documented a variety of biological activities. Oxidative stress imposed by reactive oxygen species (ROS) plays a crucial role in the pathophysiology associated with kidney stone disease. Oxalate increases the production of free radicals, which can induce a cell death process, crystal deposition in the renal tubules, which further leads to growth of calcium oxalate stones. The human, animal, explants and in vitro studies indicate that raised oxidative stress is often present in urolithiasis and oxalate promotes oxidative stress, which is substantially retarded by antioxidants (Kubo et al., Citation1997). A well-known antioxidant, vitamin E along with mannitol, has been reported to prevent the deposition of CaC2O4 crystals in the kidneys of rats injected with sodium oxalate (Thamilselvan & Menon, Citation2005).
Therefore, treatment with natural antioxidants could be a suitable way for mitigating the hyperoxaluria induced oxidative stress and renal injury. Chemical principles from natural sources have become much simpler and have contributed significantly to the development of new drugs from medicinal plants (Butterweck & Khan, Citation2009).
Bergenia ciliata (Haw) sternb., commonly known as Pasanbheda in folk medicine, is a perennial herbaceous plant of family Saxifragaceae. Tea prepared from this plant is used to cure kidney stones (Pant et al., Citation2009). Medicinal values of this plant in the treatment of a large number of human ailments including fever, cough and pulmonary infections have been reported (Islam et al., Citation2002). The rhizomes have been used for centuries in herbal formulation for dissolving of kidney and bladder stones and for treatment of leucorrhea, piles and pulmonary infections. The rhizomes also reported to have antibacterial (Islam et al., Citation2002), hypoglycemic (Islam et al., Citation2002), antioxidant (Rajkumar et al., Citation2010), and anti-inflammatory activities (Islam et al., Citation2002). The roots are used as decoction by boiling in water and addition of table salt by human adults for the treatment of asthma (Singh, Citation1995).
The major chemical constituents reported from B. ciliata are gallic acid, bergenin, (+)-afzelechin (Reddy et al., Citation1999), 11-O-galloyl bergenin (Vaishali et al., Citation2008), pashaanolactone (Reddy et al., Citation1999), β-sitosterol (Reddy et al., Citation1999) and β-sitosterol-d-glucoside (Reddy et al., Citation1999).
Cystone is a marketed composite herbomineral formulation specifically developed for the management of urolithiasis or renal calculi. This formulation has been approved by regulatory authorities in India as an Ayurvedic formulation and is available for clinical practice for the past 60 years (Mohanty et al., Citation2010). Therefore, we have compared our extract with cystone at same dose levels.
In the present study, an effort has been made to establish the scientific validity for the preventive effect of B. ciliata rhizome extract in experimentally induced calcium oxalate nephrolithiasis and hyperoxaluric oxidative stress in rat kidney.
Materials and methods
Plant material and extraction
The plant material was collected from Uttarakhand district, India, and was taxonomically identified and authenticated as B. ciliata rhizomes by Dr. V. P. Bhatt, Scientist, Herbal Research & Development Institute, Uttarakhand, India (Ref. no. 414/HRDI/114/2012-13). The hydro-methanol extract of B. ciliata rhizomes was prepared so that it can extract both polar and non-polar active components present in the rhizomes. The rhizomes of B. ciliata were thoroughly cleaned, dried under shade and coarsely powdered and the extract was prepared according to the WHO protocol CG-06. Briefly, 5 g of powder and 100 mL of methanol:water (70:30, v/v) were mixed and stirred on a magnetic stirrer and then filtered twice with Whatman filter paper No. 1. After evaporation of the solvent, the crude extract was dried under vacuum and stored in airtight container at 4 °C. The yield of dry hydro-methanol extract was 8.5% (w/w). The dried extract of B. ciliata (BCE) was dissolved in Milli-Q water (Waters, Milford, MA) and used for further study.
Preliminary phytochemical screening
Qualitative analysis for determining the presence of major active compounds in the extract was carried out using standard methods (Sofowara, Citation2009).
Standardization of extract
The 70% hydro-alcoholic extract of B. ciliata rhizomes was standardized by using gallic acid as a standard. For reverse-phase separation of gallic acid from other constituents, a Spectra-Physics HPLC-UV system (San Jose, CA) with Spectra system P1000 pump, a Spectra system UV1000 detector and a Rheodyne 7125 injector with 20 µL loop was used. Chromatographic separation was achieved on a COSMOSIL C18 column (250 mm × 4.6 mm, length × inner diameter with 5 µm particle size), maintained at 35 °C in a column oven. The mobile phase consisted of deionized water:acetonitrile:acetic acid (88:10:2, v/v/v). For isocratic elution, the flow rate of the mobile phase was kept at 1.0 mL/min and the wavelength of detector was set at 280 nm.
Standard stock, calibration standards and quality control sample preparation
The standard stock solution of gallic acid (1 mg/mL) was prepared by dissolving the requisite amount in methanol. Calibration standards (CS, 1–9) were made at 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, 100, 150 and 200 µg/mL concentrations, while quality control samples were prepared at three levels, namely 175 µg/mL (HQC, high-quality control), 50 µg/mL (MQC, medium-quality control) and 5.50 µg/mL (LQC, low-quality control) in methanol.
Validation procedures for HPLC analysis
System suitability experiment was performed by injecting six consecutive injections using MQC (50.0 µg/mL) sample at the start of each batch during method validation. System performance was studied by injecting one CS-1 sample at the beginning of each analytical batch and before re-injecting any sample during method validation. The carryover effect of the autosampler was evaluated by sequentially injecting solutions of standard gallic acid equivalent to highest standard (CS-9) in the calibration range and the mobile phase. The linearity of the method was determined by analysis of five linearity curves containing nine non-zero concentrations. Standard plots were drawn by plotting mean peak area against the amount injected. Each calibration curve was analyzed individually by using least square linear regression. Precision (% coefficient of variation) was examined by performing the intra- and inter-day analysis of three replicates at LQC, MQC and HQC levels. Intra-day assay was performed with an interval of 3 h in 1 d, while inter-day precision was evaluated over five consecutive days. Accuracy of the method was checked from the recovery studies by the standard addition method. Known amounts of standard gallic acid (80, 100 and 120%) were added to pre-analyzed powdered extract material and the amounts were calculated from the standard calibration curve.
Experimental animals
Healthy female rats of Wistar strain weighing between 200 and 250 g of equivalent age groups were obtained from Torrent Research Centre, Ahmedabad, India. They were acclimatized for 15 d in polypropylene cages under controlled conditions (temperature 25 ± 2 °C; relative humidity 50–55%; 12 h light/dark cycle) in the Animal House of Zoology Department, Gujarat University, Ahmedabad, India. Animals were maintained on certified pelleted rodent feed supplied by Amrut Feeds, Pranav Agro Industries Limited, Pune, India and water ad libitum. The experimental procedures were approved by “The Committee for the Purpose of Control and Supervision of Experiment on Animals” (Reg – 167/1999/CPCSEA), New Delhi, India. Ethylene glycol (EG) induced hyperoxaluria model was used to assess the antinephrolithiatic activity in Wistar rats. The dose of EG was determined by the pilot study with three different doses of EG, namely 0.4, 0.75 and 1%, in drinking water in female rats. Nephrolithiasis was finally induced by 0.75% EG in drinking water for 28 d (Tsai et al., Citation2008).
Experimental design and treatment schedule
The doses of BCE were determined by the pilot study and calculated from the lethal dose (LD50) of the plant extract. The acute minimum LD50 was found to be 3000 mg/kg body weight for the extract. Therefore, 1/10th and 1/20th dosage of LD50, which was found to be 150 and 300 mg/kg body weight/day, respectively, were selected for the study. For the treatment of marketed polyherbal drug, cystone was maintained at the same dose levels as that of extract to compare the potency of the extract at identical doses. In the experiment, a total of 40 rats were randomly divided into eight groups containing five animals in each group. Group I served as untreated control (without EG or herbal treatment). Animals of groups II and III were given 300 mg/kg body weight/day of cystone and BCE, respectively, without EG to determine any lethality associated with extract and polyherbal drug, cystone treatment, served as cystone and plant controls. EG (0.75%, v/v) in drinking water was administered to animals of groups IV–VIII for induction of renal calculi till 28th day. Animals of groups V and VI were given polyherbal drug, cystone (150 and 300 mg/kg body weight/day), simultaneously for 28 d along with EG. Similarly, groups VII and VIII received BCE (150 and 300 mg/kg body weight/day) simultaneously along with EG for 28 d and served as a preventive regimen. Cystone and extract were given once daily by the oral route.
Collection and analysis of urine
The 24 h urine samples were collected on early morning on 14th and 28th day of calculi induction treatment and analyzed microscopically for the presence of CaC2O4 crystals and biochemically for calcium (Ballentine et al., Citation1957), oxalate (Hodgkinson, Citation1970), phosphate (Fiske & Subbarow, Citation1925), magnesium (Neill & Neely, Citation1956) and total protein (Lowry et al., Citation1951) on 14th and 28th day of treatment.
Serum analysis
Blood was collected from the retro-orbital sinus under mild ether anesthetic conditions on the 14th and 28th day of treatment and serum was separated by centrifugation at 10 000 × g for 10 min and analyzed for calcium (Ballentine et al., Citation1957), phosphate (Fiske & Subbarow, Citation1925), magnesium (Neill & Neely, Citation1956) and electrolytes (sodium and potassium) by flame photometry.
Kidney analysis
At the end of the 28th day of treatment after the experimental protocol, animals were sacrificed under ether anesthesia. The kidneys were homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4) for assaying tissue calcium (Ballentine et al., Citation1957), oxalate (Hodgkinson, Citation1970), phosphate (Fiske & Subbarow, Citation1925), lipid peroxidation (LPO) (Ohkawa et al., Citation1979), glutathione content (GSH) (Moron et al., Citation1979), total ascorbic acid (TAA) (Roe & Kuether, Citation1943), catalase (CAT) (Sinha, Citation1972), superoxide dismutase (SOD) (Kakkar, Citation1984), glutathione reductase (GR) (Moron et al., Citation1979) and glutathione peroxidase (GPx) (Rotruck et al., Citation1973).
von Kossa staining
The von Kossa (Citation1901) method specifically detects calcium deposits. The kidney sections were fixed in 10% buffered neutral formalin and then dehydrated by passing through ascending grades of alcohol, cleared in xylene, embedded in paraffin wax, sectioned and stained with silver nitrate solution and treated with sodium thiosulfate solution for 2 min. Then the sections were rinsed briefly in water, counterstained in nuclear fast red solution for 5 min, blotted, cleared in xylene and mounted in DPX. They were then examined under a light microscope.
Pizzolato staining
Renal specimens of rats treated with EG and 300 mg/kg body weight of cystone/extract were also fixed in 10% buffered neutral formalin, and embedded in paraffin and were stained with the Pizzolato (Citation1964) staining method to detect oxalate-containing crystals.
Statistical analysis
The results were expressed as mean ± SEM. Statistical analysis was performed using GraphPad Instat, software, version 3.0 (GraphPad software Inc., La Jolla, CA). The values were analyzed by a one way analysis of variance (ANOVA) followed by Tukey–Kramer’s multiple comparison test.
Results
Phytochemical analysis
Phytochemical analysis indicated the presence of tannins, saponins, flavonoids and alkaloids in the hydro-methanol extract of B. ciliata rhizomes.
Standardization of extract of B. ciliata (BCE)
Gallic acid was used as a marker for standardizing the BCE. The higher anti-nephrolithiatic effect of BCE can be related to the presence of gallic acid as evident from the HPLC studies for plant extracts and standard gallic acid sample. The chromatogram of extract in methanol: water (70:30, v/v) showed gallic acid as the major component of BCE, corresponding to 70% based on peak area (). The retention time of gallic acid (in BCE) on the reversed phase HPLC column was 2.77 min was comparable with that observed for standard gallic acid sample (2.78 min). During the method validation, the precision (% CV) of system suitability test was observed in the range of 0.05–0.12% for the retention time and 0.09–1.1% for the area response of gallic acid. The signal-to-noise ratio for system performance was ≥80 for gallic acid. Carry-over evaluation was performed in each analytical run to ensure that it does not affect the accuracy and the precision of the proposed method. There was negligible carry-over (less than 0.4% of CS-1) observed during autosampler carryover experiment. All five calibration curves were linear over the concentration range of 2.0–200 µg/mL (). A straight-line fit was made through the data points by least square regression analysis to give the mean linear equation y = 18 977x − 167.8, with correlation coefficient (r2) of 0.9997. The standard deviation value for slope, intercept and correlation coefficient observed was 0.010, 0.127 and 0.0006, respectively. The accuracy and precision (% CV) observed for the calibration curve standards ranged from 98.7 to 100.4% and 0.2 to 1.5, respectively. The intra-day and inter-day precision and accuracy were established from validation runs performed at HQC, MQC and LQC levels (). The intra-day precision (% CV) ranged from 0.9 to 1.2% and the accuracy was within 98.6 to 99.5%. For the inter-day experiments, the precision varied from 1.1 to 1.4 and the accuracy was within 100.2 to 100.4%. The recovery of extracted samples ranged from 99.2 to 100.1% for all the three samples with % CV values less than 0.6 ().
Figure 1. (A) HPLC chromatographic profiles of standard gallic acid sample. (B) HPLC chromatographic profiles of hydro-methanol extract of Bergenia ciliata rhizomes.
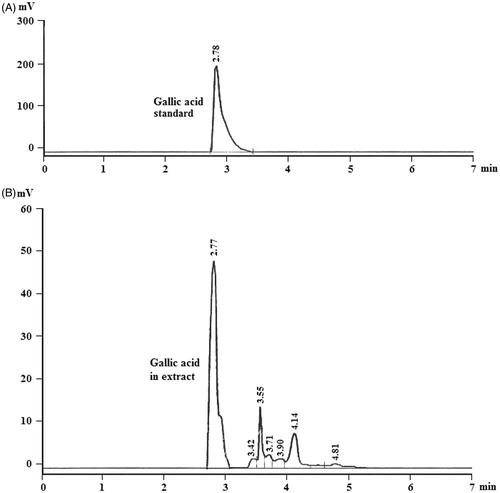
Table 1. Mean peak areas obtained for gallic acid from five calibration curves containing nine calibration standards (CS, 1–9).
Table 2. Intra-day and inter-day precision and accuracy for gallic acid.
Table 3. Recovery results of gallic acid added to pre-analyzed samples (n = 3).
Urine analysis
Microscopic observation revealed that urine of untreated control rats was devoid of any CaC2O4 crystal (). revealed the presence of CaC2O4 crystals in urine of EG induced nephrolithiatic rats whereas oral administration of BCE showed significantly less number of CaC2O4 crystals with reduced size at both the dose levels () on 28th day of treatment. However, numerous crystals were seen in urine of cystone-treated rats at both the dose levels (). Moreover, EG treatment caused a significant increase in calcium (87% on 14th and 125% on 28th day), oxalate (77% on 14th and 244% on 28th day) and phosphate (301% on 14th day and 650% on 28th day) excretion levels with a significant reduction in excretion of magnesium (42% on 14th day and 77% on 28th day). However, simultaneous treatment with BCE at both dose levels significantly prevented these changes (). Moreover, the protection observed by BCE was found to be higher than the cystone treatment at the same dose levels.
Figure 2. Light microscopic images of calcium oxalate crystals in urine. (A) Control, (B) nephrolithic, (C) prophylactic treatment with cystone (150 mg/kg), (D) prophylactic treatment with cystone (300 mg/kg), (E) prophylactic treatment with extract of B. ciliata (150 mg/kg), and (F) prophylactic treatment with extract of B. ciliata (300 mg/kg).
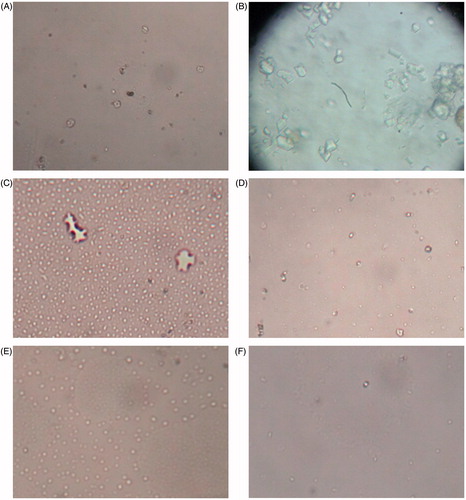
Table 4. Prophylactic effect of oral administration of B. ciliata on biochemical parameters in urine.
Serum analysis
depicts the EG induced changes in calcium, phosphate, sodium, potassium and magnesium levels in serum. The treatment of EG caused significant reduction in serum calcium, magnesium and sodium levels by 41%, 46% and 14% on 14th day and 68%, 72% and 21% on 28th day, respectively. Whereas serum levels of potassium and phosphate were increased by 46% and 74% on 14th day and 86% and 135% on 28th day, respectively, due to EG treatment in group IV (p < 0.001). A simultaneous administration with BCE significantly restored these serum parameters ().
Table 5. Prophylactic effect of oral administration of B. ciliata on biochemical parameters of serum.
Kidney analysis
EG treatment caused a significant increase in calcium (266%), oxalate (191%) and phosphate (122%) retention in kidney of rats, which were reversed in the animals receiving a simultaneous treatment with BCE ().
Table 6. Prophylactic effect of oral administration of B. ciliata on biochemical parameters in kidney of rats.
EG treatment enhanced LPO (220%) and total protein content (41%) and reduced the activities of enzymatic antioxidants, namely CAT (63%), SOD (72%), GPx (70%) and GR (61%) as well as in non-enzymatic antioxidants, namely GSH (63%) and TAA (83%) contents in kidney as compared to untreated control rats (p < 0.001; group I; ). However, the oral administration of BCE protected against the oxidative changes induced by EG treatment.
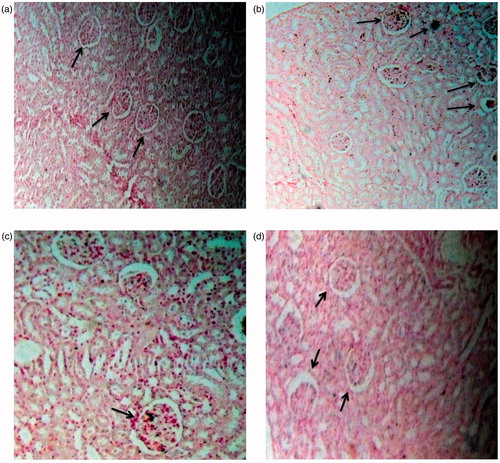
The von Kossa stained renal sections of untreated control rats showed no calcifications (). However, a significantly increased number of calcium deposits were found attached to the renal tubules in EG-treated rat kidneys (). The rats treated with hydro-methanol extract of BCE along with EG showed only mild calcifications in glomeruli and normal tubular structures (), whereas cystone treatment showed moderate calcifications in glomerulo-tubular structures which were comparatively more than that found in the kidney of rats treated with the extract ().
Figure 3. Light microscopic architecture and calcification in the kidney section with von Kossa stained: ×100 of (A) control, (B) nephrolithic, (C) prophylactic treatment with cystone (300 mg/kg), and (D) prophylactic treatment with extract of B. ciliata (300 mg/kg).
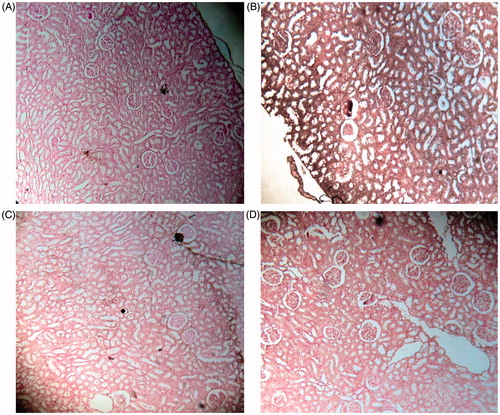
The Pizzolato staining method revealed black-stained CaC2O4 deposits in the glomeruli and tubular region of the EG induced nephrolithiatic rats (), whereas the simultaneous administration with BCE along with EG showed only few stray areas of calcification in glomeruli and normal tubular structures (). The administration of polyherbal drug, cystone, showed mild calcifications (). No crystal deposits were seen in the kidney of untreated control rats ().
Discussion
The medical management of urolithiasis has undergone a great change in the modern era. In the present time, the management of urolithiasis includes not only stone removal but also prevention of recurrence. The current market potential of herbal medicine is estimated to be about $80–250 billion in Europe and the USA (Meena et al., Citation2009). The indispensible medicinal properties of different plants are due to the presence of several chemical entities such as saponins, tannins, alkaloids, alkenyl phenols, flavonoids, sesquiterpenes lactones, terpenoids and phorbol esters (Meena et al., Citation2009). In this regard, many traditional medicinal plants such as Kampo medicine from Japanese traditional herbal system, Takusya (Alismatis rhizome Sam.) and Wullingsan with a combination of dried herbs including Alisma orientalis Sam. (which provide the Alisma Rhizoma or Takusya), Polyporus umbellatus Pers., Poria cocos (Schw.) Wolf and Cinnamomon cassia (L.) Presl., Jin Qian Cao (Desmodium styracifolium Merr.) are reported to be beneficial for the treatment of urolithiasis worldwide (Miyaoka & Monga, Citation2009). Therefore, it is worthwhile to investigate traditional herbs for alternative and complementary medicine.
Renal CaC2O4 deposition induced by EG is associated with proximal tubule cell necrosis leading to production of several metabolites (glycolaldehyde, glycolate, glyoxylate and oxalate, in order) and accumulation of large CaC2O4 crystals in tubular lumen (Tsai et al., Citation2008). However, it has been proven that oral intake of EG in rats caused significant hyperoxaluria with CaC2O4 crystalluria rather than the formation of papillary plaques or stones (Chen et al., Citation2011). As traditional medicines are usually taken by the oral route, the same route of administration was followed in our study for the evaluation of preventive effect of the B. ciliata rhizomes against EG induced nephrolithiasis in rats. The results of many epidemiological studies have stated that males are more prone to CaC2O4 urolithiasis than females although they are found in both sexes. In South East Asia including many regions of India, the scenario is, however, a little different from the rest of the studies. Reports showed that females are more prone to renal stones, whereas males are more prone to uretral, vesical and urethral stones (Singh et al., Citation1978). Therefore, the present study was aimed to investigate the effect of our rhizome extract directly on females. Thus, the gender-based differences in the mechanism of action of these traditional plant extracts can also be resolved, if any. In a recent study, phenolic compound isolated from the leaves of B. ciliata were shown to be effective in dissolving calcium oxalate and calcium phosphate stones (Byahatti et al., Citation2010). In our previous study, EG caused disrupted renal parenchyma, degenerative changes in glomeruli and focal calcification in glomerulo-tubular structures, whereas animals exposed to BCE reduced these changes and normalized the renal architecture (Saha & Verma, Citation2011). Therefore, we have further evaluated the medicinal effect of B. ciliata rhizomes on CaC2O4 deposition in rats using this model.
In the present study, BCE administration led to a significant reduction in CaC2O4 crystal formation. Increased urinary calcium is a factor favoring the nucleation and precipitation of CaC2O4 from urine and subsequent crystal growth (Selvam et al., Citation2001). In the view of increased excretion of phosphorus, initially calcium phosphate precipitates in basement membrane of loop of Henle and protrude to the uroepithelium, forming a nidus which provides a platform for CaC2O4 crystal attachment and more centers for nucleation of new crystals. Oxalic acid, a metabolite of EG, chelates serum calcium and precipitates as crystals in renal tubules thereby causing depletion of serum calcium level (Scalley et al., Citation2002). The decrease in oxalate excretion due to extract might be either due to inhibition of formation of oxalate or interference with the oxalate metabolism. Oxalate excretion has been reported to decrease with the administration of Aerva lanata Juss., in EG fed rats, by decreasing the activities of oxalate synthesizing enzymes such as glycolic acid oxidase in liver and lactate dehydrogenase in liver and kidney (Soundararajan et al., Citation2006). Many studies showed stone formation in EG fed animals due to hyperoxaluria and subsequent hypercalciuria, which further resulted in increased renal retention and excretion of oxalate (Verma et al., Citation2009). Hyponatremia results when water intake exceeds the water excretion. EG has also been reported to increase the water intake and subsequent hyponatremia that was significantly reversed by B. ciliata (Bashir & Gilani, Citation2011). In the present investigation, increase in serum potassium level could be due to the fact that EG caused acidosis followed by hyperkalemia because of shift of potassium from the intracellular to the extracellular compartment. Similar hyperkalemia due to EG administration has been reported earlier (Perez et al., Citation1981). However, these changes were significantly restored in extract treated rats, probably due to the presence of polyphenolic compounds such as gallic acid.
The deposition of the crystalline components in the renal tissue, namely oxalate, phosphate and calcium, were significantly increased in the EG-treated group. The EG treatment caused increase in oxalate production by way of increasing substrate availability that induces the activity of oxalate-synthesizing enzyme. Glycolic acid oxidase and lactate dehydrogenase catalyse the oxidation and reduction of glyoxalate results in the formation of glycolate and oxalate (Soundararajan et al., Citation2006). These changes facilitate the hyperoxaluria and subsequent CaC2O4 crystal adherence and retention in renal tubules (Khan, Citation2005).
It has already been proven that EG-induced hyperoxaluria caused LPO with a concomitant decrease in activities of antioxidant enzymes, namely SOD and CAT (Celik & Suzek, Citation2007). It was reported that hyperoxaluria enhances the production of free radicals with subsequent LPO in the kidneys, leading to renal tubular epithelial cell injury and calcium oxalate stone formation (Moriyama et al., Citation2009). Oxidative tissue damage caused by ROS results in structural alteration of membrane with the release of cell and organelle contents, loss of essential fatty acids with the formation of cytosolic aldehyde and peroxide products (Kato et al., Citation2007). These generated toxic responses in renal epithelial cells, including altered membrane surface properties, changes in gene expression (NF-κB), disruption of mitochondrial function and formation of ROS (Jonassen et al., Citation2005). Mitochondria are a major site of ROS formation and oxalate-induced activation of NADPH oxidase is another source of ROS in renal cells. Animal model studies have provided evidence for the hyperoxaluria-induced activation of the renin–angiotensin system (RAS) and angiotensin II; implicated in causing oxidative stress by activating membrane associated NADPH oxidase, which leads to the production of ROS (Khan, Citation2005). Reduction of angiotensin II production by inhibiting angiotensin-converting enzymes (ACE) or blocking angiotensin receptors has been shown to significantly reduce renal CaC2O4 crystal deposition as well as the development of interstitial inflammation (Toblli et al., Citation2002). Actis et al. (2006) have attributed the inhibition in ACE activity by isolated polyphenols including gallic acid, chlorogenic acid, catechin and epicatechin in rat kidney membranes. The inhibition of the LPO after post-treatment of rhizome extract might be due to the fact that extract scavenge the ROS and indirectly inhibit the phospholipase A2 through inactivation of NF-κB. Gallic acid, an important constituent of the B. ciliata, is accredited with NF-κB inactivation activity (Ho et al., 2010). Polyphenols and flavonoids have been reported to attenuate hyperoxaluria-induced oxidative stress and subsequent CaC2O4 stone formation (Selvam et al., Citation2001).
Normal urine contains many inorganic and organic inhibitors of crystallization, magnesium is one such inhibitor. Magnesium complexes with oxalate and reduces the supersaturation of calcium oxalate by reducing the saturation of CaC2O4 and as a consequence reduces the growth and nucleation rate of CaC2O4 crystals (Gershoff & Andrus, Citation1962). Moreover, oral intake of magnesium has been reported to decrease the oxalate absorption and urinary excretion, in a manner similar to calcium by binding to oxalate in the gut (Gershoff & Andrus, Citation1962). Diets high in magnesium have been found to protect against deposition of CaC2O4 in the kidneys of vitamin B6 deficient rats (Gershoff & Andrus, Citation1962).
In conclusion, administration of the hydro-methanol extract of B. ciliata rhizomes along with EG reduced and prevented the growth of nephrolithiasis, supporting folk information regarding antinephrolithiatic activity of the plant which might be due to the various phytochemical constituents present in the plant. As chromatographic studies showed the presence of gallic acid as major active component present in the extract, therefore, it may be responsible for the antinephrolithiatic activity of the extract, thus further work on the purification of isolated bioactive component can reveal the potential of the rhizome extract. The mechanism underlying this effect is apparently related to lowering of urinary concentrations of stone-forming constituents.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The financial assistance to Sarmistha Saha from Lady Tata Memorial Trust in the form of Senior Research Fellowship (LTMT/AD/Q2/2011-2013) is highly acknowledged with thanks. The authors are thankful to Dr. V. P. Bhatt, Herbal Research & Development Institute, Uttarakhand, India, and Mr. Sanjay Parikh from Advanced Analytical Research Institute for HPLC analysis.
References
- Actis LG, Ottaviani JI, Fraga CG. (2006). Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem 54:229–34
- Ballentine R, Burford DD, Colowick SP, Kaplan NO. (1957). Methods in Enzymology. New York, America: Academic Press
- Barros ME, Lima R, Mercuri LP, et al. (2006). Effect of extract of Phyllanthus niruri on crystal deposition in experimental urolithiasis. Urol Res 34:351–7
- Bashir S, Gilani AH. (2011). Antiurolithiatic effect of berberine is mediated through multiple pathways. Eur J Pharmacol 651:168–75
- Butterweck V, Khan SR. (2009). Herbal medicines in the management of urolithiasis: Alternative or complementary? Planta Med 75:1095–103
- Byahatti VV, Pai KV, D'Souza MG. (2010). Effect of phenolic compounds from Bergenia ciliata (Haw.) Sternb. leaves on experimental kidney stones. Anc Sci Life 30:14–17
- Celik I, Suzek H. (2007). Effects of subacute treatment of ethylene glycol on serum marker enzymes and erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Chem Biol Interact 167:145–52
- Chen YH, Liu HP, Chen HY, et al. (2011). Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: A Drosophila model for nephrolithiasis/urolithiasis. Kidney Int 80:369–77
- Fiske CH, Subbarow Y. (1925). The colorimetric determination of phosphorus. J Biol Chem 66:375–81
- Gershoff SN, Andrus SB. (1962). Effect of vitamin B6 and magnesium on renal deposition of calcium oxalate induced by ethylene glycol administration. Proc Soc Exp Biol Med 109:99–102
- Hodgkinson A. (1970). Determination of oxalic acid in biological material. Clin Chem 16:547–57
- Ho HH, Chang CS, Ho WC, et al. (2010). Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappa B activity and down regulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol 48:2508–16
- Islam M, Iqbal A, Khan U, et al. (2002). Bioactivity evaluation of Bergenia Ciliata. Pak J Pharm Sci 15:15–33
- Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M. (2005). Oxalate toxicity in renal cells. Urol Res 33:329–39
- Kakkar P, Das B, Viswanathan PN. (1984). A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21:130–2
- Kato Y, Miura E, Matsushima R, Sakamoto W. (2007). White leaf sectors in yellow variegated are formed by viable cells with undifferentiated plastids. Plant Physiol 144:952–60
- Khan SR. (2005). Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33:349–57
- Kubo K, Saito M, Tadocoro T, Maekawa A. (1997). Changes in susceptibility of tissues to lipid peroxidation after ingestion of various levels of docosahexanoic acid and vitamin E. Br J Nutr 78:655–69
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with folin-phenol reagent. J Biol Chem 193:265–75
- Meena AK, Bansal P, Kumar S. (2009). Plants-herbal wealth as a potential source of Ayurvedic drugs. J Trad Med 4:152–70
- Miyaoka R, Monga M. (2009). Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol 35:396–405
- Mohanty NK, Nayak RL, Patki PS. (2010). Safety and efficacy of an Ayurvedic formulation cystone in management of ureteric calculi: A prospective randomized placebo controlled study. Am J Pharm Toxicol 5:58–64
- Moriyama MT, Suga K, Miyazawa K, et al. (2009). Inhibitions of urinary oxidative stress and renal calcium level by an extract of Quercus salicina Blume/Quercus stenophylla Makino in a rat calcium oxalate urolithiasis model. Int J Urol 16:397–401
- Moron MS, Depierre JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta 582:67–78
- Neill DW, Neely RA. (1956). The estimation of magnesium in serum using titan yellow. J Clin Pathol 9:162–3
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Pant S, Samant SS, Arya SC. (2009). Diversity and indigenous household remedies of the inhabitants surrounding Mornaula reserve forest in West Himalaya. Ind J Trad Knowledge 8:606–10
- Pendse AK, Singh PP. (1986). The etiology of urolithiasis in Udaipur (Western Part of India). Urol Res 14:59–62
- Perez GO, Oster JR, Vaamonde CA. (1981). Serum potassium concentration in acidemic states. Nephron 27:233–43
- Pizzolato P. (1964). Histochemical recognition of calcium oxalate. J Histochem Cytochem 12:333–6
- Rajkumar V, Guha G, Kumar AR, Mathew L. (2010). Evaluation of antioxidant activities of Bergenia ciliata rhizome. Records Nat Prod 4:38–48
- Reddy UDC, Chawla AS, Deepak M, et al. (1999). High pressure liquid chromatographic determination of bergenin and (+)-afzelechin from different parts of Paashaanbhed (Bergenia ligulata Yeo). Phytochem Anal 10:44–7
- Roe JH, Kuether CA. (1943). The determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazine derivative of dehydroascorbic acid. Biol Chem 147:399–407
- Rotruck JT, Pope AL, Ganther HE, et al. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science 179:588–90
- Saha S, Verma RJ. (2011). Bergenia ciliata extract prevents ethylene glycol induced histopathological changes in the kidney. Acta Pol Pharm Drug Res 68:711–15
- Scales CD, Smith AC, Hanley JM, Saigal CS. (2012). Prevalence of kidney stones in the United States. Euro Urol 62:1–30
- Scalley RD, Ferguson DR, Piccaro JC, et al. (2002). Treatment of ethylene glycol poisoning. Am Fam Physician 66:807–12
- Selvam R, Kalaiselvi P, Govindaraj A, et al. (2001). Effect of Aerva lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 43:89–93
- Singh PP, Singh LBK, Prasad SN, Singh MG. (1978). Urolithiasis in Manipur (north eastern region of India): Incidence and chemical composition of stones. Am J Clin Nutr 31:1519–25
- Singh V. (1995). Traditional remedies to treat asthma in North West and Trans Himalayan region in J&K state. Fitoterapia 66:507–9
- Sinha AK. (1972). Calorimetric assay of catalase. Anal Biochem 47:389–94
- Sofowara A. (2009). Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd, 289
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. (2006). Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Ind J Exp Biol 44:981–6
- Thamilselvan S, Menon M. (2005). Vitamin E therapy prevents hyperoxaluria induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. British J Urol Int 96:117–26
- Toblli JE, Ferder L, Stella I, et al. (2002). Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol 168:1550–5
- Trinchieri A. (2006). Epidemiological trends in urolithiasis: Impact on our health care systems. Urol Res 34:151–6
- Tsai CH, Chen YC, Chen LD, et al. (2008). A traditional Chinese herbal antilithic formula, Wilingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res 36:17–24
- Vaishali AS, Vikas MD, Krishnapriya M, Sanjeevani G. (2008). Identification of potential antioxidants by in vitro activity guided fractionation of Bergenia ligulata. Pharmacog Mag 4:79–84
- Verma NK, Patel SS, Saleem TSM, et al. (2009). Modulatory effect of noni-herbal formulation against ethylene glycol-induced nephrolithiasis in albino rats. J Pharm Sci Res 1:83–9
- von Kossa J. (1901). Über die im Organismus künstlich erzeugbaren Verkalkungen. Beitr path Anat 29:163–202