Abstract
Context: Despite the usage of Nerium oleander L. (Apocynaceae) for anticancer studies and traditional remediation, the regulatory effect of N. oleander leaf distillate on cholesterol metabolism is not disclosed sufficiently.
Objective: Cholesterol is an important biological molecule and the synthesis rate is regulated by the amount of cholesterol uptake from the diet. The aim of this study was to investigate the regulation of cholesterol metabolism in response to a high-fat diet (HFD) and the effects of N. oleander leaf distillate-supplemented diet (NOHFD) in rats.
Materials and methods: Microarray technology was used to clarify the regulation of cholesterol mechanism in HFD and NOHFD-fed rats (375 μg/0.5 mL distilled water applied by gavage). The treatment period was 90 days. Rat liver tissues were used for microarray analysis using the Affymetrix GeneChip Rat Genome platform. Results of groups were statistically analyzed with the Partek 6.6 bioinformatic program.
Results: The HFD group exhibited alterations in the expression levels of about 1945 genes with respect to the normal diet (ND) group. The results showed that expression levels of 47 genes were altered related to cholesterol metabolism in HFD and NOHFD groups. The expression levels of seven genes in the NOHFD group were significantly closer to those in the ND group than those of the HFD group.
Discussion and conclusion: To conclude, findings suggest that N. oleander leaf distillate-supplemented food has considerable beneficial effects on cholesterol metabolism-related gene expression levels.
Introduction
Cholesterol is a biological molecule that is the component of the cell membrane and precursor of steroid hormones, bile acids, and vitamin D. This special type of lipid is essential for life and is synthesized in the body (Babin & Gibbons, Citation2009). Acetyl-CoA, which is the precursor of cholesterol and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), is reduced to mevalonate by the enzyme HMG-CoA reductase. This is the regulated, rate-limiting and irreversible step in cholesterol synthesis. Mevalonate is decarboxylated to isopentenyl pyrophosphate, which is a key metabolite for various biological reactions. Farnesyl pyrophosphate is synthesized from three molecules of isopentenyl pyrophosphate by the action of geranyl transferase. Two molecules of farnesyl pyrophosphate then form squalene by the catalysis of squalene synthase in the endoplasmic reticulum. After lanosterol is formed, it is converted to the cholesterol through a 19-step process (Akhtar et al., Citation1977).
The synthesis and the use of cholesterol in the body are tightly regulated because the sustained production of cholesterol and its accumulation is of great importance for the body. Deposition of cholesterol around vessels is clinically abnormal. A high accumulation of cholesterol is prevented by decreasing cholesterol synthesis the in liver and uptake from the diet. As medical treatment, statins are used to block cholesterol synthesis in liver to lower blood cholesterol level (Harris et al., Citation2004). Furthermore, traditional phytotherapy strategies to regulate the blood cholesterol level were investigated and accepted by the scientific community (Han et al., Citation2013).
Nerium oleander L. (Apocynaceae) is a known poisonous plant, which is used for the treatment of cancer, asthma, and various diseases traditionally. Some types of N. oleander leaf extracts prepared in different solvents were found to be anti-proliferative in cancer cells (Fu et al., Citation2005). Nerium oleander leaf distillate, however, was declared by our group as non-toxic to normal and cancer cells (Kars et al., Citation2013; Yazihan et al., Citation2013).
Liver tissue samples from animal models (rats), which were fed with high-fat diet (HFD), N. oleander leaf distillate-supplemented high-fat diet (NOHFD) and normal diet (ND), were used in this study. The effects of diet types were analyzed at the transcriptional level by a whole genome cDNA microarray method to confirm the previous findings of Bas et al. (Citation2012) that showed that N. oleander leaf distillate lowered blood fatty acid levels in rats. Whole genome cDNA expression analysis was performed using rat liver RNA samples to determine the molecular effects of NPHFD on the expression levels of genes related to the cholesterol metabolism. Affymetrix GeneChip Rat Genome Array (Affymetrix, Santa Clara, CA) and a bioinformatic program (Partek, PGS 6.6, Partek Inc., St. Louis, MO) were used to determine and enlighten the nutrigenomic effects of HFD and NOHFD in the rat liver with respect to ND.
Materials and methods
Plants and preparation of distillate
Nerium oleander leaves were previously collected from the Mersin region (Latitude: 36°47′42″ N Longitude: 34°37′04″ E) in April–May 2011 period. The plant was identified and authenticated at the Department of Biology by Prof. Dr. Yavuz Bağcı (voucher specimen was deposited at the Herbarium at Selçuk University, Turkey: Bagci 4153). The leaves were washed and then chopped. The plant material was boiled in water (100 g/1000 ml) and the distillate was collected. The distillate was lyophilized in 350 µg portions (FDT-8618 Freeze Dryer, Operon, Korea). Dried extracts were stored at room temperature in dark vials until use. The distillation method was original and submitted to the Turkish Patent Institute (Application No: 2009/00312) and Patent Cooperation Treaty (Application No: PCT/TR2009/000013).
Animal tissue material
Liver tissues from male Sprague–Dawley rats (6–8 weeks old) which were fed ND-control, HFD and NOHFD were used (Bas et al., Citation2012). The experimental protocol was approved by the Ethics Committee in Animal Experimentation of Selcuk University, Turkey, with the permission date 20 March 2013 and number 2013/14. Healthy rats were fed normally for two weeks and the blood serum cholesterol levels of the rats were evaluated before the diets. ND rats were fed with a 2850 kcal/kg diet containing 21% protein, 5% cellulose and 11% minerals. HFD rats were fed with a diet containing a high amount of fat with 5387 kcal/kg (1% cholesterol, 5% animal fat, 5% soy fat, 20% casein, 35.1% corn starch, 20% sucrose, 3.5% mineral, 1% vitamin, 0.4% cholin, and 9% methyl α-cellulose) (Bas et al., Citation2012). NOHFD rats were fed with HFD, and N. oleander distillate supplementation was applied by gavage with 375 µg distillate in 0.5 ml distilled water. The treatment was 90 days of daily feeding. After the feeding period, blood serum cholesterol levels were measured as per the instructions given in Bas et al. (Citation2012) and it was found that N. oleander distillate decreased the high blood cholesterol level even when applied together with HFD (). After the autopsy of rats, the liver tissue of each animal was stored at −80 °C for microarray analysis in this study.
Table 1. Blood serum cholesterol levels of the rats selected for cDNA microarray analysis.
RNA isolation, cDNA synthesis, and target preparation
The liver tissues (50–100 mg) of two different rats from each group (ND, HFD, NOHFD) were selected. Tissue was minced with a homogenizator (Heidolph, Germany) and RNA extractions from tissues were performed using TRI Reagent (Sigma, St. Louis, MO), according to the manufacturer’s instructions (Chomczynski, Citation1987). Absorbance values (260 nm, 280 nm) were measured for RNA quantification with a spectrophotometer (Biochrom Libra, UK). RNA intactness was checked by 1% w/v agarose gel electrophoresis at 70 V, 60 min. RNA concentration in each sample was adjusted to 100 ng with an OD260 nm/OD280 nm ratio of 1.8:2.0. All RNA samples were prepared as duplicates. cDNA was synthesized from total RNA by One-Cycle Target Labelling Assay® (Affymetrix, Santa Clara, CA), according to manufacturer’s instructions. Second-strand cDNA synthesis, biotin-labelled cRNA synthesis (IVT Labelling) and cRNA fragmentation were performed by Affymetrix GeneChip® kit reagents, according to the procedure described in the Affymetrix GeneChip® Expression Analysis Technical Manual (Santa Clara, CA).
Target hybridization and scanning
Biotin-labelled and fragmented target cRNA samples were loaded into 49/64 format-type Affymetrix GeneChip® (Rat Genome 230 2.0 Array) together with control cRNAs and oligo B2. Hybridization procedure was conducted at 45 °C, 60 rpm for 17 hours in Affymetrix GeneChip® Hybridization Oven 640. Washing and staining procedure was performed in Affymetrix GeneChip® Fluidics Station 450 with Euk Ge-WS2v5 fluidics script according to the instructions in the technical manual. Affymetrix GeneChip® Scanner 3000 was used for scanning the arrays.
Data analysis, preparation of gene list, and pathways
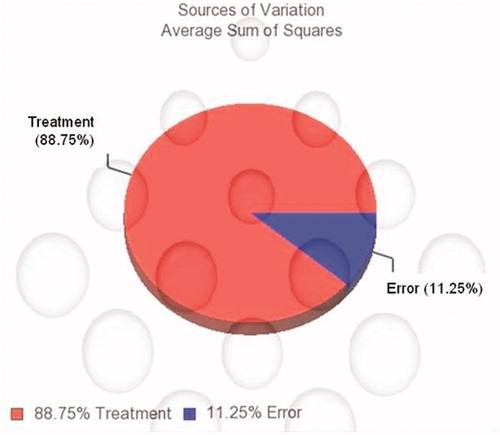
Preliminary and advanced analysis of the scanned chips was performed using Partek® Genomics Suite™ 6.6 (Partek Inc., St. Louis, MO). The quality of gene expression data was checked according to quality control criteria (Yilmaz et al., Citation2008). The Partek Genomics Suite platform is designed to break through bottlenecks in the analysis process and to help identify genes that are truly relevant to the biological question by comparing analysis results from expression, genotyping, protein, metabolite and other data types. The data was normalized by 75 percentile normalization algorithms. Statistically significant data were selected by ANOVA (α = 0.05) between duplicate data for groups. ND data was compared to HFD while HFD data was compared to NOHFD in terms of statistically significant alterations in the gene expression levels. The genes that we up-regulated and down-regulated more than two-fold were considered in preparing gene lists. Average Sum of Squares analysis revealed that the difference in gene expression levels was due to the treatment/diet type (88.5%) (). The genes were classified in terms of gene ontology. Finally, the genes that encode proteins related to lipid and cholesterol metabolism were selected from the gene list. Up-regulated and down-regulated genes were evaluated in terms of hyperlipidemia and effects of N. oleander distillate, as dietary supplement, on cholesterol and lipid metabolism. The important pathways related to our findings were evaluated by Kyoto Encyclopedia of Genes and Genomes (KEGG, Citation2013a,Citationb,Citationc,Citationd,Citatione) pathway database available at: http://www.genome.jp/kegg/.
Results
Quality and quantity of RNA samples and chip scan data
Spectrophotometric measurements and electrophoresis results demonstrate that RNA quality and quantity were suitable for microarray analysis. OD260 nm/OD280 nm ratios were 1.8–2.0 for all the RNA samples. Chip scan data were also passed from quality check.
Analysis of microarray data
The whole genome analysis obtained from microarray is given as Supplementary material to provide whole data demonstrating alterations of gene expression levels in response to HFD and NOHFD. The genes of interest related to lipid and cholesterol metabolism were selected from the list of up- and down-regulated genes. The genes of interest are listed in . Positive values indicate up-regulation levels while negative values indicate down-regulation levels. The cellular supply of cholesterol is maintained at a steady level by three distinct mechanisms: (1) regulation of HMGR activity and level, (2) regulation of excess intracellular cholesterol through the activity of cholesterol acyltransferase, (3) regulation of plasma cholesterol level via LDL receptor-mediated uptake and HDL-mediated reverse transport. represents the alterations in lipid metabolism-related genes in rat liver tissue. Generally, the HFD seems to have changed the metabolism so as to decrease cholesterol synthesis in the rats. The microarray data confirms the analytical clinic data obtained from blood serum, which showed that N. oleander leaf distillate supplementation decreased the high blood cholesterol level (). This study shows that NOHFD affected the expression levels of some of the genes in opposite manner when compared to HFD treatment. Specifically, expression levels of the genes Adh7, Hmgcs1, Cyp39a1, Fabp1, Acox2, Pla2g2d and Baat were regulated by N. oleander supplementation to HFD in liver tissue. Expression levels of these important genes in NOHFD-fed rats were closer to the expression levels of ND-fed rats than that of HFD-fed rats.
Table 2. The gene list that represents the fold change of genes encoding lipid/cholesterol metabolism related proteins in rat livers.
Discussion
The expression levels of genes related to cholesterol metabolism are discussed by relating the genes with KEGG pathways and providing the pictures of regulatory effects of N. oleander distillate supplementation.
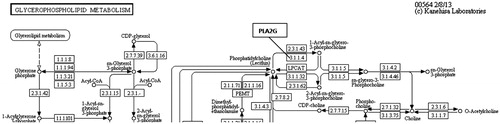
Alcohol dehydrogenase enzymes metabolize ethanol, retinol, aliphatic alcohols and hydroxysterol (Satre et al., Citation1994). Omega oxidation is a process of fatty acid metabolism and alcohol dehydrogenase enzymes catalyze the conversion of fatty omega hydroxyl-acid to omega aldoacid. The results demonstrated that HFD caused an 18-fold down-regulation in Adh7 gene (alcohol dehydrogenase 7) that may have caused a decrease in fatty acid synthesis. When N. oleander was supplemented to the same fatty diet, the expression level of the same gene increased to the normal level three-fold to favor fatty acid metabolism. A related pathway is demonstrated in . HMGCS1 gene encodes 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 that is a crucial enzyme in cholesterol synthesis (Chan et al., Citation2008). HMGCS1 enzyme is localized in the mitochondrial matrix. Expression of the gene was significantly down-regulated because increased cholesterol level resulting from HFD may have inhibited cholesterol biosynthesis (). NOHFD rats expressed the gene two-fold closer to the normal level. CYP39A1 gene encodes one of the members of cytochrome P450 family enzymes (Henegouwen et al., Citation2001). CYP39A1 enzyme is required for the synthesis of 24-hydroxycholesterol in human and rat (). HFD rats express the gene 11.5-fold lower than the ND rats, interestingly NOHFD caused four-fold up-regulation in the gene expression level, closer to the normal value. Fabp1 gene encodes fatty acid-binding protein which plays a significant role in fatty acid uptake, transport and metabolism (Chen et al., Citation1986; Weickert et al., Citation2007). Fatty acid-binding protein activity is tightly regulated by peroxisome proliferator-activated-receptor (Ppar) signaling pathway (). Microarray results demonstrated that the expression of the Fabp1 gene was down-regulated five-fold due to HFD and NOHFD up-regulated the expression to the normal level significantly (about three-fold). The decreased level of Fabp1 gene expression may consequently lead to the down-regulation of fatty acid uptake and lipid metabolism. In a report, our group previously suggested that N. oleander distillate supplement had an effect on the expression levels of Ppar genes (Bas et al., Citation2012). In conclusion, N. oleander distillate normalized the effects of HFD to some extent. Acox2 gene encodes acyl-CoA oxidase that functions in cholesterol catabolism and bile acid metabolism (). Low level of Acox2 enzyme results in accumulation of dihydroxycholestenoyl-CoA and down-regulation in cholesterol catabolism. HFD-fed rats expressed the gene five-fold lower than the ND-fed rats. This situation may have caused down-regulation of bile acid synthesis in HFD-fed rats. Interestingly, NOHFD-fed rats expressed the gene three-fold closer to the ND-fed rats, which means N. oleander supplement has decreased the effect of HFD. Phospholipase A2 enzyme, encoded by Pla2g2d, catalyzes a reaction in the hydrolysis of 3-phosphoglyceride (). This enzyme was reported to hydrolyze phospholipids in high-density lipoprotein (HDL) and low-density lipoprotein (LDL) (Sato et al., Citation2008). Increased PLA2 subtype levels increases the atherosclerosis lesions in human and mice (Sato et al., Citation2008). Pla2g2d gene expression level increased nine-fold in HFD-fed rats, which may be a sign of atherosclerosis risk due to accumulated lipid. Interestingly, N. oleander addition decreased the effect of HFD about three-fold in NOHFD-fed rats. BAAT gene encodes bile acid-CoA:amino acid N-acyltransferase that catalyzes the conjugation of bile acids to glycine and taurine for excretion into bile. It has a critical role in cholesterol homeostasis and biliary secretion of cholesterol (O'Byrne et al., Citation2003). The gene was down-regulated in HFD-fed rats two-fold and up-regulated towards the ND values about 11-fold after N. oleander supplementation. The N. oleander supplement resulted in an over-expression of the gene to decrease blood cholesterol level and increase bile acid synthesis ().
Figure 2. Adh7 enzyme in KEGG pathway: fatty acid metabolism (http://www.genome.jp/ kegg-bin/show_pathway?map00071+M00086).
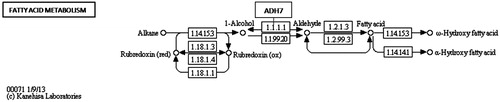
Figure 3. Hmgcs1 enzyme in KEGG pathway: synthesis and degradation of ketone bodies (http://www.genome.jp/kegg-bin/show_pathway?ko00072+K00626).
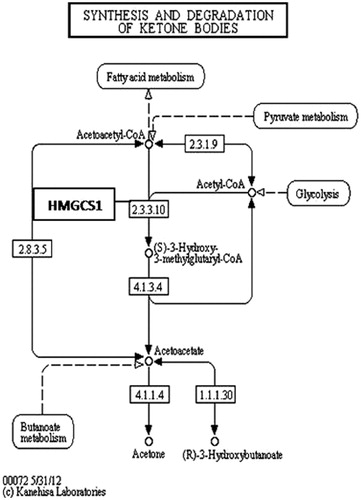
Figure 4. Cyp39a1, acox1 and baat enzymes in KEGG pathway: primary bile acid biosynthesis (http://www.genome.jp/kegg-bin/show_pathway?ko00120+K00489).
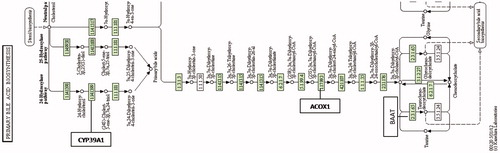
Figure 5. Fabp1 enzyme in KEGG pathway: PPAR signaling pathway (http://www.genome.jp/kegg-bin/show_pathway?ko03320+K00489).
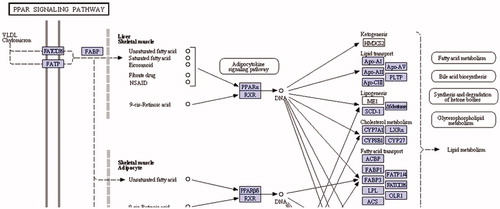
Figure 6. Pla2g enzyme in KEGG pathway: glycerophospholipid metabolism (http://www.genome.jp/kegg-bin/show_pathway?map00564).
Conclusions
To conclude, this study reveals the cholesterol metabolism related genes affected by HFD in rat liver. It is also demonstrated that N. oleander leaf distillate has some impact on the regulation of gene expression levels in rat liver, specifically towards the normalization of HFD. This effect may be more pronounced in N. oleander leaf distillate supplemented normal fat diet-fed animals. This report is the first nutrigenomic study that exhibits the regulation of cholesterol and lipid metabolism by N. oleander leaf distillate. The results obtained from microarray analysis comply with the biochemical data obtained from blood serum. Regulation of lipid metabolism which is controlled by very complex metabolic pathways and by diet type is enlightened to some extent at the gene expression level. It is also revealed that non-toxic N. oleander leaf distillate (Kars et al., Citation2013; Yazihan et al., Citation2013) may be used as a supplement to regulate cholesterol metabolism in animal models. Further functional and pharmacological assessments will be conducted by our group to suggest the unique N. oleander leaf distillate as a medicinal food supplement.
Declaration of interest
All authors fully disclose that there is no financial or ethical conflict of interest. This study was supported by the Selcuk University Research Fund with the project number 12201031. Also, the distillation method was submitted to Turkish Patent Institute (Application No: 2009/00312) and Patent Cooperation Treaty (Application No: PCT/TR2009/000013).
Supplementary Material
Download MS Excel (792 KB)References
- Akhtar M, Freeman CW, Wilton DC, et al. (1977). The pathway for the removal of the 15α-methyl group of lanosterol. Bioorg Chem 6:473–81
- Babin PJ, Gibbons GF. (2009). The evolution of plasma cholesterol: Direct utility or a ‘spandrel’ of hepatic lipid metabolism? Prog Lipid Res 48:73–91
- Bas AL, Demirci S, Yazihan N, et al. (2012). Nerium oleander distillate improves fat and glucose metabolism in high-fat diet-fed streptozotocin-induced diabetic rats. Int J Endocrinol 2012:947187
- Chan J, Donalson LM, Kushwaha RS, et al. (2008). Differential expression of hepatic genes involved in cholesterol homeostasis in high- and low-responding strains of laboratory opossums. Metabolism 57:718–24
- Chen SH, Van Tuinen P, Ledbetter DH, et al. (1986). Human liver fatty acid binding protein gene is located on chromosome 2. Somat Cell Mol Genet 12:303–6
- Chomczynski PS. (1987). Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–9
- Fu L, Zhang S, Li N, et al. (2005). Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J Nat Prod 68:198–206
- Han K, Kim S, Shimada K, et al. (2013). Purple potato flake reduces serum lipid profile in rats fed a cholesterol-rich diet. J Func Food 5:905–13
- Harris JI, Hibbeln JR, Mackey RH, Muldoon MF. (2004). Statin treatment alters serum n-3 and n-6 fatty acids in hypercholesterolemic patient. Prostaglandins Leukot Essent Fatty Acids 71:263–9
- Henegouwen GP, Keppler D, Leuschner U, et al. (2001). Biology of bile acids in health and disease. XVI International Bile Acid Meeting Germany, 7–8
- Kars MD, Gunduz U, Uney K, Bas AL. (2013). Exploring a natural MDR reversal agent: Potential of medicinal food supplement, Nerium oleander leaf distillate. Asian Pac J Trop Biomed 3:644–9
- KEGG. (2013a). Glycerophospholipid metabolism. Available from: http://www.genome.jp/ kegg-bin/show_pathway?map00564 [last accessed 05 May 2013]
- KEGG. (2013b). Fatty acid degradation pathway. Available from: http://www.genome.jp/kegg-bin/show_pathway?map00071+M00086 [last accessed 05 May 2013]
- KEGG. (2013c). PPAR signalling pathway. Availale from: http://www.genome.jp/kegg-bin/show_pathway?ko03320+K00489 [last accessed 05 May 2013]
- KEGG. (2013d). Primary bile acid biosynthesis. Available from: http://www.genome.jp/kegg-bin/show_pathway?ko00120+K00489 [last accessed 05 May 2013]
- KEGG. (2013e). Synthesis and degradation of ketone bodies. Available from: http://www.genome.jp/kegg-bin/show_pathway?ko00072+K00626 [last accessed 05 May 2013]
- O’Byrne J, Hunt MC, Rai DK, et al. (2003). The human bile acid-coa:amino acid N-acyltransferase functions in the conjugation of fatty acids to glycine. J Biol Chem 278:34237–44
- Sato H, Kato R, Isogai Y, et al. (2008). Analyses of group III secreted phospholipase a2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J Biol Chem 283:33483–97
- Satre MA, Zgombic-Knight M, Duester G. (1994). The complete structure of human class IV alcohol dehydrogenase (retinol dehydrogenase) determined from the ADH7 gene. J Biol Chem 269:15606–12
- Weickert MO, Loeffelholz CV, Roden M, et al. (2007). A Thr94Ala mutation in human liver fatty acid-binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucose levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab 293:1078–84
- Yazihan N, Bas AL, Ermis E, et al. (2013). Increased glucose uptake and insulin binding activity of nerium oleander in hepatocytes and adipocytes. Kafkas Univ Vet Fak Derg 19:25–30
- Yilmaz R, Yücel M, Öktem HA. (2008). Quality assessment of gene expression data for an affymetrix platform with the two sample t-tests statistical analysis. Int J Biotechnol Biochem 4:101–8