Abstract
Context: Otostegia persica (Burm.) Boiss. (Lamiaceae), “Goldar” in Persian, is widely used in the folk medicine of south Iran for control of diabetes mellitus.
Objective: In the present study, hypoglycemic and antioxidant effects of different fractions of the O. persica extract were investigated and constituents of effective fractions were elucidated.
Materials and method: Different concentrations (100–400 mg/kg) of aqueous infusion (AI) of flowering aerial parts of the plant (traditional preparation) and all fractions of the O. persica extract (i.p. injection) were tested for antidiabetic activity in streptozocin-induced diabetic NMRI mice. Blood glucose level was measured at time 0 and intervals of 1, 2, 4, and 6 h later. Antioxidant activities of different fractions of the plant extract and pure compounds (0.1, 0.5, and 1 mg/ml) were determined with the DPPH method. Four compounds were isolated and identified from potent fractions.
Results and discussion: Antidiabetic activity demonstrated that the effect of the methanol fraction at a dose of 300 mg/kg was equivalent with glibenclamide, and at a dose of 400 mg/kg was comparable with glibenclamide and insulin (p > 0.05). The EC50 of the methanol fraction was 307.12 mg. Methanol and ethyl acetate fractions showed antioxidant activities (both IC50 equal to 0.49 mg/ml), so these fractions were selected for the purification of compounds. Chrysoeriol from ethyl acetate and three apigenin derivatives (6-methylapigenin, apigenin-7-O-glucoside, and echinaticin) from the methanol fraction were isolated and identified (new for the species). Chrysoeriol exhibited potent antioxidant activity comparable with vitamin E and BHT (p > 0.05).
Conclusion: The present study confirmed the folklore usage of O. persica for antidiabetic properties.
Introduction
Diabetes mellitus is a chronic metabolic disorder that afflicts 6.4% of the population in the current world and is expected to grow up to 7.7% of the adult population by 2030. In the last 20 years, there has been a three-fold increase in the prevalence of diabetes (Shaw et al., Citation2010). Around 95% of diabetic patients are diagnosed with type 2 diabetes, demonstrating its hyperglycemia feature by insulin resistance and/or insulin deficiency (Attele et al., Citation2002; Laakso, Citation2001).
Since current antidiabetic drugs usually have adverse side effects, are high in cost, and have decreased efficacy over time (De Melo et al., Citation2002), the interest for the discovery and application of natural compounds is increasing.
There were about 1200 species of antidiabetic plants in folk medicine (Marles & Farnsworth, Citation1995). Otostegia persica (Burm.) Boiss. (Lamiaceae) is an endemic plant of Iran and Pakistan (Recshinger, Citation1982). It is used in the folk medicine of Sistan and Baluchestan Province for the treatment of diseases such as antidiabetic disorder. The previous antidiabetic evaluations showed that oral administration of O. persica extract has a strong dose-dependent reduction in the blood glucose level and significant enhancement of serum insulin level. In addition, the O. persica extract showed insulin secretory properties, anti-lipid peroxidation, and improvement of renal function in diabetic animals (Ebrahimpoor et al., Citation2011; Hedayati & Pouraboli, Citation2012; Manzari-Tavakoli et al., Citation2013).
Other biological effects identified in the plant are: antirheumatic and analgesic agents used in toothache (Sharififar et al., Citation2005), treatment of oral infections and tooth pain (Asadipour et al., Citation2004), reduction of signs of morphine withdrawal syndrome (Hajhashemi et al., Citation2004), and antimicrobial activity against Gram positive strains including Listeria monocytogens, Enterococcus fecalis, Staphylococcus aureus, and Staphylococcus epidermidis (Asghari et al., Citation2006).
The previous phytochemical studies demonstrated the elucidation of five phenol compounds – morin, kaempferol, quercetin, isovitexin (C-glycoflavone), and cinnamic acid – from O. persica grown in the Kerman Province (Yassa et al., Citation2005). 3′,7-Dihydroxy-4′,6,8-trimethoxy-flavone, another flavonoid of this plant, prevents glycation formation, which implicated in the pathogenesis of diabetic complications (Ayatollahi et al., Citation2010). Eleven compounds belonging to phenolic acids and triterpenoids/steroids and four diterpenoids belonging to clerodane and tetracyclic diterpene types were isolated from O. persica in other investigations (Ayatollahi et al., Citation2007, Citation2009).
This study sought to determine an effective antidiabetic fraction of O. persica and isolation of its constituents. In addition, the free-radical scavenging activities of all fractions and pure compounds were investigated.
Materials and methods
Chemicals
Vitamin E 97% (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Fluka, Buchs, Switzerland), butylhydroxytoluene (BHT) (Merck, Hohenbrunn, Germany), methanol (Merck, Darmstadt, Germany), streptozotocin (STZ) (Sigma Chemical Co., Saint Louis, MO), glibenclamide (Pursina, Tehran, Iran), NPH insulin (Exir Co., Tehran, Iran), normal saline 0.9% solution (Samen Pharmaceutical Co., Mashhad, Iran), and Glucometer (ACCU-CHEK, Roche, Mannheim, Germany).
Plant material
Otostegia persica was collected at the flowering stage in May 2011, around the Taftan mountain of Sistan and Baluchestan Province, Iran. The aerial parts were dried in shade and powdered. A voucher specimen has been deposited at the Herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences (THE-6684; identified by Dr. Gh. Amin).
Extraction and fractionation
The powder of dried aerial parts of O. persica (1256 g) was extracted with 80% methanol by maceration to obtain a crude extract (631.51 g, yield 50.3%). The crude extract was fractionated with petroleum ether (PE), chloroform (CH), ethyl acetate (EA), butanol (BU), and methanol (ME) to yield 20.24, 114.95, 8.51, 22.6, and 148.89 g, respectively.
Preparation of the aqueous infusion (AI) extract
Aqueous extract of the plant (20 g) was obtained by infusion with 800 ml of water. After concentrating the solvent, a brown powder residue (4.25 g) was achieved.
Antioxidant activity
The DPPH method was used for the determination of free-radical scavenging antioxidant activity (Tofighi et al., Citation2009). Different fractions (1 ml each) and pure compounds (0.1, 0.5, and 1.0 mg/ml) were added to 2 ml of DPPH solution (4 × 10−5 g/ml methanol). Control consisted of sample that was added to methanol up to 3 ml; blank contained 1 ml of methanol with no sample, which was added to 2 ml of DPPH solution. A Shimadzu UV/Vis model 160A spectrophotometer (Shimadzu, Kyoto, Japan) was used for the measurement of absorbance at 517 nm in 0, 5, 10, 15, 20, 25, and 30 min. The inhibition activity was calculated using the following formula:
All tests and analyses were carried out in triplicate.
Antidiabetic assay
Animals
Male NMRI mice (25–30 g) were obtained from the Pasteur Institute of Iran, Tehran, Iran. The animals were housed under the following environmental conditions: temperature (21 ± 2 °C), humidity (51 ± 10%), and 12 h light-dark cycles with standard pellet diet and water ad libitum. The study was permitted by the Institutional Animal Ethics Committee (Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran) and was performed according to the international rules considering the animal experiments and biodiversity right.
Induction of experimental diabetes and biological assays
After 12 h fasting, mice were administrated a single intra-peritoneal (i.p.) dose of 200 mg/kg STZ dissolved in 0.05 M phosphate buffer (pH = 4) (Dogrul et al., Citation2004; Hayashi et al., Citation2006). Three days later, blood glucose level of mice reached more than 200 mg/dl and stabilized.
On the day of the experiment, overnight-fasted diabetic mice were divided into 10 groups of six animals each that received i.p. injections of different concentrations (100–400 mg/kg) of AI extract, PE, CH, EA, BU, and ME fractions. The fractions were dissolved in normal saline and, if necessary, Tween 80 was added.
Group 1 served as a control and received 0.5 ml normal saline; group 2 received 0.5 ml of normal saline plus Tween 80; groups 3–6 received ME fraction (100–400 mg/kg); groups 7–8 were administrated AI extract (300 and 400 mg/kg); groups 9–10 received glibenclamide and NPH insulin as reference (3 mg/kg and 12.5 IU/kg) (Sarkhail et al., Citation2007; Stammberger et al., Citation2002).
The blood glucose level of mice was measured exactly before and after the injection of fractions at time 0 and intervals of 1, 2, 4, and 6 h later using an Accu-Chek apparatus (ACCU-CHEK, Roche, Mannheim, Germany).
Statistical analyses
The results were presented as mean ± S.D. Statistical significance between the treated and control groups were evaluated by Student’s t-test, and p < 0.05 was considered significant.
Isolation and purification of compounds
According to the results of biological tests, ME and EA fractions showed most antidiabetic and antioxidant activities. So, these fractions were selected for the isolation of compounds.
ME fraction (10 g) was separated on a silicagel column (reverse phase, 2.5 × 17.5 cm) with H2O–methanol (80: 20 → 0: 100, V/V) as a gradient mobile phase to afford four subfractions. Subfraction 1 (519.5 mg) was selected for further separation on Sephadex-LH20 CC (Sigma Chemical Co., St. Louis, MO) (2.1 × 67 cm) and methanol was used as a solvent. Compounds 2 (14.8 mg), 3 (129.6 mg), and 4 (54.5 mg) were isolated and purified.
The EA fraction (5 g) was fractionated with a silicagel column (normal phase, 4 × 24 cm) and chloroform:methanol (100:0 → 0:100, V/V) as a mobile phase to yield six subfractions. Subfraction 1 (108.1 mg) was subjected to a silicagel column (normal phase, 1.2 × 30 cm) using BAW; butanol:acetic acid:H2O (3:1:1) as an eluent to obtain compound 1 (9.8 mg). This compound was applied to a Sephadex-LH20 CC (1.2 × 67 cm) eluted with methanol for further purification.
Results
Spectral analysis of isolated compounds
The isolated compounds were identified using different spectroscopic methods ().
Figure 1. Structure of pure compounds; chrysoeriol 1, 6-methylapigenin 2, apigenin7-O-glucoside 3, echinaticin 4.
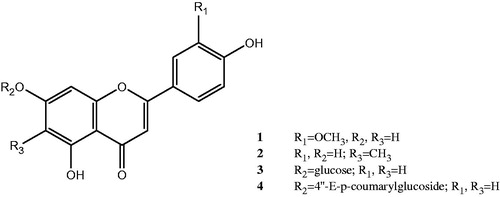
Chrysoeriol (1): Pale yellow amorphous powder: UV λmax nm (log) MeOH: 346, 270, 256sh; +AlCl3: 390, 360sh, 296, 275, 259; +AlCl3/HCl: 388, 351, 295, 278, 258; +NaOMe: 400, 328sh, 275sh, 260; +NaOAc: 394, 320sh, 272; +NaOAc/H3BO3: 348, 268; 1H NMR (500 MHz, DMSO-d6/CD3OD): δ 7.43 (1H, d, J = 8.4 Hz, H-6′), 7.39 (1H, s, H-2′), 6.88 (1H, d, J = 8.4 Hz, H-5′), 6.61 (1H, s, H-3), 6.35 (1H, bs, H-8), 6.08 (1H, bs, H-6), 3.87 (3H, s, OMe-3′); 13C NMR (DMSO-d6/CD3OD): δ 182.1 (C-4), 167.3 (C-7), 164.7 (C-2), 162.2 (C-5), 158.3 (C-9), 148.3 (C-3′), 147.2 (C-4′), 121.0 (C-1′), 119.5 (C-6′), 116.9 (C-5′), 113.7 (C-2′), 103.7 (C-3), 102.8 (C-10), 100.2 (C-6), 94.9 (C-8), 56.5(OCH3); EIMS (m/z %): 300 [M]+(17), 286 [M − Me] (70), 151 [B2] (18), 153 [A1] (30), 148 [B1] (10), 134 [B1 − Me] (20).
6-Methylapigenin (2): Yellow amorphous powder: UV λmax nm MeOH: 346, 312sh, 273; +AlCl3: 388sh, 347, 306, 279, 262sh; +AlCl3/HCl: 388sh, 347, 306, 279, 262sh; +NaOMe: 397, 335, 277; +NaOAC: 378, 306sh, 281; +NaOAC/H3BO3: 345, 274; 1H NMR (500 MHz, DMSO-d6/CD3OD): δ 7.90 (2H, d, J = 8.2 Hz, H-2′,6′), 6.90 (2H, d, J = 8.2 Hz, H-3′,5′), 6.62 (1H, s, H-3), 6.60 (1H, s, H-8), 2.5 (3H, s, Me-6); 13C NMR (DMSO-d6/CD3OD): δ 181.7 (C-4), 164.2 (C-7), 163.7 (C-2), 161.4 (C-4′), 159.0 (C-5), 157.3 (C-9), 128.7 (C-2′, 6′), 122.3 (C-1′), 115.9 (C-3′, 5′), 108.5 (C-6), 106.8 (C-3), 103.0 (C-10), 94.0 (C-8), 7.8 (CH3); EIMS (m/z %): 285 [M]+ (8), 269 [M − Me] (100), 167 [A1 + Me] (9), 152 [A1] (13), 121 [B2] (40).
Apigenin-7-O-glucoside (3): Pale yellow amorphous powder: UV λmax nm (log) MeOH: 335, 269.5; +AlCl3: 385, 347, 299, 276; +AlCl3/HCl: 386, 346, 298, 276; +NaOMe: 386, 298, 270, 250sh; +NaOAC: 386, 348, 267, 256sh; +NaOAC/H3BO3: 342, 268; 1H NMR (500 MHz, DMSO-d6/CD3OD): δ 7.95 (2H, d, J = 8.5 Hz, H-2′,6′), 7.06 (2H, d, J = 8.5 Hz, H-3′,5′), 6.87 (1H, bs, H-8), 6.83 (1H, s, H-3), 6.58 (1H, bs, H-6), 5.17 (1H, d, J = 7.6 Hz, H-1″), 3.5–4.5 (5H, m, H-2″-6″); 13C NMR (DMSO-d6/CD3OD): δ 182.3 (C-4), 163.7 (C-7), 163.3 (C-2), 161.7 (C-4′), 159.1 (C-5), 158.2 (C-9), 128.7 (C-2′, 6′), 121.3 (C-1′), 116.0 (C-3′, 5′), 106.2 (C-10), 105.3 (C-3), 103.2 (C-1″), 99.5 (C-6), 95.4 (C-8), 76.6 (C-5″), 74.7 (C-3″), 73.2 (C-2″), 70.3 (C-4″), 61.2 (C-6″); EIMS (m/z %): 270 [M-glucose] (100), 153 [A1 + H] (27), 121 [B2] (36), 118 [B1] (20).
Apigenin 7-(4″-E-p-coumarylglucoside): echinaticin (4): Yellow amorphous powder: UV λmax nm (log) MeOH: 319, 290sh, 269, 228sh; +AlCl3: 384, 327, 300, 277; +AlCl3/HCl: 384, 326, 300, 277; +NaOMe: 376, 308sh, 268, 240sh; +NaOAC: 380sh, 317, 290sh, 268; +NaOAC/H3BO3: 319, 290sh, 268; 1H NMR (500 MHz, DMSO-d6/CD3OD): δ 7.87 (2H, d, J = 8.7 Hz, H-2′, 6′), 7.53 (1H, d, J = 15.7 Hz, H-7′′′), 7.28 (2H, d, J = 8.5 Hz, H-2′′′, 6′′′), 7.00 (2H, d, J = 8.7 Hz, H-3′, 5′), 6.72 (2H, d, J = 8.5 Hz, H-3′′′, 5′′′), 6.71 (1H, d, J = 2 Hz, H-8), 6.68 (1H, s, H-3), 6.50 (1H, d, J = 2 Hz, H-6), 6.29 (1H, d, J = 15.7 Hz, H-8′′′), 5.09 (1H, d, J = 7.1 Hz, H-1″), 3.5–4.5 (6H, m, H-2″, 3″, 4″, 5″, 6″); 13C NMR (DMSO-d6/CD3OD): δ 182.3 (C-4), 167.0 (C-9′′′), 163.7 (C-2), 163.3 (C-7), 161.5 (C-4′), 160.4 (C-5), 159.4 (C-4′′′), 158.2 (C-9), 145.1 (C-7′′′), 130.5 (C-2′′′, 6′′′), 128.9 (C-2′, 6′), 126.8 (C-1′′′), 122.3 (C-1′), 117.0 (C-8′′′), 116.1 (C-3′′′, 5′′′), 115.9 (C-3′, 5′), 107.5 (C-10), 105.7 (C-3), 101.0 (C-1″), 99.8 (C-6), 95.2 (C-8), 77.2 (C-5″), 76.9 (C-3″), 73.4 (C-2″), 72.9 (C-4″), 61.9 (C-6″); EIMS (m/z %): 270 [M-glucose–coumaric acid]+ (100), 269 (18), 242 (16), 164 [coumaric acid] (7), 153 [A1] (25), 147 (26), 121 [B2] (22), 120 (32), 119 (17), 118 [B1] (13).
Antioxidant activity
Antioxidant activities of different fractions of the plant extract (PE, CH, EA, BU, and ME) and pure compounds were determined with the DPPH method ( and ). ME fraction showed potent activity of free-radical scavenging with 91.53% inhibition percent comparable with vitamin E (94.69%) and BHT (90.6%) (p > 0.05). EA and BU fractions demonstrated moderate antioxidant activity with 81.96 and 81.50%, respectively. The IC50 of ME, BU, and EA fractions was equal to 0.49 mg/ml (Y = 89.49 x + 5.37, r2 = 0.97; Y = 67.21 x + 16.32, r2 = 0.98; Y = 69.83 x + 15.87, r2 = 0.95, respectively). The free-radical scavenging activity of chrysoeriol was more than other pure compounds, which was comparable with vitamin E and BHT (p > 0.05).
Figure 2. Comparison of antioxidant activity of different fractions and pure compounds of O. persica (1 mg/ml) with BHT (200 µg/ml) and vitamin E (50 µg/ml).
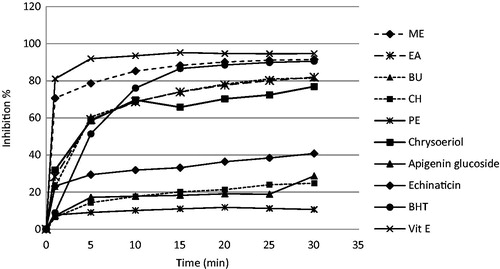
Table 1. Radical scavenging activity of different fractions and pure compounds of O. persica.
Antidiabetic activity
Antidiabetic activity of AI extract and all fractions of O. persica are shown in . AI extract at a dose of 400 mg/kg demonstrated a significant reduction of blood glucose level in diabetic mice. ME fraction at a dose of 100 and 200 mg/kg showed equal response with NaCl (p = 0.1), but its antidiabetic effect at a dose of 300 mg/kg was equivalent with glibenclamide (p = 0.07) and weaker than insulin (p = 0.003). There was no significant difference between this fraction at a dose of 400 mg/kg and glibenclamide (p = 0.45) or insulin (p = 0.06). The EC50 of methanol fraction was 307.12 mg (Y = 0.1481x + 4.515; r2 = 0.92). Other fractions did not show hypoglycemic effect in diabetic mice.
Table 2. Antidiabetic activity of AI extract and different fractions of O. persica extract.
Discussion
Otostegia persica is an endemic plant of Iran used as an antidiabetic remedy in Sistan and Baluchestan Province. The aim of the present study was to investigate antidiabetic and antioxidant effects of different fractions of O. persica and elucidation of pure compounds of effective fractions.
According to the results of antioxidant assay, ME fraction showed potent activity of free-radical scavenging which was comparable with vitamin E and BHT (p > 0.05). This result was confirmed by the previous study which exhibited strong antioxidant activity of ME fraction using beta-carotene bleaching and lipid peroxidation methods (Sharififar et al., Citation2003). The other investigation remarked that the antioxidant activity of the essential oil of O. persica was more potent than vitamin E and BHA (Tofighi et al., Citation2009).
AI extract and all fractions of O. persica were subjected to antidiabetic experiments. It was demonstrated that AI extract at a dose of 400 mg/kg and ME fraction at doses of 300 and 400 mg/kg could generate a significant reduction of blood glucose level in diabetic mice. However, AI extract and ME fraction indicated hypoglycemic effects but the difference between them was obvious (p < 0.05), which means the existence of various phytochemical components. It may be due to the omission of very polar compounds such as mono- and oligo-sacharides, amino acids, poly-sacharides, and proteins from ME fraction of the plant. This consequence proved the consumption of this herb in folk medicine for hypoglycemic properties.
The phytochemical screening of the O. persica crude extract in our investigation revealed the presence of flavonoids, phenolic acids, and steroids/triterpenoids. EA, BU, and ME fractions showed the presence of flavonoids and phenolic acids. Chrysoeriol from EA fraction and three apigenin derivatives, namely 6-methylapigenin, apigenin-7-O-glucoside, and echinaticin, were isolated from the ME fraction.
Chrysoeriol exhibited potent antioxidant activity which was comparable with vitamin E and BHT, and this result was confirmed by previous studies (Demirtas et al., Citation2013; Mishra et al., Citation2003). The other flavonoids purified from the ME fraction of this plant showed significant lipid peroxidation activity (Sharififar et al., Citation2005; Yassa et al., Citation2005).
The antidiabetic effect of the ME fraction of O. persica may be due to the presence of one or more antihyperglycemic principles which demonstrated synergistic properties. Phenolics and flavonoids, the abundant compounds of ME fraction, are found as effective antihyperglycemic agents and they regenerate the damaged beta cells in alloxan diabetic rats (Chakravarthy et al., Citation1980; Manickam et al., Citation1997). A previous study reported that total flavonoids in Leucaena seeds had a hypoglycemic effect in diabetic mice (Li et al., Citation2005). Other researchers identified that the oral administration of the methanol extract of Cleome droserifolia, which contained three flavonoids (quercetin, kaempferol, and isorhamnetin) and three phenolic acids (sinapinic, ferulic, and 4-coumaric acid), restored the blood glucose level, plasma malondialdehyde, and urine sugar near the physiological values (p < 0.05) (El Naggar et al., Citation2005).
Rauter et al. (Citation2010) and Chayarop et al. (Citation2011) showed that apigenin derivatives and chrysoeriol significantly lowered the blood glucose levels of diabetic animals; these compounds were isolated and identified in our study.
Conclusion
According to the results, the antihyperglycemic activity caused by glibenclamide in STZ-induced diabetic rats could indicate the presence of some beta cells. Since glibenclamide is known to stimulate insulin secretion from beta cells, the ME fraction of O. persica may stimulate the extant beta cells. Therefore, further experiments are required to distinct the exact mechanism of action and evaluation of antidiabetic activity of the isolated compounds.
Declaration of interest
The authors report no declarations of interest. This research was supported by Tehran University of Medical Sciences and Health Services Grant (No. 5692).
References
- Asadipour A, Khazaeli P, Mahmudi M, Saber Amoli S. (2004). The study of components of essential oil of Otostegia persica. Clin Exp Pharmacol Physiol 31:A51–202
- Asghari G, Nourallahi H, Havaie SA, Issa L. (2006). Antimicrobial of Otostegia persica Boiss. extracts. Res Pharm Sci 1:53–8
- Attele AS, Zhou YP, Xie JT, et al. (2002). Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 51:1851–8
- Ayatollahi SA, Kobarfard F, Asgarpanah J, et al. (2009). Diterpenoids of Otostegia persica (Burm.) Boiss. Daru 17:290–3
- Ayatollahi SAM, Kobarfard F, Asgarpanah J, Ahmed Z. (2007). Chemical constituents from Otostegia persica. J Chem Soc Pakistan 29:61–3
- Ayatollahi SAM, Kobarfard F, Asgarpanah J, Choudhary MI. (2010). Antiglycation activity of Otostegia persica (Burm.) Boiss. Afr J Biotechnol 9:3645–8
- Chakravarthy BK, Gupta S, Gambhir SS, Gode KD. (1980). Pancreatic beta-cell regeneration – a novel antidiabetic mechanism of Pterocarpus marsupium Roxb. Indian J Pharmacol 12:123–7
- Chayarop K, Temsiririrkkul R, Peungvicha P, et al. (2011). Antidiabetic effects and in vitro antioxidant activity of Pseuderanthemum palatiferum (Nees) Radlk. ex Lindau leaf aqueous extract. MU J Pharm Sci 38:13–22
- De Melo Junior EJM, Raposo MJ, Lisboa Neto JA, et al. (2002). Medicinal plants in the healing of dry socket in rats: Microbiological and microscopic analysis. Phytomedicine 9:109–16
- Demirtas I, Erenler R, Elmastas M, Goktasoglu A. (2013). Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem 136:34–40
- Dogrul A, Gul H, Yildiz O, et al. (2004). Cannabinoids blocks tactile allodynia in diabetic mice without attenuation of its antinociceptive effect. Neurosci Lett 368:82–6
- Ebrahimpoor MR, Khaksar Z, Noorafshan A. (2011). Anti-diabetic effect of orally administered Otostegia persica extract on streptozotocin diabetic rats. Comp Clin Path 20:523–5
- El Naggar EMB, Kov LB, Zemlicka M, et al. (2005). Antidiabetic effect of Cleome droserifolia aerial parts: Lipid peroxidation-induced oxidative stress in diabetic rats. Acta Vet Brno 74:347–52
- Hajhashemi VA, Rabbania M, Asghari GR, Karami-Saravi Z. (2004). Effects of Otostegia persica (Burm.) Boiss on morphine withdrawal syndrome in mice. Iran J Pharm Res 3:171–5
- Hayashi K, Kojima R, Ito M. (2006). Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull 29:1110–19
- Hedayati M, Pouraboli I. (2012). The effect of methanolic extract of Otostegia persica on serum glucose level and renal function indicators in streptozotocin induced diabetic rats. Zahedan J Res Med Sci 14:12–15
- Laakso M. (2001). Insulin resistance and its impact on the approach to therapy of type 2 diabetes. Int J Clin Pract 121:8–12
- Li XJ, Deng JG, Qin ZL, Huang HB. (2005). Experimental study on antidiabetic effect of the total flavonoids in Leucaena seeds. Zhongguo Zhongyao Zazhi 30:842–4
- Manickam M, Ramanathan M, Farboodniay Jahromi MA, et al. (1997). Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod 60:609–10
- Manzari-Tavakoli A, Pouraboli I, Yaghoobi MM, et al. (2013). Antihyperglycemic, antilipid peroxidation, and insulin secretory activities of Otostegia persica shoot extract in streptozotocin-induced diabetic rats and in vitro C187 pancreatic b-cells. Pharm Biol 51:253–9
- Marles RJ, Farnsworth NR. (1995). Antidiabetic plants and their active constituents. Phytomedicine 2:137–89
- Mishra B, Priyadarsini KI, Kumar MS, et al. (2003). Effect of O-glycosylation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorg Med Chem 11:2677–85
- Rauter AP, Martins A, Borges C, et al. (2010). Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother Res 24:S133–8
- Recshinger K. (1982). Otostegia persica (Labiatae). In: Rechinger K, ed. Flora Iranica. Akademische Druck-u, Verlagsanstalt, Graz-Austria, 347–8
- Sarkhail P, Rahmanipour S, Fadyevatan S, et al. (2007). Antidiabetic effect of Phlomis anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacol Res 56:261–6
- Sharififar F, Yassa N, Mozafarrian V, Shafiee A. (2005). In vitro evaluation of antioxidant activity of Otostegia persica L. Paper Presented at the First Seminar of Medicinal & Natural Products Chemistry, Shiraz, Iran
- Sharififar F, Yassa N, Shafiee A. (2003). Antioxidant activity of Otostegia persica (Labiatae) and its constituents. Iran J Pharm Res 2:235–9
- Shaw JE, Sicree RA, Zimmet PZ. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pr 87:4–14
- Stammberger I, Bube A, Durchfeld-Meyer B, et al. (2002). Evaluation of the carcinogenic potential of insulin glargine (Lantus) in rats and mice. Int J Toxicol 21:171–9
- Tofighi Z, Alipour F, Yassa N, et al. (2009). Chemical composition and antioxidant activity of Otostegia persica essential oil from Iran. Int J Essent Oil Ther 3:45–8
- Yassa N, Sharififar F, Shafiee A. (2005). Otostegia persica as a source of natural antioxidants. Pharm Biol 43:33–8

