Abstract
Context: Boerhaavia diffusa Linn. (Nyctaginaceae) roots possess potent antioxidant, antistress, and anticonvulsant activities. It is used as a medicinal plant in Ayurvedic and natural herbal medicines.
Objective: To evaluate the effect of Boerhaavia diffusa root ethanol extract and its active constituent, punarnavine, on depression in Swiss albino mice.
Materials and methods: Ethanol extract (50, 100, and 200 mg/kg, p.o.) and punarnavine (20 and 40 mg/kg, p.o.) were separately administered to 22 and 17 groups of mice, respectively, for 14 successive days followed by testing in the tail suspension and forced swim tests (FST). About 2% w/v gum acacia and double distilled water were used as controls for the extract and punarnavine, respectively.
Results: Antidepressant-like effect of the lowest dose (50 mg/kg) of the extract and lower dose (20 mg/kg) of punarnavine were found to be comparable to fluoxetine. The ED50 value of the ethanol extract was 26.30 mg/kg (FST) and 33.11 mg/kg (tail suspension test); and of punarnavine was 15.14 mg/kg (FST) and 17.38 mg/kg (tail suspension test). The drugs did not show any significant effect on locomotor activities of mice. Prazosin (α1-adrenoceptor antagonist), sulpiride (selective D2-receptor antagonist), para-chlorophenylalanine) (p-CPA) (tryptophan hydroxylase inhibitor), and baclofen (GABAB agonist) significantly attenuated the extract and punarnavine induced-antidepressant-like effect in the tail suspension test. The extract and punarnavine also significantly reduced mouse brain monoamine oxidase (MAO)-A levels, but there was no significant effect on plasma corticosterone levels.
Conclusion: Ethanol extract of Boerhaavia diffusa and punarnavine produced an antidepressant-like effect in mice probably through interaction with monoaminergic and GABAergic systems.
Introduction
Depression is a chronic neuropsychiatric disease that affects people worldwide. Depression is estimated to affect 350 million people (World Health Organization, Citation2012). The main biochemical theory of depression is the monoamine hypothesis, which states that depression is caused by a functional deficit of monoamines (norepinephrine, serotonin, and dopamine) at certain sites in the brain (Gold et al., Citation1988). Monoamine oxidase (MAO) is an enzyme responsible for metabolizing monoamines such as nor-epinephrine, dopamine, and serotonin. MAO is found in nearly all tissues. MAO exists in two similar molecular forms: MAO-A and MAO-B. MAO-A has substrate preference for serotonin and is the main target for the antidepressant monoamine oxidase inhibitors. MAO-B has substrate preference for phenylethyl amine. Both enzymes act on nor-adrenaline and dopamine. In case of depression, the levels of MAO in brain increase which in turn reduces the levels of monoamines. Experimentally, selective MAO-A inhibitors are thought to be more effective in treating major depression than type B inhibitors (Krishnan, Citation1998). In addition to the monoaminergic system, the GABAergic system also contributes to the pathophysiology of depression. Acute and chronic treatment with CGP56433A, a selective GABAB receptor antagonist, decreased immobility in the forced swim test (FST). Thus, GABAB receptor antagonism may serve as a basis for the generation of novel antidepressants (Mombereau et al., Citation2004). Antidepressants and mood stabilizers appear to up-regulate frontal–cortical GABAB but not GABAA receptors (Kimber et al., Citation1987; Lloyd et al., Citation1985). A number of drugs are available for the management of depression, but they impose a variety of side-effects including cardiac toxicity, hypoplasia, sexual dysfunction, body weight gain, and sleep disorder (Antai-Otong, Citation2004; Baldwin et al., Citation2006; Khurana & Baudendistel, Citation2003). The use of alternative medicines is increasing worldwide day by day. Therefore, there is a constant need to explore plants for antidepressant activity and to explore their potential over synthetic antidepressants.
Boerhaavia diffusa Linn. (Nyctaginaceae), commonly known as Punarnava, is a herbaceous perennial plant. It is widely distributed in the tropics and subtropics and used as a medicinal plant in Ayurvedic and in natural herbal medicines (Dhar et al., Citation1968). An ethanol extract of the plant has been reported to possess antidiabetic, antioxidant (Chauhan et al., Citation2011), and antistress (Desai et al., Citation2011) activities. A methanol extract of B. diffusa roots showed anticonvulsant activity (Kaur & Goel, Citation2011). An aqueous extract of B. diffusa roots showed anti-inflammatory (Bhalla et al., Citation1971), hepatoprotective (Rawat et al., Citation1997), antiurolithiatic (Pareta et al., Citation2010), and nephroprotective (Pareta et al., Citation2011) activities. Punarnavine, isolated from an ethanol extract of B. diffusa, has antimetastatic (Manu & Kuttan, Citation2009a) and immunomodulatory (Manu & Kuttan, Citation2009b) activities. It also shows a protective effect in immobilization stress-induced gastric ulceration in rats (Bhatia et al., Citation2011).
Boerhaavia diffusa contains a large number of compounds such as flavonoids, alkaloids, steroids, triterpenoids, lipids, lignins, carbohydrates, protein, and glycoproteins. The active principles reported in roots are punarnavine (Manu & Kuttan, Citation2009a), boeravinone A–F (Kadota et al., Citation1989; Lami et al., Citation1990, Citation1991a), hypoxanthine-9-l-arabinofuranoside (Ahmad & Hossain, Citation1968), hentriacontane, β-sitosterol, ursolic acid (Mishra & Tiwari, Citation1971), a phenolic glycoside, punarnavoside (Jain & Khanna, 1989), and two known lignans namely liriodendrin and syringaresinol mono-β-D-glycoside (Lami et al., Citation1991b).
Stress has been observed to play a key role in the etiology of various psychiatric disorders (Esch et al., Citation2002). Stressful experiences have been reported to favor the development of depression in humans (Kendler et al., Citation1999). As mentioned above, B. diffusa has been reported to possess antistress activity (Desai et al., Citation2011), but its antidepressant-like activity and the possible modulation of monoaminergic and GABAergic systems in this activity have not been explored previously. So the aims of the present study were to explore the antidepressant potentials of ethanol extract of B. diffusa and its isolated active constituent, punarnavine; and to investigate the probable underlying mechanisms of antidepressant-like action.
Materials and methods
Collection of plant material
The dried roots of B. diffusa were purchased from a commercial market, New Delhi, and were authenticated by Dr. H.B. Singh as B. diffusa Linn. from Raw Materials, Herbarium and Museum Division, National Institute of Science Communication and Information Resources, New Delhi (Ref. no. NISCAIR/RHMD/Consult/2009-10/1205/09).
Preparation of ethanol extract
The dried roots were grounded to coarse powder. About 2 kg of powdered drug was extracted with ethanol (95%) using a Soxhlet apparatus (Labco, New Delhi, India) at 70 °C untill the siphoning solution became colorless. The solvent was recovered by distillation and the extract was dried by using a water bath at 50–60 °C. The dried ethanol extract was brownish in color and the yield was 4.25% w/w. The dried extract was stored in an airtight container, which was kept in a refrigerator (4–8 °C) for future use. The doses of extract were selected based on the literature (Sumanth & Mustafa, 2007).
Isolation of punarnavine
The alkaloid punarnavine was isolated from the plant B. diffusa as reported previously (Manu & Kuttan, Citation2009b). About 3 kg of powdered drug was extracted with ethanol (95%) using a Soxhlet apparatus at 70 °C until the siphoning solution became colorless. The extract was concentrated to 20% of its volume, and boerhaavic acid was filtered off. The filtrate was concentrated to dryness leaving a sticky material. This was extracted with hot water and concentrated to yield potassium nitrate. It was filtered and the filtrate was made ammonical and extracted repeatedly with chloroform. The chloroform extract was evaporated and the residue was macerated with diethyl ether. It was then evaporated and gave amorphous punarnavine. A further amount of punarnavine was obtained from the sticky material, remaining from the water extraction by extraction with dilute hydrochloric acid. Amorphous punarnavine was then crystallized from a small volume of ethyl alcohol and was purified again by recrystalization. The overall yield of punarnavine was 0.016% w/w. The melting point of crystallized punarnavine is 235 °C. The isolated compound gave positive results for the alkaloid giving a red color with HNO3, green color with FeCl3, and a brown precipitate with Dragendroff’s reagent. The thin-layer chromatography for crystallized punarnavine was performed using a solvent system comprised of diethyl amine:cyclohexane (30:70). It showed a single band (Rf = 0.87), which confirmed the presence of a single constituent, punarnavine. The Rf value was calculated by using the following formula:
Experimental animals
Swiss albino mice of either sex, weighing around 20–25 g, were purchased from Disease Free Small Animal House, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar (Haryana, India). Animals were housed separately in groups of 10 per cage (polycarbonate cage size: 29 × 22 × 14 cm3) under laboratory conditions with alternating light and dark cycle of 12 h each having free access to food and water. The animals were kept fasted 2 h before and 2 h after drug administration. The animals were acclimatized for at least 5 d before behavioral experiments that were carried out between 09:00 and 17:00 h. The experimental protocol was approved by IAEC and animal care was taken as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (Registration no. 0436).
Drugs and chemicals
Fluoxetine hydrochloride (Ranbaxy laboratories Ltd., Gurgaon, India), prazosin hydrochloride, (±) sulpiride, DL-CPA, p-nitroso-N,N-dimethylaniline, and baclofen (Sigma-Aldrich, St. Louis, MO), sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dihydrate, Tris, EDTA disodium salt AR, sucrose, 5-hydroxy-tryptamine creatinine sulfate monohydrate (Hi Media Laboratories Pvt. Ltd., Mumbai, India), acetic acid, boric acid, chloroform, hydrochloric acid, gum acacia, potassium hydroxide, sodium hydroxide (CDH Lab Reagents, New Delhi, India), total protein measurement kit (Coral Industries Ltd., Uttarakhand, India) were used in this study.
Vehicles
Fluoxetine, prazosin, sulpiride, and baclofen were separately dissolved in normal saline (0.9% NaCl). p-CPA was dissolved in minimum quantity of 0.1 N sodium hydroxide solution and pH was adjusted to 7.0 with 0.1 N hydrochloric acid. The dried extract of B. diffusa was suspended in 2% gum acacia each time before administration. Isolated punarnavine was dissolved in double distilled water before administration. Doses of prazosin, sulpiride, p-CPA, and baclofen were selected on the basis of the literature (Dhingra & Kumar, Citation2008; Dhingra & Valecha, Citation2007).
Laboratory models employed for evaluation of antidepressant-like activity
Tail suspension test
Tail suspension test (TST) is a commonly employed behavioral model for screening antidepressant-like activity in mice (Steru et al., Citation1985). The test was conducted as previously followed in our laboratory (Dhingra & Kumar, Citation2008). Animals were moved from their housing colony to laboratory in their own cages and allowed to adapt to the laboratory conditions for 1–2 h. Each mouse was individually suspended to the edge of a table, 50 cm above the floor, by adhesive tape placed approximately 1 cm from the tip of the tail. The total period of immobility was recorded manually for 6 min. An animal was considered to be immobile when it did not show any body movement, hung passively, and completely motionless. The test was conducted in a dim-lighted room, and each mouse was used only once in the test.
Forced swim test
FST is another commonly used behavioral model for screening antidepressant-like activity in rodents (Porsolt et al., Citation1977). The procedure was the same as previously followed in our laboratory (Dhingra & Kumar, Citation2008). Animals were moved from their housing colony to the laboratory in their own cages and allowed to adapt to the laboratory conditions for 1–2 h. Mice were individually forced to swim in an open glass chamber (25 × 15 × 25 cm3) containing fresh water to a height of 15 cm and maintained at 26 ± 1 °C. Each animal showed vigorous movement during the initial 2 min period of the test. The duration of immobility was manually recorded during the next 4 min of the total 6 min testing period. Mice were considered to be immobile when they ceased struggling and remained floating motionless in water, making only those movements necessary to keep their head above water. The test was conducted in a dim-lighted room, and each mouse was used only once in the test.
Biochemical estimations
Measurement of MAO-A
Mouse brain mitochondrial fraction was prepared following the procedure described previously (Schurr & Livne, Citation1976). Briefly, the brain samples were collected immediately on an ice plate. Mouse brain mitochondrial fraction was prepared by cutting the brain sample into small pieces and rinsed in cold 0.25 M sucrose, 0.1 M Tris, and 0.02 M EDTA (pH 7.4) to remove blood. The pieces were homogenized for 45 s in a homogenizer with 400 mL of the same medium. The homogenate was centrifuged (Remi Centrifuge, Mumbai, India) at 800 rpm for 10 min at 4 °C and the pellets were discarded. The supernatant was then centrifuged at 12 000 rpm for 20 min in the same medium. The precipitate was washed twice more with 100 mL of sucrose-Tris-EDTA buffer and resuspended in 50 mL of the medium (Pan et al., Citation2005). MAO activity was assessed spectrophotometrically as described previously (Yu et al., Citation2002) using UV–visible–NIR spectrophotometer (Varian Cary-5000, Christ, The Netherlands).
Estimation of total protein content
Total protein was estimated in brain homogenate (Henry et al., Citation1974) by using total protein kit from Coral Industries Ltd., Uttarakhand, India, using colorimeter (Digital Photocolorimeter, Biomed, Ambala, Haryana, India).
Estimation of corticosterone levels
Corticosterone levels were estimated in plasma by method of Bartos and Pesez (Citation1979) using UV–visible–NIR spectrophotometer (Varian Cary-5000, Christ, The Netherlands).
Measurement of locomotor activity
To rule out the effects of the extract on immobility period, horizontal locomotor activities of control and test animals were recorded for a period of 10 min using photoactometer (INCO, Ambala, India).
Experimental protocol
Animals were divided into 39 groups and each group composed of a minimum of 6–10 mice. Only for studying the effects of drugs on locomotor activity, the number of animals in each group was 6; for all the other groups, the number of animals in each group was 10. The experimental protocol was divided into the following groups.
Groups for TST (n = 10 each)
Groups 1 and 2: (control group for ethanol extract and punarnavine), 2% w/v gum acacia and double distilled water, respectively, were administered orally for 14 consecutive days, and after 60 min of administration on the 14th day, immobility periods of mice were recorded in TST.
Groups 3, 4, 5, 6, 7, and 8: fluoxetine (20 mg/kg), ethanol extract (50, 100, and 200 mg/kg) of B. diffusa, and punarnavine (20 and 40 mg/kg), respectively, were administered orally for 14 consecutive days, and after 60 min of administration on the 14th day, immobility periods of mice were recorded using TST.
Groups for FST (n = 10 each)
Groups 9–16 were similar to those mentioned under TST (Groups 1–8) except the immobility period was recorded using FST.
Groups for investigating mechanisms of action of ethanol extract of B. diffusa by co-administration of various drugs modulating levels of monoamines and GABA using TST (n = 10 each)
Groups 17 and 18: vehicle (2% gum acacia solution) and ethanol extract (50 mg/kg, p.o.) of B. diffusa, respectively, were administered orally for 14 consecutive days, and after 45 min of administration on 14th day, sulpiride (50 mg/kg, i.p.) was injected. After 45 min of injection, the animals were subjected to TST.
Groups 19 and 20: vehicle and ethanol extract (50 mg/kg, p.o.) of B. diffusa, respectively, were administered for 14 consecutive days, and after 45 min of administration on 14th day, prazosin (62.5 μg/kg, i.p.) was injected, and 45 min after the injection, the animals were subjected to TST.
Groups 21 and 22: vehicle and ethanol extract (50 mg/kg, p.o.) of B. diffusa, respectively, were administered for 14 consecutive days. Then, p-CPA (100 mg/kg, i.p.) was injected 45 min after administration of respective vehicle and extract from 11th day to 14th day. On the 14th day, 45 min after the injection of p-CPA, the animals were subjected to TST.
Groups 23 and 24: vehicle and ethanol extract (50 mg/kg, p.o.) of B. diffusa, respectively, were administered for 14 consecutive days, and after 45 min of administration on the 14th day, baclofen (10 mg/kg, i.p.) was injected, and 45 min after the injection, the animals were subjected to TST.
Groups for investigating mechanisms of action of punarnavine by co-administration of various drugs modulating levels of monoamines and GABA using TST (n = 10 each)
Groups 25–32 were the same as Groups 17–24 except punarnavine (20 mg/kg, p.o.) was administered in place of ethanol extract (50 mg/kg, p.o.) of B. diffusa and vehicle for punarnavine is double distilled water.
Groups for estimating brain MAO-A levels and plasma corticosterone levels
After carrying out FST on the animals of Groups 9–16 on the 14th day, these were sacrificed by cervical dislocation on the 15th day, and immediately brain samples were collected and analyzed for MAO-A and protein levels. At the same time, blood samples were collected by carotid bleeding and plasma corticosterone levels were estimated.
Groups for measuring locomotor activity (n = 6 each)
Groups 33, 34, 35, 36, 37, 38, and 39: vehicle for ethanol extract and punarnavine, ethanol extract (50, 100, and 200 mg/kg, p.o.) of B. diffusa and punarnavine (20 and 40 mg/kg, p.o.), respectively, were administered for 14 consecutive days; and on the 14th day, 60 min after administration, locomotor activity was measured.
Statistical analysis
All the results were expressed as mean ± standard error mean (SEM). The data were analyzed by using a one-way ANOVA followed by Tukey’s test for multiple comparisons using the software Graphpad Instat (GraphPad Software, San Diego, CA). In all the tests, the criterion for statistical significance was p < 0.05.
Results
Effect of ethanol extract of B. diffusa and punarnavine on immobility periods of mice in TST and FST
Ethanol extract (50, 100, and 200 mg/kg, p.o.) of B. diffusa as well as punarnavine (20 and 40 mg/kg, p.o.) administered for 14 successive days to mice significantly decreased the immobility periods in both TST and FST, indicating significant antidepressant-like activity. The lowest dose of ethanol extract (50 mg/kg) decreased the immobility period to the greatest extent (p < 0.001) in FST, thus showed most potent antidepressant-like action. ED50 value of ethanol extract using FST and TST were 26.30 and 33.11 mg/kg, respectively. Similarly, the lower dose of punarnavine (20 mg/kg) showed better antidepressant-like action than the higher dose (40 mg/kg), as indicated by a greater decrease (p < 0.001) in the immobility period as compared to the higher dose. The ED50 values of punarnavine using FST and TST were 15.14 and 17.38 mg/kg, respectively. The efficacies of both punarnavine as well as ethanol extract were comparable to the standard antidepressant drug, fluoxetine ().
Figure 1. Effect of ethanol extract of Boerhaavia diffusa on immobility period of mice using forced swim test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Eth ext stands for ethanol extract. F(4, 45) = 8.554, p < 0.0001. ap < 0.001, bp < 0.05 as compared to the vehicle-treated group.
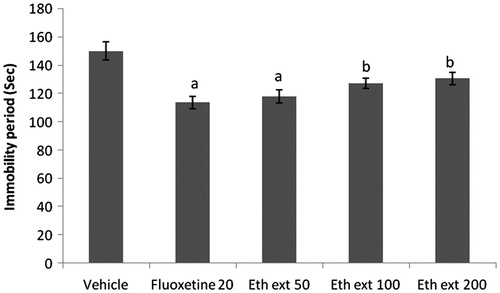
Figure 2. Effect of ethanol extract of Boerhaavia diffusa on immobility period of mice using the tail suspension test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Eth ext stands for ethanol extract. F(4, 45) = 39.238, p < 0.0001. ap < 0.01 as compared to the vehicle-treated group.
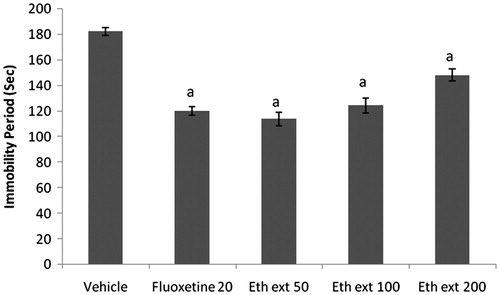
Figure 3. Effect of punarnavine on the immobility period of mice using forced swim test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. F(3, 36) = 8.184, p = 0.0003. ap < 0.05, bp < 0.001, cp < 0.01 as compared to the vehicle-treated group.
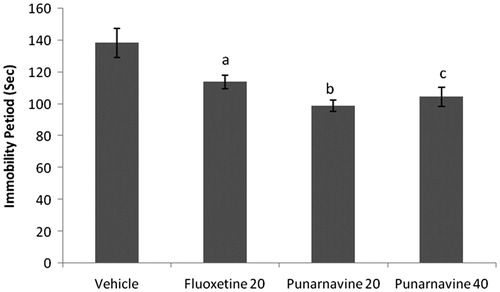
Figure 4. Effect of punarnavine on the immobility period of mice using tail suspension test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. F (3, 36) = 75.757, p < 0.0001. ap < 0.01 as compared to the vehicle-treated group.
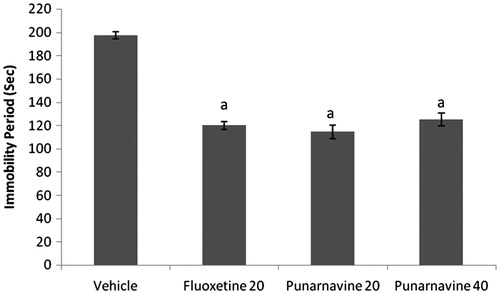
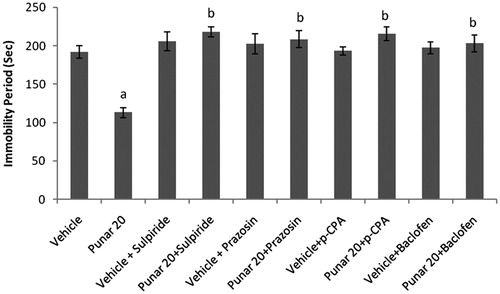
Effects of co-administration of ethanol extract (50 mg/kg) and punarnavine (20 mg/kg) with sulpiride, prazosin, p-CPA, and baclofen on immobility period of mice in TST
Sulpiride (50 mg/kg, i.p.), prazosin (62.5 µg/kg, i.p.), p-CPA (100 mg/kg, i.p.), and baclofen (10 mg/kg, i.p.) significantly increased the immobility period as compared to the vehicle-treated group. Pretreatment of animals with sulpiride/prazosin/p-CPA/baclofen significantly reversed the decrease in immobility period elicited by ethanol extract (50 mg/kg) and punarnavine (20 mg/kg) ( and ).
Figure 5. Effect of combination of ethanol extract of Boerhaavia diffusa with sulpiride, prazosin, p-CPA, and baclofen on immobility periods of mice using the tail suspension test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Eth ext stands for ethanolic extract. F (9, 90) = 19.498; p < 0.0001. ap<0.001 as compared to vehicle treated group; bp<0.001 as compared to ethanolic extract (50 mg/kg) treated group.
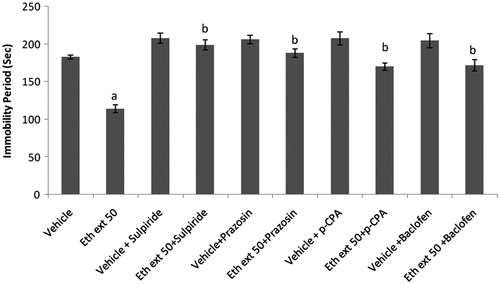
Figure 6. Effect of combination of punarnavine with sulpiride, prazosin, p-CPA, and baclofen on immobility periods of mice using tail suspension test. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Punar stands for punarnavine. F(9, 90) = 10.426. p < 0.0001. ap < 0.001 as compared to the vehicle-treated group; bp < 0.001 as compared to punarnavine (20 mg/kg) group.
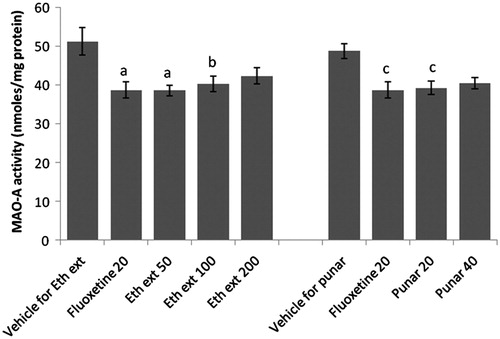
Effect of ethanol extract and punarnavine on brain MAO-A levels
Ethanol extract (50 and 100 mg/kg, p.o.) as well as punarnavine (20 mg/kg, p.o.), administered for 14 consecutive days to mice, significantly reduced the brain MAO-A levels as compared to the respective vehicle-treated groups. The efficacies of both were found to be comparable to fluoxetine ().
Figure 7. Effect of ethanol extract of Boerhaavia diffusa and punarnavine on MAO-A activity. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Eth ext stands for ethanol extract and punar stands for punarnavine. F(7, 72) = 5.326, p < 0.0001. ap < 0.01, bp < 0.05 as compared to the vehicle group for ethanol extract; cp < 0.05, as compared to the vehicle group for punararnavine.
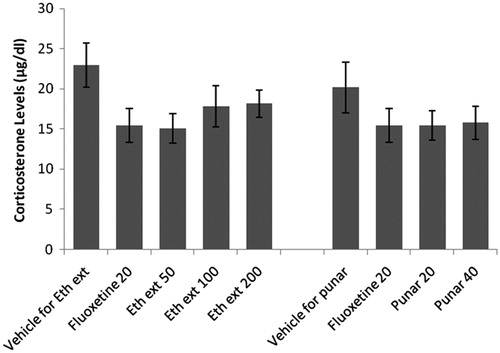
Effect of ethanol extract and punarnavine on plasma corticosterone levels
Ethanol extract as well as punarnavine administered for 14 consecutive days to mice did not significantly affect corticosterone levels as compared to the respective vehicle-treated controls ().
Figure 8. Effect of ethanol extract of Boerhaavia diffusa and punarnavine on plasma corticosterone levels. n = 10 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s post hoc test. Eth ext stands for ethanol extract and punar stands for punarnavine. F(7, 72) = 1.477, p = 0.1891.
Effect of ethanol extract and punarnavine on locomotor activity
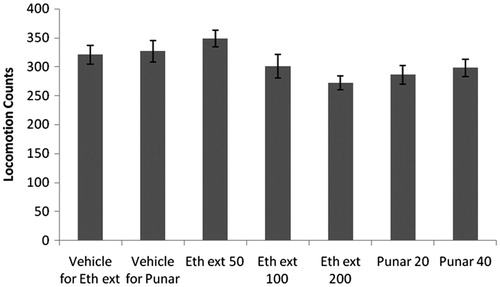
Ethanol extract and punarnavine administered for 14 successive days did not significantly affect locomotor activities of mice as compared to respective vehicle-treated mice ().
Figure 9. Effect of ethanol extract of Boerhaavia diffusa and punarnavine on locomotor activity. n = 6 in each group; values are in mean ± SEM. Doses are listed in mg/kg. Data were analyzed by a one-way ANOVA followed by Tukey’s test. Eth ext stands for ethanol extract and punar stands for punarnavine. F(6, 35) = 2.614, p = 0.0251.
Discussion
In the present study, the ethanol extract (50, 100, and 200 mg/kg, p.o.) of B. diffusa and its isolated constituent, punarnavine (20 and 40 mg/kg, p.o.), administered for 14 successive days to mice produced a significant antidepressant-like effect in both FST and TST. This is the first study showing antidepressant-like effect of ethanol extract of B. diffusa and punarnavine. Antidepressant-like effect of ethanol extract and punarnavine seems not to be associated with any motor effects, as there was no significant change in the locomotor activity of mice by any dose of ethanol extract or punarnavine. This indicates that antidepressant effect of the extract and punarnavine was not due to any effect on locomotor activity.
Animal models of depression are typically based on the exposure of animals to a stressful condition (a potential or an actual threatening situation) and a specific test for measuring behavioral responses. FST and TST are widely employed in rodents to predict antidepressant potential by decrease of immobility period produced by several different classes of antidepressant drugs (Porsolt et al., Citation1977; Steru et al., Citation1985). These tests are quite sensitive and relatively specific to all major classes of antidepressant drugs including tricyclics, serotonin-specific reuptake inhibitors, MAO inhibitors, and atypical antidepressants (Cryan et al., Citation2005; Detke et al., Citation1995). The efficacies of the lowest dose (50 mg/kg) of the ethanol extract and lower dose of punranavine (20 mg/kg) were found to be comparable (p < 0.001) to the standard drug, fluoxetine (20 mg/kg, p.o.). Further, the role of noradrenaline in the pathophysiology of depression is well established. Some antidepressant drugs increase the synaptic concentration of noradrenaline or act directly on noradrenergic receptors (Elhwuegi, Citation2004). In addition, it was demonstrated that NA-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors (SSRIs) (Cryan et al., Citation2004).
In our study, prazosin (a α1-adrenoceptor antagonist) was able to reverse the antidepressant-like effect of ethanol extract and punarnavine separately. This shows that both showed the antidepressant-like effect by interacting with α1-adrenoceptor. The dopaminergic system is also implicated in the regulation of mood (Dailly et al., Citation2004). Clinical studies have shown that plasma levels of dopamine metabolites are significantly lower in depressed patients, indicating diminished dopamine turnover (Mitani et al., Citation2006). The anti-immobility effects of the ethanol extract and punarnavine were significantly reversed by sulpiride (D2 antagonist), suggesting that the antidepressant effect of both might be mediated through interaction with D2 dopamine receptors.
The involvement of the 5-HT system was investigated by inhibiting 5-HT synthesis with the tryptophan hydroxylase inhibitor, p-CPA. The reversal of the antidepressant-like effect of ethanol extract and punarnavine, by the pretreatment of mice with p-CPA, suggested that the antidepressant effect might be dependent on the availability of 5-HT in the synaptic cleft. Thus, our findings provide convincing behavioral evidence that p-CPA treatment was effective in depleting 5-HT stores.
Baclofen (GABAB agonist) also antagonized the effect of ethanol extract and punarnavine, showing involvement of GABAB receptors in antidepressant-like action. A decrease in GABAB neurotransmission may contribute to action of antidepressants (Ghose et al., Citation2011). Therefore, antidepressant-like effect of ethanol extract and punarnavine might be through interaction with α1-adrenoceptors, dopamine D2-receptors, serotonergic, and GABAB receptors. Levels of monoamines like norepinephrine, serotonin, and dopamine are decreased in depression, so antidepressant drugs enhance the levels of these monoamines. The ethanol extract as well as punarnavine also reduced brain MAO-A activity as compared to respective vehicle-treated controls, so it indicated that both extract and punarnavine inhibited the metabolism of monoamines, hence increasing the levels of norepinephrine, dopamine, and serotonin.
MAO inhibitors are an important class of drugs used for the treatment of depression (O’Donnell & Shelton, Citation2011). The ethanol extract and punarnavine did not significantly reduce plasma corticosterone levels of mice as compared to respective control groups, so their antidepressant-like action might not be due to reduction of corticosterone levels. This finding is supported by an earlier study where onion powder showed significant antidepressant activity without affecting baseline levels of plasma corticosterone in rats (Sakakibara et al., Citation2008).
In conclusion, the ethanol extract of Boerhaavia diffusa as well as its isolated constituent, punarnavine, exerted significant antidepressant-like effect in mice probably through interaction with α1-adrenoceptors, dopamine D2 receptors, serotonergic, and GABAB receptors, and through inhibition of brain MAO-A activity. The efficacies of the extract and punarnavine were comparable to that of fluoxetine. Thus, the extract and punarnavine may have potential therapeutic value for the management of mental depression.
Declaration of interest
The authors do not have any conflict of interests with the content of the paper.
Acknowledgements
The authors are thankful to Ranbaxy Research Laboratories Ltd., Gurgaon, India, for providing a gift sample of fluoxetine.
References
- Ahmad K, Hossain A. (1968). Isolation, synthesis and biological action of hypoxanthine-9-l-arabinofuranoside. J Agr Biol Sci 11:41–4
- Antai-Otong D. (2004). Antidepressant-induced insomnia: Treatment options. Perspect Psych Care 40:29–33
- Baldwin D, Bridgman K, Buis C. (2006). Resolution of sexual dysfunction during double-blind treatment of major depression with reboxetine or paroxetine. J Psychopharmacol 20:91–6
- Bartos J, Pesez M. (1979). Colorimetric and fluorimetric determination of steroids. Pure Appl Chem 51:2157–69
- Bhalla TN, Gupta MB, Bhargava KP. (1971). Anti-inflammatory activity of Boerhaavia diffusa. J Res Indian Med 6:11–15
- Bhatia N, Kumar A, Tuli A, et al. (2011). Beneficial role of punarnavine against immobilization stress induced gastric ulceration in rats. Der Pharmacia Lett 3:92–101
- Chauhan SK, Thapliyal RP, Ojha SK, et al. (2011). Antidiabetic and antioxidant effect of ethanol root extract of Boerhaavia diffusa in streptozotocin-induced diabetic rats. J Pharm Res 4:446–8
- Cryan JF, O’Leary OF, Jin SH, et al. (2004). Norepinephrine deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Nat Acad Sci USA 101:8186–91
- Cryan JF, Mombereau C, Vassout A. (2005). The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
- Dailly E, Chenu F, Renard CE, Bourin M. (2004). Dopamine, depression and antidepressants. Fundam Clin Pharmacol 18:601–7
- Desai SK, Desai SM, Arya P, et al. (2011). Antistress activity of Boerhaavia diffusa root extract and a polyherbal formulation containing Boerhaavia diffusa using cold restraint stress model. Int J Pharm Pharm Sci 3:130–2
- Detke MJ, Rickels M, Lucki I. (1995). Active behavior in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
- Dhar ML, Dhar MM, Dhawan BN, et al. (1968). Screening of Indian plants for biological activity. Indian J Exp Biol 6:232–47
- Dhingra D, Kumar V. (2008). Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of garlic extract in mice. Indian J Pharmacol 40:175–8
- Dhingra D, Valecha R. (2007). Evaluation of antidepressant-like activity of Terminalia bellirica Roxb. fruits in mice. Indian J Exp Biol 45:610–14
- Elhwuegi AS. (2004). Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry 28:435–51
- Esch T, Stefano GB, Fricchione GL, Benson H. (2002). The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett 23:199–208
- Ghose S, Winter MK, McCarson KE, et al. (2011). The GABAB receptor as a target for antidepressant drug action. Br J Pharmacol 165:1–5
- Gold PW, Goodwin FK, Chrousus GP. (1988). Clinical and biochemical manifestations of depression in relation to the neurobiology of stress: Part 1. N Engl J Med 319:348–53
- Henry RJ, Cannon DC, Winkelman JW. (1974). Clinical Chemistry, Principles and Techniques, 2nd ed. New York: Harper and Row, 118
- Jain GK, Khanna NM. (1989). Punarnavoside: A new antifibrinolytic agent from Boerhaavia diffusa Linn. Indian J Chem 28B:163--6
- Kadota S, Lami N, Tezuka Y, Kikuchi T. (1989). Constituents of the roots of Boerhaavia diffusa Linn. Examination of sterols and structures of new rotenoids (boeravinones A and B). Chem Pharm Bull 37:3214–20
- Kaur M, Goel RK. (2011). Anticonvulsant activity of Boerhaavia diffusa: Plausible role of calcium channel antagonism. Evid Based Complement Alternat Med 2011:310420 (1--7)
- Kendler KS, Karkowski LM, Prescott CA. (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–41
- Khurana RN, Baudendistel TE. (2003). Hypertensive crisis associated with venlafaxine. Am J Med 115:676–7
- Kimber JR, Cross JA, Horton RW. (1987). Benzodiazepines and GABA (A) receptors in rat brain following chronic antidepressant drug administration. Biochem Pharmacol 36:4173–5
- Krishnan KRR. (1998). Monoamine oxidase inhibitors. In: Schatzberg AF, Nemeroff CB, eds. The American Psychiatric Press Textbook of Psychopharmacology, 2nd ed. Washington DC: American Psychiatric Press, 239–49
- Lami N, Kadota S, Tezuka Y, Kikuchi T. (1990). Constituents of the roots of Boerhaavia diffusa Linn. structure and stereochemistry of a new rotenoid boeravinone C2. Chem Pharm Bull 38:1558–62
- Lami N, Kadota S, Kikuchi T. (1991a). Constituents of the roots of Boerhaavia diffusa Linn. IV. Isolation and structure determination of boeravinones D, E and F. Chem Pharm Bull 39:1863–5
- Lami N, Kadota S, Tezuka Y, Kikuchi T. (1991b). Constituents of the roots of Boerhaavia diffusa Linn. identification of Ca2+ channel antagonistic compound from the methanol extract. Chem Pharm Bull 39:1551–5
- Lloyd KG, Thuret F, Pile A. (1985). Upregulation of gamma aminobutyric acid GABAB binding sites in rat frontal cortex. J Pharmacol Exp Ther 235:191–9
- Manu KA, Kuttan G. (2009a). Anti-metastatic potential of punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology 214:245–55
- Manu KA, Kuttan G. (2009b). Immunomodulatory activities of punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol Immunotoxicol 31:377–87
- Mishra A, Tiwari HP. (1971). Constituents of the roots of Boerhaavia diffusa. Phytochemistry 10:3318–19
- Mitani H, Shirayama Y, Yamada T, Kawahara R. (2006). Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 30:531–4
- Mombereau C, Kaupmann K, Froestl W, et al. (2004). Genetic and pharmacological evidence of a role for GABA (B) receptors in the modulation of anxiety and antidepressant-like behavior. Neuropsychopharmacology 29:1050–62
- O’Donnell JM, Shelton RC. (2011). Drug therapy of depression and anxiety disorders. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12th ed. New York: McGraw-Hill, 397–416
- Pan Y, Kong L, Xia X, et al. (2005). Antidepressant-like effect of icariin and its possible mechanism in mice. Pharmacol Biochem Behav 82:686–94
- Pareta SK, Patra KC, Mazumder PM, Sasmal D. (2010). Boerhaavia diffusa Linn. aqueous extract as curative agent in ethylene glycol induced urolithiasis. Pharmacologyonline 3:112–20
- Pareta SK, Patra KC, Mazumder PM, Sasmal D. (2011). Aqueous extract of Boerhaavia diffusa root ameliorates ethylene glycol-induced hyperoxaluric oxidative stress and renal injury in rat kidney. Pharm Biol 49:1224–33
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn 229:327–36
- Rawat AK, Mehrotra S, Tripathi SC, Shome U. (1997). Hepatoprotective activity of Boerhaavia diffusa Linn. roots – A popular Indian ethnomedicine. J Ethnopharmacol 56:61–6
- Sakakibara H, Yoshino S, Kawai Y, Terao J. (2008). Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioural model of depression. Biosci Biotechnol Biochem 72:94–100
- Schurr A, Livne A. (1976). Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochem Pharmacol 25:1201–3
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berlin) 85:367–70
- Sumanth M, Mustafa SS. (2007). Antistress, adaptogenic and immunopotentiating activity of roots of Boerhaavia diffusa in mice. Int J Pharmacol 3:416–20
- World Health Organization. (2012). World Suicide Prevention Day. Washington, DC: WHO
- Yu ZF, Kong LD, Chen Y. (2002). Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol 83:161–5

