Abstract
Context: Consumption of high fructose is associated with metabolic abnormalities, insulin resistance, and hypertension. It is not known whether this hypertensive effect of fructose is related to metabolic abnormalities or due to the direct effect of fructose on blood vessels.
Objective: Here, we investigated the direct effect of fructose on rat isolated aorta and the possible protective effect of curcumin.
Materials and methods: The isolated rat thoracic aorta rings were used to measure the contractile responses to different concentrations of both phenylephrine and KCl, and the relaxant response to acetylcholine (Ach). The effect of curcumin (1 µM) alone or in combination with tempol (1 mM), a superoxide dismutase mimetic agent, and N-{[3(amino-methyl)-phenyl]-methyl} ethanimidamide dihydrochloride (1400 W), a specific inducible nitric oxide synthase (iNOS) inhibitor, (1 µM) on fructose-treated aorta was compared. The aortic rings were incubated with different treatments for 60 min before starting the experiment. Changes in the intracellular calcium in response to KCl and nitric oxide levels were also measured.
Results and discussion: Fructose strongly increased the contractile response of aortic rings to both phenylephrine and KCl (Emax was increased by 147.3% and 150.5%, respectively) but it did not affect the relaxant response to Ach. Curcumin significantly decreased the hyper responsiveness of arterial rings to both vasopressors (for phenylephrine, Emax decreased from 147.3% in fructose incubated aorta to 81%, and for KCl, Emax decreased from 150.5% in fructose-incubated aorta to 77.24% respectively). Curcumin also reduces the intracellular calcium level (85% reduction in intracellular calcium). A 1400 W was the only agent that potentiates the effect of curcumin.
Conclusion: Fructose has a direct deleterious effect on aortic vascular reactivity. Curcumin can partially protect against fructose-induced impairment in vascular contractility via an antioxidant effect and reduction of elevated intracellular calcium.
Introduction
Disturbances in aortic vascular reactivity are important components of diabetes-induced macrovascular complications and hypertension (El-Bassossy et al., Citation2011a). Oxidative stress plays an important role in this process (Bhagya et al., Citation2012). High fructose consumption has increased in western countries over the last two centuries (Marriott et al., Citation2009). It is associated with insulin resistance, hypertriglyceridemia, hepatic steatosis, oxidative stress, and endothelial dysfunction (Hwang et al., Citation1987; Nyby et al., Citation2007; Shinozaki et al., Citation1999, Citation2004; Suwannaphet et al., Citation2010; Tran et al., Citation2009). Several studies relate the deterioration of endothelial function associated with high fructose consumption to metabolic abnormalities (Carranza et al., Citation2011; Patel et al., Citation2009). Others relate it to fructose-induced oxidative stress (Oudot et al., Citation2010; Rebolledo et al., Citation2010). Our previous studies (El-Bassossy et al., Citation2011a; Mahmoud et al., Citation2012) showed that fructose feeding to rats induce an inflammatory process in aorta which impairs vascular reactivity. However, no data were reported on the direct effect of fructose on vascular reactivity and endothelial function.
Curcumin is the major active constituent of the spice, turmeric (Pistelli et al., Citation2012). It has several beneficial effects including anti-inflammatory and antioxidant effects (Klawitter et al., Citation2012; Lin et al., Citation2012). It may also contribute to the improvement of insulin resistance (Shao et al., Citation2012). In a previous study, curcumin protects endothelial function of pulmonary artery from TNF-α-induced injury via induction of heme oxygenase-1 and antioxidant effect (El-Bassossy et al., Citation2009). Curcumin treatment attenuated phenylephrine-induced increase in contractility during early stage of streptozotocin (STZ)-induced diabetes via increasing antioxidant enzymes levels (Majithiya & Balaraman, Citation2005). Administration of bis (curcumino) oxo vanadate complex to STZ diabetic rats restored blood pressure and vascular reactivity to nor-adrenaline and acetylcholine (Ach) to normal by improving metabolic abnormalities (Majithiya et al., Citation2005).
Thus, this study investigated whether fructose directly alters vascular function. In addition, curcumin was tested either alone or in combination with a superoxide dismutase mimetic agent, tempol or iNOS inhibitor, 1400 W as a promising agent against the effects of fructose.
Materials and methods
Chemicals
Curcumin, tempol, and 1400 W were purchased from Sigma Chemical Co. (St. Louis, MO). Fructose was obtained from El-Nasr Company, Cairo, Egypt. All chemicals of physiological solution were of high purity and analytical grade.
Animals and diets
All animal procedures were approved by the Ethical Animal Research Committee of Zagazig University. Male Wistar rats aged 8 weeks were housed in temperature-controlled rooms (25 ± 2 °C) under a 12-h light:dark cycle. The rats were fed standard commercial chow diet ad libitum. The rat diet was composed of 62% starch, 23% protein, 4% fat, 7% cellulose, standard vitamins, and salt mixture. After acclimatization for 1 week, the rats were sacrificed and aorta was isolated, freed from fatty tissue and cut into pieces (3–4 mm length).
Preparation of thoracic aortae and measurement of vascular reactivity
The descending thoracic aortae of the rats were carefully isolated through an opening in the abdomen and immediately placed into a cold Petri dish filled with cold Krebs–Henseleit buffer containing (in mM), NaCl, 118.1; KCl, 4.69; KH2PO4, 1.2; NaHCO3, 25.0; MgSO4, 0.5, and CaCl2, 2.5. The control group solution contained 11 mM glucose while in other groups fructose in the same concentration was placed instead of glucose. The aorta was cleansed of excess connective tissue and fat, then cut into rings of approximately 3–4 mm in length and mounted in a 30-ml organ bath containing Krebs–Henseleit solution at 37 °C and aerated with 95% O2 and 5% CO2 to study contractile responses. Four to six rings were prepared from each aorta and studied in parallel. Force displacement of the rings was measured by Power Lab Data Interface Module connected to a PC running Chart software (v7.3, AD Instruments, Oxon, UK). Rings were equilibrated for 60 min, during which time the bath solution was changed every 15 min. In the aortic rings of rats, a passive stretch of 1 g was determined to be optimal tension for maximal responsiveness to phenylephrine (10−6 M). The viabilities of the preparations were checked by KCl (40 mM) and the rings that produced a tension of less than 1 g were not included in the experiments.
To prove the standardization of the aortic rings, two reproducible contractions were obtained with KCl (40 mM). The presence of the endothelium was tested functionally by applying Ach (10−6 M) on phenylephrine (10−6 M)-precontracted aortic rings and preparations demonstrating <70% relaxations in control group were discarded (Akar et al., Citation2012; Soylemez et al., Citation2011). The cumulative concentration–response curves of phenylephrine (10−9–10−4 M) were constructed in aortic rings. The relaxant effects of Ach (10−9–10−4 M) were studied in arterial rings constricted sub-maximally with phenylephrine (10−6 M). The aortic rings were incubated with different treatments for 60 min before starting the experiment and placed in the organ bath throughout the experiment (glucose 11 mM, fructose 11 mM, fructose + curcumin (1 µM), fructose + curcumin + tempol (1 mM) or fructose + curcumin + N{[3(amino methyl) phenyl]methyl} ethanimidamide dihydrochloride (1400 W) (1 µM).
Ach-induced nitric oxide (NO) generation and KCl-stimulated Ca2+ influx
The real-time generation of intracellular NO following Ach stimulation and the intracellular Ca2+ level following KCl stimulation were measured with the fluorescence probes 4-amino-5-methylamino-20, 70-difluorofluorescein diacetate (DAF-FM) and Calcium Green TM-2 (Molecular Probes, Paisley, UK) according to the method described by El-Bassossy et al. (Citation2011b). The aorta rings were loaded for 30 min at 37 °C with 5 mM of DAF-FM diacetate or Calcium Green TM-2 plus 0.1% Pluronic F-127 in Krebs–Henseleit buffer containing 25 mM HEPES. Aorta rings were washed twice and then cut and placed in a chamber designed especially for fluorescence measurement in aortic segments with the endothelial side up in case of NO measurements and with the smooth muscle side up in case of calcium measurements. Hundred milliliters of Krebs–Henseleit buffer containing HEPES were added quickly to the chamber. DAF fluorescence was measured by LS45 Perkin–Elmer fluorescence spectrophotometer (Perkin Elmer, New York, NY) with remote fiber optic which allows measurement of tissue fluorescence in the used chamber. Readings (λex = 485 or 503 nm and λem = 515 or 536 nm) were recorded before and every 5 s after the addition of Ach (10−4) or KCl (100 mM). The NO donor diethyl amine NONOate (100 nM) was used as a positive control for DAF while calcium ionophore was used as a positive control for Calcium Green TM-2.
Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was performed by the analysis of variance followed by Bonferroni’s complementary analysis. The maximal response (Emax) and the agonist pD2 value (−log EC50) of the agents were determined by Sigmoidal curve fitting using 3-parameter non-linear regression using Graph pad prism 5 software (La Jolla, CA). Values were considered to be significantly different when the p value was less than 0.05.
Results
The results of the current study showed clearly that fructose strongly increased the contractile response of aortic rings to both phenylephrine and KCl, but did not affect the relaxant response to Ach when applied directly to isolated aorta of normal rats. Curcumin significantly decreased the hyper responsiveness of arterial rings to both vasopressors and reduced the intracellular Ca2+ level. Among the drugs studied, only 1400 W potentiated the effect of curcumin.
Fructose impaired vascular reactivity; the protective effect of curcumin
The vasopressor responses to both phenylephrine and KCl were more pronounced in aortic rings incubated with fructose (p less than 0.05) compared with the glucose control group (Emax was increased by 147.3% and 150.5%, respectively). Curcumin, either alone or in combination with either tempol or 1400 W treatment, significantly decreased the vasopressor responses to both phenylephrine (Emax decreased from 147.3% in fructose incubated aorta to 81, 73.8, and 63.4% with either curcumin alone or its combinations with both tempol and 1400 W, respectively) and KCl (Emax decreased from 150.5% in fructose incubated aorta to 77.24, 79.5, and 60.83% with curcumin alone or its combination with tempol or 1400 W, respectively), as shown in and and presented in .
Figure 1. Effect of curcumin alone (1 µM) or in combination with tempol (1 mM) or 1400 W (1 µM) on fructose-treated isolated rat aorta responsiveness to KCl. Symbols indicate mean ± SEM for n = 6–10 aortic rings; ***p < 0.001, compared with the corresponding control group values; ###p < 0.001 compared with the corresponding fructose group values; aap < 0.001 compared with curcumin group values.
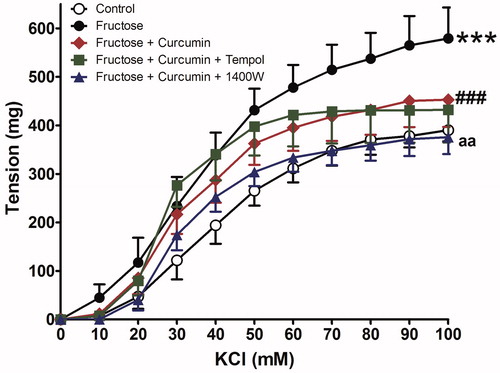
Figure 2. Effect of curcumin alone (1 µM) or in combination with tempol (1 mM) or 1400 W (1 µM) on fructose-treated isolated rat aorta responsiveness to phenylephrine (PE). Symbols indicate mean ± SEM for n = 6–10 aortic rings; ***p < 0.001, compared with the corresponding control group values; ##p < 0.001 compared with the corresponding fructose group values; ap < 0.01 compared with curcumin group values.
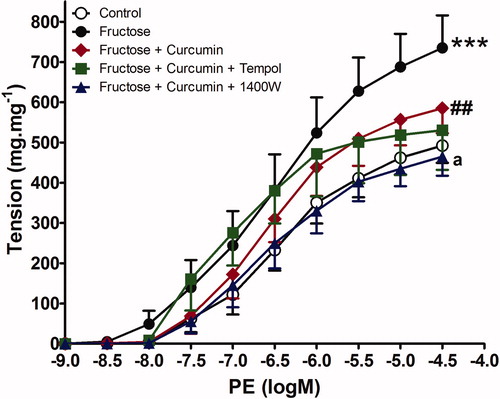
Table 1. Effect of fructose and curcumin either alone or in combination with either tempol or 1400 W on the maximal response (Emax) and pD2 (−Log EC50) values of phenylephrine (PE), potassium chloride (KCl), and acetyl choline (Ach), dose–response curves.
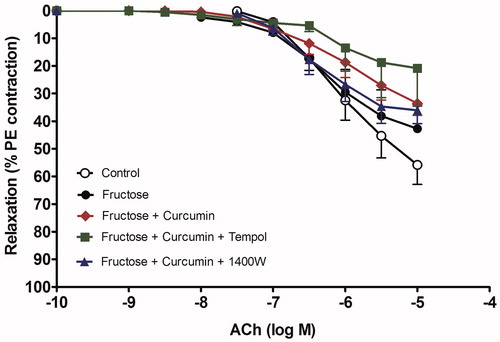
The combination of 1400 W with curcumin normalized the vasopressors response to control values and had a significant effect compared with curcumin alone (p less than 0.05). In contrast, tempol did not provide any additional benefit over curcumin in reducing the exaggerated aorta response to either phenylephrine or KCl in the presence of fructose incubation. Fructose did not affect the endothelial relaxation to Ach, in terms of Emax as graphically presented in and shown in .
Fructose-increased intracellular calcium; suppressive effect of curcumin
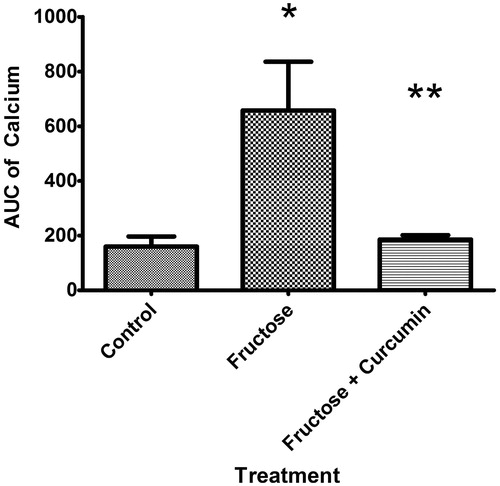
KCl induced an elevation in the intracellular calcium levels which was higher in tissues of fructose-treated aortic rings than those of controls (area-under-calcium curve was elevated from 226.1 ± 43.49 to 1216.0 ± 308.9 in fructose-treated aorta). Curcumin supplementation significantly suppressed the increased KCl-induced elevation in calcium level when incubated along with fructose (85% reduction in intracellular calcium, and ).
Figure 4. Effect of curcumin (1 µM) on intracellular calcium in fructose-treated isolated rat aorta. Symbols indicate mean ± SEM for n = 6–10 aortic rings; *p < 0.05, compared with the corresponding control group values; **p < 0.05 compared with the corresponding fructose group values.
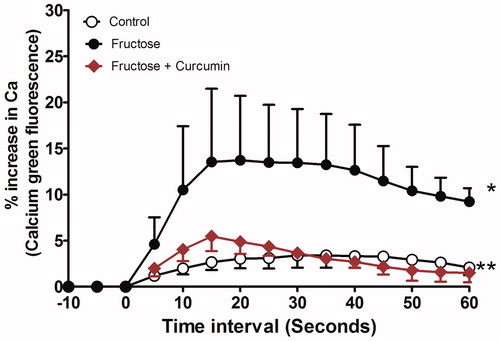
Figure 5. Effect of curcumin (1 µM) on area under the calcium curve (AUC) intracellular calcium in fructose-treated isolated rat aorta. Symbols indicate mean ± SEM for n = 6–10 aortic rings; *p < 0.05, compared with the corresponding control group values; **p < 0.05 compared with the corresponding fructose group values.
Fructose effect on intracellular nitric oxide level
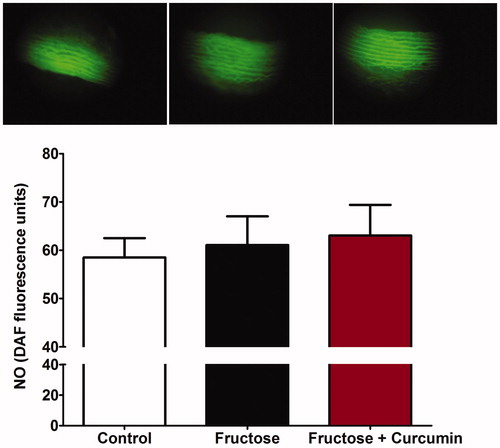
The basal intracellular NO levels were neither affected by the incubation with fructose nor by the addition of Ach to fructose-treated aorta (). Curcumin also had no significant effect on Ach-induced NO release compared with fructose-treated aortae (p more than 0.05).
Discussion
Herein, we have tried to investigate the direct influence of fructose on vascular reactivity of rat thoracic aorta. The study also investigated the possible protective effect of curcumin on fructose-induced vascular impairment. Replacement of glucose (11 mM) in the physiological solution by the same concentration of fructose caused an increase of the contractile response of aorta to both KCl and phenylephrine. In addition, fructose did not affect the response of aorta to Ach. The addition of curcumin reduced the exaggerated contractile response of fructose-treated aorta to both KCl and phenylephrine but did not affect the relaxant response to Ach. High-fructose diet is known to induce metabolic abnormalities and endothelial dysfunction in rats (Nyby et al., Citation2007; Shinozaki et al., Citation2004; Tran et al., Citation2009). However, whether these effects on endothelium are mediated via metabolic changes or direct effect of fructose on the endothelium is unknown. It was previously shown that in vivo administration of 10% fructose in drinking water for 6 weeks increased serum total cholesterol and insulin levels but did not induce hyperglycemia in rats (El-Bassossy et al., Citation2011a; Mahmoud et al., Citation2012). In these studies, fructose-induced inflammation and oxidative stress in rats and exaggerated the contractile response of aorta of fructose fed rats to both KCl and phenylephrine. These effects of fructose were attributed to different metabolic changes elicited by fructose (Huang et al., Citation2010). In the current study, fructose directly exaggerated the contractile response of aorta to KCl and phenylephrine. This was accompanied by an increase of intracellular calcium of isolated aorta during the addition of KCl compared with control.
NO was previously known as endothelium-dependent relaxing factor. It is the major mediator of vasorelaxation in medium- and large-sized blood vessels. It mediates the relaxant effect of both Ach- and the direct-acting vasodilator, sodium nitroprusside (Furchgott & Zawadski, Citation1980; El-Bassossy et al., Citation2009). In the present study, there was no significant increase in the basal intracellular NO in aorta exposed to fructose that may explain the normal relaxant response of aorta to Ach. Similarly, it was reported in our previous study that fructose feeding did not change the relaxant response of aorta to Ach (El-Bassossy et al., Citation2011b). However, Akar et al. (Citation2012) showed that 20% but not 10% of high fructose corn syrup reduced aorta endothelial and nitric oxide-mediated relaxation to acetylcholine in rats, compared with controls. This indicated that high concentration of fructose is required to impair the relaxant response to Ach.
As oxidative stress and inflammation may be responsible for the shown fructose-induced vascular injury, we tested whether curcumin, a well-known antioxidant and anti-inflammatory agent, can protect fructose-treated aorta from vascular injury. We found some evidence that curcumin is able to overcome the disturbances in vasopressors response of fructose-treated aortae, as verified by reduced contractile response for both KCl and phenylephrine. It was reported previously that tetrahydrocurcumin, a major metabolite of curcumin, improved aortic elasticity concomitant with reduction of oxidative stress in l-NAME-induced hypertension (Nakmareong et al., Citation2012). Chronic curcumin administration attenuated the phenylephrine-induced increase in contraction of aorta of STZ-diabetic rats at early stage of diabetes but not at the late stage (Majithiya & Balaraman, Citation2005). Similarly, vanadium complex with curcumin exerts protective effect against vascular impairment in STZ diabetic rats (Majithiya et al., Citation2005).
In order to understand the mechanism of action of curcumin on fructose-induced vascular impairment, we add tempol, a superoxide dismutase mimetic agent to curcumin during the incubation of aorta with fructose; we found that tempol did not provide any additional protection when compared with curcumin alone. This indicates the protective effect of curcumin is mediated, at least in part; by scavenging superoxide radical as no additional effect was observed by tempol. This finding is supported by a previous study of Fleenor et al. (Citation2013) who found that tempol did not provide further protection when given with curcumin in old mice. It was also reported previously that oral administration of curcumin decreased superoxide production in diabetic vasculature (Rungseesantivanon et al., Citation2010).
In contrast, the addition of 1400 W, a specific iNOS inhibitor, to curcumin synergized its effects and normalized the vasopressors response to both KCl and phenylephrine. This finding indicates that, fructose stimulates iNOS and produces excessive NO which together with free radicals impaired the vascular reactivity of aorta. However, a previous study showed that curcumin may protect the blood–brain barrier ultra-structure, and thus decrease brain edema following brain ischemia by down-regulating ischemia-induced increase in NOS activities (Yu et al., Citation2012). This controversy may be related to the difference in experimental model and organ studied. The present study revealed that both tempol and 1400 W had no effect on the relaxant response of aorta to Ach or Ach-induced elevation of intracellular NO levels.
Taken together, it is likely that curcumin contributes to the restoration of vascular dysfunction in fructose-treated aortae, at least in part, by superoxide scavenging effects. Moreover, the present study clearly showed that NO produced by iNOS is involved in the deleterious effects of fructose on vascular contractility and curcumin could not normalize the vasopressor’s responses of aorta as it did not affect NO level.
Conclusion
Fructose has a direct exaggerating effect on aorta contractility while curcumin can protect against this fructose-induced vascular injury by a mechanism involving superoxide scavenging effects and via inhibition of calcium influx.
Declaration of interest
The authors report no declarations of interest.
References
- Akar F, Uludağ O, Aydın A, et al. (2012). High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats: Protective effect of resveratrol. Food Chem Toxicol 50:2135–41
- Bhagya D, Prema L, Rajamohan T. (2012). Therapeutic effects of tender coconut water on oxidative stress in fructose fed insulin resistant hypertensive rats. Asian Pac J Trop Med 5:270–6
- Carranza A, Litterio MC, Prince PD, et al. (2011). Lipopolysaccharide (LPS) induction of nitric oxide synthase-2 and cyclooxygenase-2 is impaired in fructose overloaded rats. Life Sci 88:307–13
- El-Bassossy HM, El-Maraghy NN, El-Fayoumi HM, Watson ML. (2009). Heme oxygenase-1 induction protects against tumor necrosis factor alpha impairment of endothelial-dependent relaxation in rat isolated pulmonary artery. Br J Pharmacol 158:1527–35
- El-Bassossy HM, El-Moselhy MA, Mahmoud MF. (2011a). Pentoxifylline alleviates vascular impairment in insulin resistance via TNF-α inhibition. Naunyn Schmiedebergs Arch Pharmacol 384:277–85
- El-Bassossy HM, Fahmy A, Badawy D. (2011b). Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol 49:3007–12
- Fleenor BS, Sindler AL, Marvi NK, et al. (2013). Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48:269–76
- Furchgott RF, Zawadski JV. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–6
- Hwang I-S, Ho H, Hoffman BB, Reaven GM. (1987). Fructose-induced insulin resistance and hypertension in rats. Hypertension 10:512–16
- Huang F, Lezama MA, Ontiveros JA, et al. (2010). Effect of losartan on vascular function in fructose-fed rats: The role of perivascular adipose tissue. Clin Exp Hypertens 32:98–104
- Klawitter M, Quero L, Klasen J, et al. (2012). Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm (Lond) 9:1–14
- Lin CM, Lee JF, Chiang LL, et al. (2012). The protective effect of curcumin on ischemia-reperfusion-induced liver injury. Transplant Proc 44:974–7
- Mahmoud MF, El-Nagar M, El-Bassossy HM. (2012). Anti-inflammatory effect of atorvastatin on vascular reactivity and insulin resistance in fructose fed rats. Arch Pharm Res 35:155–62
- Majithiya JB, Balaraman R. (2005). Time-dependent changes in antioxidant enzymes and vascular reactivity of aorta in streptozotocin-induced diabetic rats treated with curcumin. J Cardiovasc Pharmacol 46:697–705
- Majithiya JB, Balaraman R, Giridhar R, Yadav MR. (2005). Effect of bis[curcumino]oxovanadium complex on non-diabetic and streptozotocin-induced diabetic rats. J Trace Elem Med Biol 18:211–17
- Marriott BP, Cole N, Lee E. (2009). National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139:1228–35
- Nakmareong S, Kukongviriyapan U, Pakdeechote P, et al. (2012). Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens Res 35:418–25
- Nyby MD, Abedi K, Smutko V, et al. (2007). Vascular angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens Res 30:451–7
- Oudot A, Behr-Roussel D, Le Coz O, et al. (2010). How does chronic sildenafil prevent vascular oxidative stress in insulin-resistant rats? J Sex Med 7:79–88
- Patel J, Iyer A, Brown L. (2009). Evaluation of the chronic complications of diabetes in a high fructose diet in rats. Indian J Biochem Biophys 46:66–72
- Pistelli L, Bertoli A, Gelli F, et al. (2012). Production of curcuminoids in different in vitro organs of Curcuma longa. Nat Prod Commun 8:1037–42
- Rebolledo A, Rebolledo OR, Marra CA, et al. (2010). Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc Diabetol 9:1–13
- Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. (2010). Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med 14:1–9
- Shao W, Yu Z, Chiang Y, et al. (2012). Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 7:e28784
- Shinozaki K, Kashiwagi A, Nishio Y, et al. (1999). Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes 48:2437–45
- Shinozaki K, Ayajiki K, Nishio Y, et al. (2004). Evidence for a causal role of the renin–angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension 43:255–62
- Soylemez S, Gurdal H, Sepici A, Akar F. (2011). The effect of long-term resveratrol treatment on relaxation to estrogen in aortae from male and female rats: Role of nitric oxide and superoxide. Vascul Pharmacol 49:97–105
- Suwannaphet W, Meeprom A, Yibchok-Anun S, Adisakwattana S. (2010). Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem Toxicol 48:1853–7
- Tran LT, Yuen VG, McNeill JH. (2009). The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332:145–59
- Yu L, Yi J, Ye G, et al. (2012). Effects of curcumin on levels of nitric oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain damage. Brain Res 1475:88–95