Abstract
Context: Cocculus hirsutus (L.) Diels (Menispermaceae) is used in Indian folk system of alternative medicine for rheumatism, eczema, diabetics, inflammation, and neuralgia.
Objective: To evaluate antitumor activities of C. hirsutus in vitro and in vivo.
Materials and methods: C. hirsutus was successively extracted using hexane, petroleum ether, chloroform, ethyl acetate, methanol, and water. In vitro cytotoxicity was assessed by the MTT assay. Phytochemical analyses were conducted with methanol extract of C. hirsutus (MECH) and in vivo antitumor activity was carried out with MECH using Dalton’s lymphoma ascites (DLA) mouse model. Antioxidant properties were assessed by estimating superoxide dismutase (SOD), catalase (CAT), and lipid peroxidation.
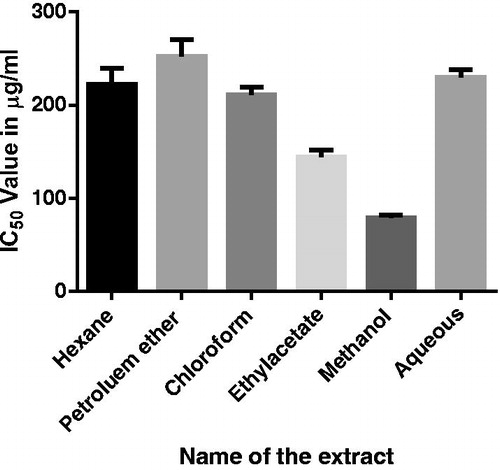
Results and discussion: Phytochemical studies indicated a high content of total alkaloid (165.6 mg/100 g), total phenolic (43.5 GAE mg/g), and total flavanoid (4.97 RE mg/g) in MECH. Anti-proliferative activity against the breast cancer cell line MCF-7 showed IC50 values of 221.5 ± 16.68, 255 ± 17.88, 213 ± 8.4, 147 ± 7.9, and 229 ± 8.02 µg/ml with hexane, petroleum ether, chloroform, ethyl acetate, methanol, and aqueous extracts, respectively. A significant (p < 0.01) decrease in packed cell volume, viable cell count, and increased lifespan (58 and 77%) was observed. Hematological and serum biochemical profiles were restored to normal levels in MECH-treated mice. MECH-treated group significantly (p < 0.001) decreased SOD, lipid peroxidation, and CAT towards normal.
Conclusion: C. hirsutus exhibited significant in vitro and in vivo antitumor activities that are reasonably attributed to endogenous antioxidant mechanisms.
Introduction
Cancer is a major health issue despite advancement in the health care sector. Present day research is aimed at identifying synthetic and natural molecules to be used in the prevention and/or treatment of cancer. Chemotherapy is considered as the most effective method among many other methods prevalent to treat cancer. Yet, chemotherapeutic agents will affect the host cells, especially bone marrow, epithelial tissues, reticulo-endothelial system, and gonads (Mascarenhas, Citation1994). There are several chemotherapeutic agents that produce serious chronic or delayed toxicities that may be irreversible, particularly in heart, lungs, and kidneys (Nitha et al., Citation2005). Therefore, a novel approach for reducing unwanted toxicity is to employ newer natural products that may act by different and distinct mechanism(s) and/or precipitate fewer, or different side effects. Hence, now natural products have been contemplated of exceptional value in the development of effective anticancer drugs with minimum host cell toxicity possessing good antioxidant potential (Gupta et al., Citation2004).
Cocculus hirsutus (L.) Diels (Menispermaceae) is a climbing shrub that grows all over India, especially in dry regions. It is widely used in the indigenous system of medicine for curing various ailments due to its different medicinal properties. The roots are used for the treatment of rheumatism, skin diseases, dyspepsia, constipation, impotency, and the leaves are used in the treatment of eczema, gonorrhea, opthalmia, neuralgia, and leucorrhoea (Warrier et al., Citation2005). Alcoholic extract of the leaves and stem is also reported to have anticancer and hypotensive activities (Khare, Citation2007). The plant is reported to have jamtine, cohirsine, hirsudiol, cohirsinine, cohirstinine, haiderine, jamantine, hirsutine, shaheenine (Rasheed et al., Citation1991a,Citationb; Viqar & Iqbal, Citation1992, Citation1993a,Citationb; Viqar et al., Citation1987a,Citationb,Citationc, Citation1991). No pharmacological investigation in the perspective of antitumor activity has yet been reported on C. hirsutus. Therefore, the present study evaluated the in vitro and in vivo anticancer properties of C. hirsutus.
Materials and methods
Collection and extraction of plant materials
The whole plant was collected in October 2011 from Kallakad, Tirunelveli District in South India. The specimen was identified by Prof. V. Chelladurai, Research Officer – Botany, C.C.R.A.S. Govt. of India (Retired). A voucher specimen was prepared in our research lab and maintained with voucher no. PSGCP/DPC/01 for further reference. Immediately after the collection, the whole plant was washed thoroughly with water and shade-dried at room temperature. The shade-dried plant was then pulverized to form coarse powder and used for extraction. The crude drug was successively extracted in a Soxhlet apparatus using hexane, petroleum ether, chloroform, methanol, ethyl acetate, and water. The solvents were removed by distillation on a water bath at atmospheric pressure. The final traces of the solvents were removed with the help of rotary evaporator under reduced pressure (Raju et al., Citation2013) and the residual solvents were found to be within the limits as per International Conference on Harmonization (ICH) guidelines 2012. All the extracts were subjected to in vitro antiproliferative activity against MCF-7 cancer cell line. The methanol extract of C. hirsutus (MECH) was used for in vivo antitumor activities.
Estimation of total alkaloid, phenolic, and flavonoid content
The total amount of alkaloids present in the crude extract was assayed as per the protocol reported earlier (Sreevidya & Mehrotra, Citation2003). Total phenolic and flavonoid contents were determined in MECH extract using Folin–Ciocalteu reagent (Chandler & Dodds, Citation1983) and the Dowd method (Arvouet-Grand et al., Citation1994), respectively.
In vitro cytotoxic studies
The human breast cancer cell line (MCF-7) was obtained from National Centre for Cell Science (NCCS), Pune, and grown in Eagles minimum essential medium containing 10% fetal bovine serum (FBS). All the cells were maintained at 37 °C, 5% CO2, 95% air, and 100% relative humidity. Maintenance cultures were passaged weekly and the culture medium was changed twice a week.
Cell treatment procedure
The monolayered cells were detached with trypsin–ethylene diamine tetra acetic acid (EDTA) to make a single cell suspension. The viable cells were counted using a hemocytometer and diluted with a medium with 5% FBS to give a final density of 1 × 105 cells/ml. Cell suspension (100 µL per well) was seeded into 96-well plates at a plating density of 10 000 cells/well and incubated to allow for cell attachment at 37 °C, 5% CO2, 95% O2, and 100% relative humidity. After 24 h, the cells were treated with serial concentrations of the extracts. They were initially dissolved in dimethylsulfoxide (DMSO) and further diluted in serum free medium to produce five concentrations. Each concentration (100 µl per well) was added to plates to obtain final concentrations of 250, 125, 62.5, 31.25, and 15.62 µg/ml. The final volume in each well was 200 ml and the plates were incubated at 37 °C, 5% CO2, 95% air, and 100% relative humidity for 48 h. The medium without samples served as a control. Triplicates were maintained for all concentrations. After 48 h of incubation, 15 ml of MTT (5 mg/ml) in phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were solubilized in 100 ml of DMSO and then the absorbance was measured at 570 nm using micro-plate reader. The % cell inhibition was determined using the following formula:
Non-linear regression graph was plotted between % cell inhibition and log10 concentration, and IC50 was determined using GraphPad Prism 6 software (GraphPad, San Diego, CA).
Acute toxicity
The acute toxicity in male Swiss albino mice was studied as per Organization for Economic Co-operation and Development (OECD) guidelines 425 (OECD, Citation2008). LD50 value of MECH was determined using the method of maximum likelihood.
Induction of cancer using DLA cells
Dalton’s lymphoma ascites (DLA) cells were obtained under the courtesy of Amala Cancer Research Center, Trissur, Kerala, India. The cells were maintained in vivo in Swiss albino mice by intraperitoneal administration. While transforming the tumor cells to the grouped animals, the DLA cells were aspirated from peritoneal cavity of the mice using saline. The cells were counted and further dilution was made so that the total cell should be 1 × 106/mouse. This dilution was given intraperitoneally.
Animals
Male and female adult Swiss Albino mice of about 8 weeks old, with an average body weight of 23 ± 2 g were procured from KM College of Pharmacy, Madurai, Tamil Nadu. They were housed in microloan boxes in a controlled environment of temperature 25 ± 2 °C and 12 h dark/light cycle with standard laboratory diet and water ad libitum. The study was conducted after obtaining Institutional Animal Ethical Committee’s clearance (Protocol no. IAEC/KMCP/92/2013). The mice were segregated based on their gender and acclimatized for 15 d before the commencement of the experiment.
Treatment protocol
The animals were divided into five groups (n = 12). All the groups except the first group received 0.1 ml cell suspension of DLA tumor cell line (1 × 106 cells/mouse, i.p.). This was considered as day ‘0’. The first group served as a normal saline control which received normal saline of 5 ml/kg body weight, orally (p.o.). The second group served as a DLA tumor cell line control. The third group received the standard drug 5-fluorouracil (20 mg/kg body weight, i.p.) for nine consecutive days. After 24 h of tumor inoculation, the fourth and fifth groups received MECH at the doses of 200 and 400 mg/kg body weight, orally (p.o.), respectively. On day 15, blood was withdrawn by retro orbital plexus method for the estimation of hematological and serum biochemical parameters. The mice were sacrificed for the study of antitumor parameters. The remaining animals of each group were kept alive with food and water ad libitum to check the increase in lifespan of the mice (Gupta et al., Citation2007). The effect of MECH on tumor growth and host’s survival time was assessed by observation of body weight, packed cell volume, viable cell count, and % increase in lifespan.
Cancer cell count
The fluid (0.1 ml) from the peritoneal cavity of each mouse was withdrawn by sterile syringe and diluted with 0.8 ml of sterile PBS and 0.1 ml of Trypan blue (0.1 mg/ml) and the total number of the living cells was counted using hemocytometer. Cell count was calculated by the formula:
Hematological parameters
Various hematological parameters including WBC count, RBC count, platelet count, hemoglobin, and packed cell volume were determined.
Body weight
All the mice were weighed from the beginning to the 15th day of the study. Average increase in body weight on the 15th day was determined.
Percentage increase in lifespan (ILS)
% ILS was calculated by the formula: % ILS = (lifespan of treated group)/(lifespan of control group) − 1 × 100.
Serum enzyme and lipid profile
The serum was analyzed for aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), total cholesterol (TC), and triglyceride (TG). All biochemical investigations were done by using COBAS MIRA PLUS-S Auto analyzer from Roche, Basel, Switzerland. Hematological tests were carried out in COBAS MICROS OT 18 from Roche (Basel, Switzerland).
Estimation of lipid peroxidation (TBARS), superoxide dismutase (SOD), and catalase (CAT)
The thiobarbituric acid reactive substances (TBARS) in the cell lysates tissue were measured as per the method reported earlier (Ohkawa et al., Citation1979). TBARS content was expressed in µmoles/mg protein. The SOD activity in cell lysate was determined as per the method followed earlier (Kakkar et al., Citation1984). Enzyme activity was expressed as 1 Unit = 50% inhibition/minute/mg of protein. The CAT activity in cell lysate was assayed as per the method reported earlier (Aebi, Citation1974). CAT was expressed in terms of μmol of hydrogen peroxide decomposed/min/mg of protein.
Statistical analysis
The values are represented as mean ± SD. The experimental data were assessed by a one-way Anova method followed by Tukey’s multiple comparison. The results were considered to be statistically significant when the p value is <0.05.
Results
Acute toxicity
MECH did not show any toxic symptoms in animals over a period of 24 h. The oral extract was non-lethal even at the single dose of 2000 mg/kg.
Secondary metabolites
Estimation of secondary metabolites showed significant content of total alkaloid (165.6 mg/100 g), total phenolic (43.5 GAE mg/g), and total flavanoid (4.97 RE mg/g) in MECH ().
Table 1. Estimation of total alkaloid, phenolic and flavanoid content of the extracts.
In vitro anti-proliferative study
MECH exhibited significant (p < 0.001) anti-proliferative activity against the breast cancer cell line MCF-7 with a mean IC50 value of 78.5 ± 3.6 µg/ml. The IC50 values of hexane, petroleum ether, chloroform, ethyl acetate, and aqueous extracts were found to be 221.5 ± 16.68, 255 ± 17.88, 213 ± 8.4, 147 ± 7.9, and 229 ± 8.02 µg/ml, respectively ().
Effect on tumor growth
The average lifespan of animals in DLA tumor control group was found to be 48% (). The average lifespan of animals treated with MECH at the doses of 200 and 400 mg/kg body weight was found to be 58 and 77%, respectively, whereas the average lifespan of 5-FU-treated group was found to be 92%. An increase in packed cell volume is observed in DLA control mice over the extract treated group. MECH-treated groups showed a significant (p < 0.001) reduction in the packed cell volume. Similarly, MECH exhibited significant (p < 0.001) decrease in the viable cell count at the dose of 400 mg/kg than (p < 0.01) MECH-treated group at the dose of 200 mg/kg. Moreover the antitumor nature of MECH at a dose of 200 and 400 mg/kg was also evident by the significant (p < 0.001) reduction in percent increase in body weight of animal treated with MECH at a dose of 200 and 400 mg/kg body weight when compared to DLA tumor-bearing mice. All these results indicate that MECH has significant (p < 0.001) activity to inhibit tumor growth induced by DLA cell line.
Table 2. Effect of MECH on body weight, packed cell volume, viable cell count, and % increased lifespan of tumor induced mice.
Effect on hematological parameters
Hematological parameters were significantly (p < 0.001) altered after 14 d of treatment when compared with the DLA control group. Total WBC count was increased in the DLA control cell whereas RBC count, hemoglobin, and platelets decreased in DLA control cells. After treating for 14 d with MECH at doses of 200 and 400 mg/kg, the body weight and the hematological parameters were normalized close to the normal group. WBC significantly (p < 0.001) decreased in both the MECH-treated groups. RBC significantly (p < 0.001) increased in the MECH group treated with 200 mg/kg than (p < 0.01) the group treated with 400 mg/kg. Hemoglobin significantly (p < 0.001) increased in both the MECH-treated groups. Platelets significantly (p < 0.001) increased in the MECH group treated with 200 mg/kg than (p < 0.05) the group treated with 400 mg/kg. All these results suggest the anticancer nature of MECH at a dose of 200 and 400 mg/kg body weight. However, the reference drug 5-FU at the dose of 20 mg/kg body weight produced significant (p < 0.001) results in all these parameters ().
Table 3. Effect of MECH on hematological parameters.
Effect on biochemical parameters
Biochemical parameters like AST, ALT, ALP, TGL, and serum cholesterol in the DAC control group were significantly (p < 0.001) elevated as compared to the normal saline group (). Treatment with MECH at the dose of 200 and 400 mg/kg body weight significantly (p < 0.001) reduced AST, ALT, ALP, TGL, and serum cholesterol towards the normal values. The treatment with standard 5-FU also gave similar results.
Table 4. Effect of MECH on serum biochemical parameters.
Effect on SOD, CAT, and lipid peroxidation
SOD and CAT activities were reduced significantly (p < 0.001) in the DLA control groups compared with that of normal group. Treatment of MECH at 200 (p < 0.01) and 400 mg/kg significantly (p < 0.001) restored SOD level towards normal values when compared with the DLA control group. Similarly, administration of MECH at 200 and 400 mg/kg significantly (p < 0.001) recovered CAT level towards normal value when compared with DLA control group ().
Table 5. Effect of MECH on lipid peroxidation, SOD, and CAT in DLA-bearing mice.
The TBARS levels expressed as MDA were significantly (p < 0.001) increased in DLA-controlled animals when compared to that of normal control group. Treatment with MECH at 200 and 400 mg/kg body weight significantly (p < 0.001) reduced the MDA levels when compared with DLA control group.
Discussion
The present study was aimed at analyzing the in vitro antitumor activity of various C. hirsutus extracts against MCF-7 cancer cell line and in vivo antitumor activity of the active extract in DLA tumor bearing mice. MECH showed significant (p < 0.001) cytotoxic activity with an IC50 value of 84.56 µg/ml when compared with other extracts. The results of in vivo study revealed that MECH with doses of 200 and 400 mg/kg body weight significantly (p < 0.001) reduced the packed cell volume, tumor cell count, and restored the hematological and serum biochemical parameters towards normal values.
Ascetic fluid is the direct nutritional source for tumor cells and, therefore, a rapid increase in ascetic fluid with tumor growth would be a means to meet the nutritional requirement of tumor cells (Pallab et al., Citation2010; Prasad & Giri, Citation1994). Enormous increase in ascetic fluid volume was observed in DLA tumor bearing hosts but the treated group with MECH shows decrease in the packed cell volume, viable tumor cell count, and increased the lifespan of the DLA tumor-bearing mice. The reliable measure for assessing the value of anticancer drug is increasing the lifespan of the tumor bearing animal (Clarkson & Burchneal, Citation1965). It can, therefore, be understood that MECH increased the lifespan of DLA bearing mice which was due to the prevention of tumor development. Thus MECH has antitumor activity against DLA-bearing mice at a dose of 200 and 400 mg/kg body weight.
Myelosupression and anemia are major problems that are encountered in the treatment of cancer chemotherapy (Hogland, Citation1982; Prince & Greenfield, Citation1958). Anemia occurring in tumor bearing mice is mainly due to reduction in erythrocytes or hemoglobin and this may happen either due to iron deficiency or due to hemolytic or myelopathic conditions (Fenninger & Mider, Citation1954). In the present study, the results indicate that MECH significantly increased the erythrocyte count and hemoglobin level when compared to those of DLA controlled mice. Moreover, the WBC count had decreased when compared to that of DLA-controlled mice. These parameters show that MECH shows less toxic effect to the hemopoietic system and reasonably had selective affinity to the tumor cell and hence it could maintain the normal hematological profile.
Increased levels of AST, ALT, ALP, and serum bilirubin are indicative of impaired liver functions due to cancer (Dortman & Lawhorn, Citation1978; Moss & Butterworth, Citation1974). The significantly increased levels of total AST, ALT, ALP, TGL, and cholesterol in the serum of tumor-inoculated animals indicated liver damage and loss of functional integrity of cell membrane. Treatment with MECH at a dose of 200 and 400 mg/kg body weight restored the above-mentioned parameters towards the normal level.
In the present study, the biochemical examination of DLA inoculated animals showed marked changes indicating the toxic effect of the tumor. MECH significantly reduced viability of tumor cells and packed cell volume, and normalized the hematological profile and serum biochemical parameters, raising the lifespan of the treated group as compared with those of DLA control mice. Also, the group treated with MECH improved the enzymatic and non-enzymatic antioxidant systems. Decrease of lipid peroxidation and augmentation of SOD and CAT in MECH treated mice showed its potential as an inhibitor of DLA-induced intracellular oxidative stress. Thus, MECH demonstrated remarkable in vitro and in vivo antitumor activity against DLA in mice plausibly by attributing lipid peroxidation and increasing endogenous antioxidant systems.
Conclusion
The methanol extract of C. hirsutus contains high amounts of alkaloids, phenolic compounds, and flavonoids and exhibited significant in vitro and in vivo antitumor activity. The antitumor activity may be due to its marked antioxidant potential.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aebi H. (1974). Catalase. In: Packer L, ed. Methods in Enzymatic Analysis, vol. II. New York: Academic Press, 673–84
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. (1994). Standardization of propolis extract and identification of principal constituents. J Pharm Belg 49:462–8
- Chandler SF, Dodds JH. (1983). The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasidine in callus cultures of Solanum lacinitum. Plant Cell Rep 2:205–8
- Clarkson D, Burchneal JH. (1965). Preliminary screening of antineoplastic drugs. Prog Clin Cancer 1:625–9
- Dortman RB, Lawhorn GT. (1978). Serum enzymes as indicators of chemical induced liver damage. Drug Chem Toxicol 1:163–71
- Fenninger LD, Mider GB. (1954). Energy and nitrogen metabolism in cancer. Adv Cancer Res 2:229–53
- Gupta M, Mazumder UK, Kumar RS, Kumar TS. (2004). Antitumor activity and antioxidant role of Bauhinia racemosa against Elrich ascites carcinoma in Swiss albino mice. Acta Pharmacol Sin 25:1070–6
- Gupta M, Mazumeder UK, Haldar PK, Kandar CC. (2007). Anticancer activity of Indigofera aspalathoides and Wedelia calendulaceae in Swiss albino mice. Iranian J Pharm Res 6:141–5
- Hogland HC. (1982). Hematological complication of cancer chemotherapy. Semin Oncol 9:95–102
- Kakkar P, Das B, Vishwanath PN. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–2
- Khare CP. (2007). Indian Medicinal Plants. New York, NY: Springer, 162–3
- Mascarenhas M. (1994). Structure-activity characterization, a quick method to screen mushrooms for the presence of antitumor glucans. Mushroom Res 3:77–80
- Moss DW, Butterworth PJ. (1974). Enzymology and Medicine. London: Pitman Medical
- Nitha B, Meera CR, Janardhanan KK. (2005). Antitumor activity of ethanolic extract of Lentinus dicholamellatus. Amala Res Bull 25:165–8
- OECD. (2008). Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. Paris: Organisation for Economic Co-operation and Development Publishing
- Ohkawa H, Onishi N, Yagi K. (1979). Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Pallab KH, Biswakanth Kar, Asis Bala, et al. (2010). Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm Biol 48:1337–43
- Prasad SB, Giri A. (1994). Antitumor effect of cisplatin against murine ascites Dalton’s lymphoma. Indian J Exp Biol 32:155–62
- Prince VE, Greenfield RE. (1958). Anemia in cancer. In: Grensstein JP, Haddaw A, eds. Advances in Cancer Research, vol. V. New York: Academic Press, 199–200
- Raju Asirvatham, Arockiasamy Josphin MC. (2013). Anticancer activity of Drosera indica L., on Dalton’s lymphoma ascites (DLA) bearing mice. J Intercult Ethnopharmacol 2:9–14
- Rasheed T, Khan MN, Zhadi SS, Durani S. (1991a). Hirsutine: A new alkaloid from Cocculus hirsutus. Pak J Nat Prod 54:582–4
- Rasheed T, Khan MN, Zhadi SS, Durrani S. (1991b). Shaheenine: A new alkaloid from Cocculus hirsutus. Fitoterapia 62:157–8
- Sreevidya N, Mehrotra S. (2003). Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff’s reagent in plant materials. J AOAC Int 86:1124–7
- Viqar UA, Atta-ur-Rahman, Rasheed T, et al. (1987a). Cohirsine – A novel isoquinoline alkaloid from Cocculus hirsutus. Tetrahedron 43:5865–72
- Viqar UA, Atta-ur-Rahman, Rasheed T, Habib-ur-Rahman. (1987b). Jamtine-N-oxide, a new isoquinoline alkaloid from Cocculus hirsutus. Heterocycles 26:1251–5
- Viqar UA, Faryal VM, Rasheed T. (1987c). Hirsudiol, a triterpenoid from Cocculus hirsutus. Phytochemistry 26:793–4
- Viqar UA, Iqbal S. (1992). Cohirstinine, a new isoquinoline alkaloid from Cocculus hirsutus. J Nat Prod 55:237–9
- Viqar UA, Iqbal S. (1993a). Haiderine, a new isoquinoline alkaloid from Cocculus hirsutus. Nat Prod Lett 2:105–9
- Viqar UA, Iqbal S. (1993b). Jamtinine, an alkaloid from Cocculus hirsutus. Phytochemistry 33:735–6
- Viqar UA, Rasheed T, Iqbal S. (1991). Cohirsinine, an alkaloid from Cocculus hirsutus. Phytochemistry 30:1350–1
- Warrier PK, Nambier VP, Ramankutty C. (2005). Indian Medicinal Plants – A Compendium of 500 Species, vol. V. Chennai: Orient Longman Pvt Ltd, 138–40