Abstract
Context: The leaf of Careya arborea Roxb. (Lecthidaceae) has been advocated in Ayurveda for the treatment of various disorders, including ulcers, healing of wounds and several skin diseases.
Objective: The 70% ethanol (EtOH) extract of C. arborea leaves (CALE) was investigated for its gastroprotective effect in different gastric ulcer models.
Materials and methods: CALE (100, 200, and 400 mg/kg body weight) was administered orally, twice daily for 5 d, for preventing aspirin (ASP)-, EtOH-, pylorus ligation (PL)-, and cold restraint stress (CRS)-induced ulcer in rats. The status of the antioxidant enzymes in CRS-induced ulcers, H+K+ATPase activity, gastric wall mucous in EtOH-induced ulcer, and gastric secretion parameters were estimated in the PL-induced ulcer model.
Results: CALE exhibited significant (p < 0.01) dose-dependent inhibition of ulcer index in ASP 12.90–51.61%, EtOH 11.97–40.35%, PL 28.63–63.92%, and CRS 38.30–66.37%, respectively. A significant (p < 0.001) decrease occurred in the level of H+K+ATPase, volume of gastric juice, and acid output. Simultaneously, the level of gastric wall mucus was increased significantly (p < 0.05). The antioxidant enzyme levels of LPO and SOD were decreased with concomitant increase in catalase activity in CRS-induced ulcers. High-performance thin-layer chromatography (HPTLC) showed the presence of quercetin, ellagic acid, and gallic acid (0.31%, 0.24%, and 0.71% w/w, respectively) in CALE.
Conclusions: Our results show that C. arborea possesses significant gastro-protective activity, probably due to its free radical scavenging activity, and validate the folklore claim.
Intoduction
Peptic ulcer disease is a major health problem with multifactorial etiology. Peptic ulcer is a lesion of gastric or duodenal mucosa occurring at a site where the mucosal epithelium is exposed to aggressive factors. In spite of the vast amount of research on ulcer, the cause of chronic peptic ulceration is still not clear. Although in most of the cases the etiology of the ulcers is unknown, it is generally accepted that they result from an imbalance between aggressive factors and the maintenance of mucosal integrity through endogenous defense mechanisms (Piper & Stiel, Citation1986). To regain the balance, different therapeutic agents including plant extracts are used.
Careya arborea Roxb. (Lecthidaceae) leaf extract is one such herbal drug currently undertaken to evaluate its anti-ulcer potential. Careya arborea, commonly known as Wild Guava and Kumbhi in Ayurveda, is a medium-sized deciduous tree, widely available in India, Sri Lanka, Malay, and Peninsula (Nadkarni, Citation2004). Ethnobotanically, the leaves are used to treat ulcer and its pulp is used as poultice to rapidly heal the wound, ulcers and root is used for the treatment of tuberculosis and skeletal fractures (Basak et al., Citation1976; Nadkarni & Nadkarni, Citation1976). The leaves and flowers are used in the form of paste to cure several skin diseases. Traditionally, the bark has been reported to be used in the treatment of tumors, bronchitis, epileptic fits, abscesses, and antidote to snake-venom (Kirtikar & Basu, Citation1975; Satish et al., Citation2010). Careya arborea is reported to possess analgesic, antidiarrhoeal, hepatoprotective, antitumor, CNS-depressant, anticoagulant, and in vitro cytotoxic activities (Gupta et al., Citation2012). Phytochemical investigation revealed the presence of careaborin-I, careyagenolide, triterpanoid sapogenols, β-amyrin, β-sitosterol, and taraxerol in C. arborea leaves (Satish et al., Citation2010).
Reactive oxygen species, especially the hydroxyl radical, plays a major role in the oxidative damage of gastric mucosa in almost all forms of gastric ulcer (Das et al., Citation1997; Phull et al., Citation1995). Since pharmacological validation of the ethnobotanical claims regarding the plant is essential to move towards the use of the plant as a drug and since the reports suggest the ethnobotanical use of the plant in ulcer, we studied the gastroprotective potential of the C. arborea leaf extract (CALE) using physical and chemical factors-induced gastric damage in rats as a model system. In addition, antioxidant activity and phytochemical analysis of the extract was carried out to identify the chemical compounds that were probably responsible for protecting the plants. High-performance thin-layer chromatography (HPTLC) fingerprinting of the total extract was also carried out using quercetin, ellagic acid and gallic acid as markers in an attempt to characterize the constituents responsible for the activities and also to standardize the extract, since antiulcer activity is reported for quercetin (De la Lastra et al., Citation1994).
Materials and methods
Plant material
The fresh leaves of C. arborea plant were collected from the Bauli jungle, Rewa district, India, in the month of March 2010, in the daytime during the peak of flowering and maturity. The plant material was identified by Dr. Tarique Hussain, Taxonomist, National Botanical Research Institute, Lucknow, India, and a voucher specimen (UIOP/M-1103) was preserved at the Herbarium Section of Departmental Museum.
Preparation of the extract
The freshly collected leaf of C. arborea was air-dried at room temperature and powdered. The air-dried powdered plant material (250 g) was percolated with petroleum ether to remove fatty substances; the marc was further exhaustively extracted with 70% ethanol (EtOH) for 3 d. The extract was filtered and concentrated on rotavapour (Buchi, New Castle, DE) under reduced pressure and lyophilized (Labconco, Kansas City, MO) to get the dry residue; the yield of CALE was 8.25% w/w.
Preliminary phytochemical screening and HPTLC analysis
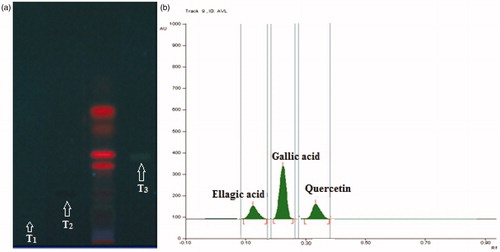
Preliminary qualitative phytochemical screening of CALE was tested for the presence of major chemical constituents using the standard procedure described by Khandelwal (Citation2008). Flavonoids were detected by the Shinoda test, while for terpenoids the Salkowski test was carried out. Ferric chloride test was used to detect the tannins while the frothing test was used for the detection of saponins. On the basis of a preliminary phytochemical test, HPTLC analysis was done to quantify the phenolic compounds. HPTLC analysis of CALE was performed on pre-activated (100 °C) silica gel 60F254 HPTLC plates (E. Merck, Mumbai, India) along with quercetin, ellagic acid and gallic acid (SD Fine-Chem Ltd, Mumbai, India). The plates were then eluted in solvent system toluene:ethyl acetate:formic acid (7:5:1). After elution, the plates were dried and densitometrically scanned at a wavelength of 278 nm (WinCats software, CAMAG, Muttenz, Switzerland). The percentage of quercetin, ellagic acid, and gallic acid in the extract was calculated by calibration using the peak height ratio.
Standardization of extract
Estimation of total phenolic content
Total phenolic content (TPC) of CALE was analyzed according to the Folin–Ciocalteu method (Ordonez et al., Citation2006). In brief, CALE (0.5 ml, 1.0 mg/ml) was well mixed with 2.5 ml of 0.2 N Folin–Ciocalteu reagent for 5 min and then 2.0 ml of Na2CO3 (75 g/l) were added to the mixture. They were then incubated at room temperature for 2 h. The absorbance was measured at 760 nm in a spectrophotometer. Phenolic content in the extract was expressed as mg gallic acid/g equivalents (GAE).
Estimation of total flavonoid content
Total flavonoid content (TFC) of CALE was determined according to a colorimetric method (Zou et al., Citation2004). In brief, 0.5 ml of the sample solution was mixed with 2 ml of distilled water, and subsequently with 0.15 ml of 5% NaNO2 solution. After 6 min of incubation, 0.15 ml of 10% AlCl3 solution was added and then allowed to stand for 6 min, followed by adding 2 ml of 4% sodium hydroxide solution to the mixture. Immediately, water was added to the sample to bring the final volume to 5 ml, the mixture was thoroughly mixed and allowed to stand for another 15 min. The absorbance of the mixture was determined at a wavelength of 510 nm. The total flavonoid content was expressed as equivalent to rutin in mg/g of the extracts.
Animals
Sprague–Dawley rats (140–180 g) and Swiss albino mice (30–35 g) of either sex were used for this study. The animals were housed under standard conditions of temperature of 25 ± 2 °C, relative humidity 45–55% and 12 h light/dark cycle, and fed with standard rodent feed (Dayal, Lucknow, India) and water. Food was withdrawn 18–24 h before the experiment though water was allowed ad libitum and allocated to different experimental groups, each of six rats. All animal experiments were conducted with the permission from Institutional Animal Ethics Committee of University Institute of Pharmacy, C.S.J.M. University, Kanpur (1589/PO/a/12/CPCSEA).
Acute toxicity study
Acute toxicity study was performed according to the OECD guidelines No. 423 (OECD, Citation2001). Swiss albino mice of either sex were divided into six groups with six animals each. CALE was administered orally as a single dose to mice at different dose levels of 250, 500, 750, 1250, 1500, and 2000 mg/kg b.w. Animals were observed periodically for the symptoms of toxicity and death within 24 h and then daily for 14 d.
Pharmacological evaluation
CALE in doses of 100, 200, and 400 mg/kg and H2 receptor blocker ranitidine (RAN) in the dose of 50 mg/kg were administered orally twice daily for 5 d for acute ulcer protective studies in various models. The control group of animals received a suspension of 1% carboxymethyl cellulose in distilled water (10 ml/kg).
Aspirin-induced ulcers
Aspirin (ASP) in a dose of 200 mg/kg (20 mg/ml) was administered to the animals on the day of the experiment and ulcers were scored after 5 h (Goel et al., Citation1985). The animals were sacrificed and the stomach was then excised and cut along the greater curvature, washed carefully with 5.0 ml of 0.9% NaCl, and ulcers were scored by a person unaware of the experimental protocol in the glandular portion of the stomach. Ulcer index (UI) was calculated by adding the total number of ulcers and the total severity of ulcers per stomach. The pooled group ulcer score was then calculated according to the method of Sanyal et al. (Citation1982) and the protection % was calculated using the following formula:
EtOH-induced ulcers
The gastric ulcers were induced in rats by administrating EtOH (1 ml/200 g) (Hollander et al., Citation1985) and after 1 h animals were sacrificed by cervical dislocation and stomach was incised along the greater curvature and examined for ulcers. The ulcer index was scored based upon the product of length and width of the ulcers present in the glandular portion of the stomach (mm2/rat). Estimation of H+K+ATPase activity and gastric wall mucous were performed.
Pylorus ligated-induced ulcers
After 5 d of drug treatment, the rats were anaesthetized using pentobarbitone (35 mg/kg, i.p.), the abdomen was opened, and pylorus ligation was done without causing any damage to its blood supply. The stomach was replaced carefully and the abdomen wall was closed in two layers with interrupted sutures. The animals were deprived of water during the post-operative period. After 4 h, stomachs were dissected out and cut open along the greater curvature. Ulcer index was calculated by adding the total number of ulcers and the total severity of ulcers per stomach (Shay et al., Citation1945).
Cold-restraint stress-induced ulcers
On day six, the experimental rats were immobilized by strapping the fore and hind limbs on a wooden plank and kept for 2 h, at temperature of 4–6 °C (Gupta et al., Citation1985). Two hours later, the animals were sacrificed by cervical dislocation and ulcers were examined on the dissected stomachs as described above. The fundic part was homogenized (5%) and centrifuged. The supernatant mitochondrial fraction was used for the antioxidant enzyme activities.
Antioxidant assay
Lipid peroxidation (LPO) was estimated by the standard method of Okhawa et al. (Citation1979) and expressed as nmol of MDA formed/min/mg protein. Superoxide dismutase (SOD) activity was estimated by the inhibition of nicotinamide adenine dinucleotide (reduced)–phenazine methosulphate–nitrobluetetrazolium reaction system as adapted by Kakkar et al. (Citation1984), and the results are expressed as units (U) of SOD activity/mg protein. Catalase (CAT) was estimated by the Aebi (Citation1974) method and the results are expressed as µmol of H2O2 consumed/min/mg protein.
Estimation of H+K+ATPase activity
The H+K+ATPase activity was assayed in EtOH-induced ulcer animals (Nagaya et al., Citation1987). The assay medium consisted of 70 mM Tris buffer, pH 6.8, 5.0 mM MgCl2, and the enzyme solution in the presence of 10 mM KCl in a total volume of 1.0 ml and was incubated for 1 h. The reaction was initiated by adding 2 mM ATP, incubated at 37 °C for 20 min, and the reaction was stopped by 10% TCA. After centrifugation, 2.5 ml of ammonium molybdate and 0.5 ml of l-amino-2-naphthol-4-sulphonic acid were added to the supernatant and the absorbance was read at 620 nm. Results are expressed as mmol of Pi liberated/min/mg/protein.
Gastric secretion studies
The gastric juice was collected 4 h after pylorus ligation and centrifuged for 5 min at 2000 rpm and the volume of the supernatant was expressed as ml/100 g body weight. Total acid output was determined by titrating with 0.01 N NaOH, using phenolphthalein as indicator and was expressed as μEq/ml concentration or μEq/4 h as output. Peptic activity was determined using hemoglobin as substrate and was expressed as mmol of tyrosine/ml as concentration or μmol of tyrosine/4 h as output (Debnath et al., Citation1974).
Estimation of gastric wall mucus
Gastric wall mucus was determined according to the method of Corne et al. (Citation1974). The glandular segments from stomachs were removed, weighed and incubated in tubes containing 1% Alcian blue solution (0.16 M sucrose in 0.05 M sodium acetate, pH 5.8) for 2 h. The Alcian blue binding extract was centrifuged and the absorbency of supernatant was measured at 498 nm. The quantity of Alcian blue extracted (µg/g of glandular tissue) was then calculated.
Statistical analysis
Values were represented as mean ± S.E.M. for six rats. Analysis of variance (ANOVA) test was followed by individual comparison by the Newmann–Keuls test using Graph Pad Prism software version 4.01 (Graph-Pad Software, Inc., San Diego, CA) for the determination of the level of significance.
Results
Phytochemical screening
Preliminary phytochemical screening revealed the presence of terpenoids, saponins, tannins and polyphenolic constituents like flavonoids as major constituents in the extract. The quantitative HPTLC determination shows the presence of 0.31, 0.24, and 0.71% w/w of quercetin, ellagic acid, and gallic acid, respectively, in CALE ().
Total phenolic and flavonoid content
The total phenolic and flavonoid content was estimated to be 172.93 ± 1.73 mg gallic acid equivalents/g of dry extract, and 37.34 ± 0.86 mg rutin equivalents/g of dry extract from triplicate measurements, respectively.
Antiulcer study
Effects of CALE at doses of 100, 200, and 400 mg/kg twice a day for 5 d prevented the acute gastric ulcers in a dose-dependent manner. The ranges of percentage protection were ASP 12.90–51.61%, EtOH-induced 11.97–40.35%, cold-restraint stress-induced (CRS) 38.30–66.37%, and pylorus ligated (PL) 28.63–63.92%, respectively. The range of percentage protection of RAN was 57.26–75.60% in various gastric ulcer models (). EtOH treated rats showed a significant increase in the H+K+ATPase activity. The H+K+ATPase activity was elevated to 1.61 ± 0.09 (p < 0.001) after EtOH administration, but CALE at 400 mg/kg dose level significantly inhibited H+K+ATPase activity to 0.74 ± 0.08 (p < 0.001) as well as increased the gastric wall mucus to 231.2 ± 11.3 (p < 0.05) ().
Figure 2. Effect of CALE on H+K+ATPase activity in gastric mucosa and gastric wall mucus in EtOH-induced ulcer group. +p < 0.001 compared to the respective normal control group. ap < 0.05, bp < 0.01 and cp < 0.001 compared to the respective EtOH-induced ulcer group.
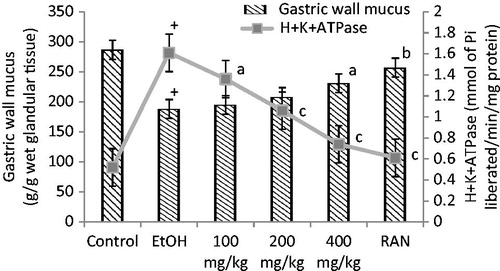
Table 1. Gastroprotective activity of CALE on aspirin-, EtOH-, CRS-, and PL-induced ulcers.
In the gastric secretion studies, compared with control, the rat treated with CALE at the dose of 400 mg/kg significantly showed a tendency to decrease in volume, acid–pepsin concentration and output. However, reference drug ranitidine, a known cytoprotective agent, has little or no effect on the volume, acid–pepsin concentration, and acid output, but showed a significant decrease in peptic output (). Acute toxicity studies of the CALE did not exhibit any sign of toxicity up to a dose of 2000 mg/kg body weight. Since there was no mortality of the animals found at high dose, these results suggest that the plant extract is not toxic and is safe.
Table 2. Effect of CALE on gastric secretion in 4h PL rats: effect on volume, acid, and pepsin.
Antioxidant study
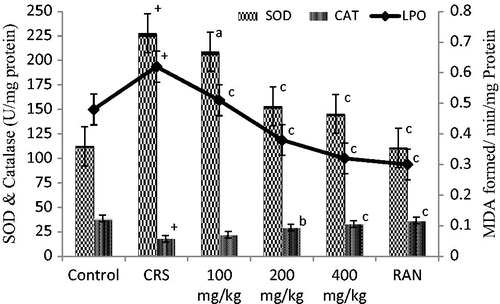
With the pretreatment of CALE 100, 200, and 400 mg/kg doses, the LPO levels dropped significantly (p < 0.001), and SOD levels decreased at 200 and 400 mg/kg dose level (p < 0.001) as compared with CRS-induced group; CAT values showed gradual, significant increase at 200 and 400 mg/kg dose levels (p < 0.001). RAN at the dose of 50 mg/kg significantly reduced the LPO, SOD (p < 0.001), and further increased CAT (p < 0.001) value respectively ().
Discussion
HPTLC analysis showed the presence of phenolic compound in CALE. The presence of high percentage of the quercetin, ellagic acid, and gallic acid in the extract justifies the potent antioxidant activity exhibited. Antioxidants play a major role in repairing the gastric damage. Quercetin and gallic acid scavenge free radicals, block •OH-mediated oxidative damage, and play an important role in the prevention and therapy of diseases. Quercetin has been reported to inhibit the lipid peroxidation of gastric cell and gastric acid secretion in the EtOH-induced ulcer model. An interesting aspect of quercetin’s antiulcer effect is that it has been shown to inhibit growth of H. pylori in dose-dependent manner in vitro (Beil et al., Citation1995). Our laboratory reported that quercetin protected gastric ulceration at least in part by scavenging the free radicals (Gupta et al., Citation2013; Rao et al., Citation2003). Ellagic acid (above 5 mg/kg) has been reported to markedly reduce the occurrence of gastric lesion in stress-induced ulcer models (Murakami et al., Citation1999). Hence, the phenolic compound was chosen for the standardization of the extract. The result of the present study showed that the hydroalcoholic extract of C. arborea leaves possesses gastroprotective activity as evidenced by its significant inhibition in the formation of ulcers induced by various physical and chemical agents. Synthetic NSAIDs, like aspirin, cause mucosal damage by interfering with prostaglandin synthesis, increasing acid secretion and back diffusion of H+ ions (Rao et al., Citation2000). The incidence of EtOH-induced ulcers predominant in the glandular part of stomach was reported to stimulate the formation of leukotriene C4 (LTC4), mast cell secretary products (Oates & Hakkinen, Citation1988) and reactive oxygen species resulting in the damage of rat gastric mucosa (Peskar et al., Citation1986). EtOH-induced depletion of gastric wall mucus has been significantly prevented by CALE due to its strong antioxidant and radical scavenging property. It is reasonable to speculate that the antioxidant potential of the extract could play an important role in the prevention and healing of gastric ulcer. The H+K+ATPase are the dimeric enzyme responsible for H+ secretion by the gastric parietal cells. H+K+ATPase are selectively blocked by the action of ranitidine, an acid blocker used to treat gastric ulcers (Nagaya et al., Citation1987). CALE reduced the level of H+K+ATPase in EtOH-induced ulcers. The possible involvement of CALE on enhancing mucosal resistance could have offered gastro-protection and is regarded as a first line of defense against gastric ulcers.
Pylorus ligation and cold-restrained stress-induced ulcers are results of auto-digestion of the gastric mucosal barrier probably due to excess production and accumulation of hydrochloric acid in the stomach (Goel & Bhattacharya, Citation1991). In pylorus-ligated rats, gastric acid is associated with severe ulceration of the rat gastric mucosa (Martin et al., Citation1993). During our observations on gastric secretion in the PL rat model, the test drug exhibited significant antisecretory and antiulcer activity. Blockade of acid secretion resulted in high healing rates of gastric and duodenal ulcers. The reduced severity of ulcers in this model could be due to its effect in reducing volume and acidity of gastric secretion (Posey et al., Citation1969).
Stress plays an important role in the causation of gastro duodenal ulceration; anti-stress drugs were found to be effective in stress-induced gastric mucosal damage. Stress-induced ulcers also involve damage by ROS apart from acid- and pepsin-related factors. Increased level of LPO is due to increase in generation of ROS during stress leading to oxidative damage. SOD converts the reactive superoxide radical to H2O2 which, if not scavenged by CAT, can by itself cause lipid peroxidation by the generation of hydroxyl radicals. Hence, decrease in CAT levels has led to increase in accumulation of these ROS, and thus has caused increased lipid peroxidation and tissue damage (Sairam et al., Citation2002). This effect was significantly reversed by prior administration of CALE, providing a close relationship between free radical scavenging activity and gastroprotective effect.
In the present study, phenolic and flavonoid contents of the hydroalcoholic extract of C. arborea were identified and quantified. It is, therefore, possible that the gastroprotective effects observed with the C. arborea leaves may be in part due to the presence of phenolic compound and potent antioxidant activities.
Conclusion
The present study strongly demonstrated that the hydroalcoholic extract of C. arborea leaves was able to protect the gastric mucosa from chemical, stress, and physically induced ulcers and inhibits gastric acid secretion probably by blocking H+K+ATPase action and offering antioxidant protection against oxidative stress-induced gastric damage. These findings corroborate the traditional indication of C. arborea, contributing for its pharmacological validation. Our future study will be directed to identify the phytoconstituents responsible for the pharmacological action of C. arborea leaves.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
The authors acknowledge Dr. S.N. Mishra and Mr. B.L. Pandey, Rewa (M.P.) for collection of the plant material.
References
- Aebi H. (1974). Catalase. In: Bergmeyer HU, ed. Methods in Enzymatic Analysis, Vol III. New York: Academic Press Inc, 673–86
- Basak A, Banerjee R, Bose L, Basu K. (1976). Chemical examination of the leaves of Careya arborea. J Indian Chem Soc 53:639–40
- Beil W, Birkholz C, Sewing KF. (1995). Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arznem Forsch 45:697–700
- Corne SJ, Morrissey SM, Woods RJ. (1974). A method for the quantitative estimation of gastric barrier mucus. J Physiol 242:116–17
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. (1997). Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med 23:8–18
- Debnath PK, Gode KD, Govinda Das D, Sanyal AK. (1974). Effect of propranolol on gastric secretion in albino rats. Br J Pharmacol 51:213–16
- De la Lastra CA, Martin MJ, Motilva V. (1994). Antiulcer and gastroprotective effects of quercetin: A gross and histologic study. Pharmacology 48:56–62
- Goel RK, Bhattacharya SK. (1991). Gastroduodenal mucosal defense and mucosal protective agents. Indian J Exp Biol 29:701–14
- Goel RK, Das GD, Sanyal AK. (1985). Effect of vegetable banana powder on changes induced by ulcerogenic agents on dissolved mucosubstances in gastric juice. Indian J Gastroenterol 4:249–51
- Gupta PC, Sharma N, Rao ChV. (2012). Pharmacognostic studies of the leaves and stem of Careya arborea Roxb. Asian Pac J Trop Biomedicine 2:404–8
- Gupta PC, Rao ChV, Sharma N. (2013). Protective effect of standardized extract of Cleome viscosa against experimentally induced gastric lesions in the rat. Pharm Biol 51:595–600
- Gupta MB, Nath R, Gupta GP, Bhargava KP. (1985). A study of the antiulcer activity of diazepam and other tranquillosedatives in albino rat. Clin Exp Pharmacol Physiol 12:61–6
- Hollander D, Taranawski A, Krause WJ, Gergely H. (1985). Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Gastroenterology 88:366–74
- OECD. (2001). OECD Guidelines for the testing of chemicals (acute oral toxicity – up and down-procedure) serial on the Internet. Available at: http://www.oecd.org/dataoecd/17/51/1948378.pdf [last accessed 10 March 2011]
- Kakkar P, Das B, Viswanathan PN. (1984). Modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 2:130–2
- Khandelwal KR. (2008). Practical Pharmacognosy. 19th ed. Pune: Nirali Publication, 149–64
- Martin MJ, Motilva V, Alarcon de la Lastra C. (1993). Quercetin and naringenin: Effects on ulcer formation and gastric secretion in rats. Phytother Res 7:150–3
- Murakami S, Muramatsu M, Tomisawa K. (1999). Inhibition of gastric H+ K+-ATPase by flavonoids: A structure-activity study. J Enzyme Inhib Med Chem 14:151–66
- Nadkarni KM. (2004). Medicinal Plants of India. Deharadun: Reprint Prakashan
- Nadkarni KM, Nadkarni AK. (1976). Indian Materia Medica. Vol I. Bombay: Popular Prakashan
- Nagaya H, Satoh H, Maki Y. (1987). Actions of antisecretory agents on proton transports in hog gastric microsomes. Biochem Pharmacol 36:513–19
- Okhawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–5
- Ordonez AAL, Gomez JD, Vattuone MA, Isla MI. (2006). Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 99:452–8
- Oates PJ, Hakkinen JP. (1988). Studies on the mechanism of ethanol induced gastric damage in rats. Gastroenterology 94:10–21
- Peskar BM, Lange K, Hoppe U, Peskar BA. (1986). Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins 31:283–93
- Phull PS, Green CJ, Jacyna MR. (1995). A radical view of stomach: The role of oxygen-derived free radicals in gastroduodenal disease. Eur J Gastroenterol Hepatol 7:265–74
- Piper DW, Stiel DD. (1986). Pathogenesis of chronic peptic ulcer current thinking and clinical implications. Med Prog 2:7–10
- Posey EL Jr, Boler K, Posey L. (1969). Inhibition of food stimulated gastrin release by a topical anaesthetic, oxethazine. Am J Dig Dis 14:797–804
- Kirtikar KR, Basu BD. (1975). Careya arborea. In: Blatter E, Caius JF, Mhaskar KS, eds. Indian Medicinal Plants. Vol II. 2nd ed. Dehradun: M/s Bishen Singh, Mahendra Palsingh, 894–5
- Rao ChV, Sairam K, Goel RK. (2000). Experimental evaluation of Bacopa monniera on rat gastric ulceration and secretion. Indian J Physiol Pharmacol 44:35–41
- Rao ChV, Ojha SK, Govindarajan R, et al. (2003). Quercetin a bioflavonoid protects against oxidative stress related gastric mucosal damage in rats. Nat Prod Sci 9:68–72
- Sairam K, Rao ChV, Babu DM, Goel RK. (2002). Antiulcerogenic activity of methanolic extract of Emblica officinalis. J Ethnopharmacol 82:1–9
- Sanyal AK, Pandey BL, Goel RK. (1982). The effect of a traditional preparation of copper, tamrabhasma, on experimental ulcers and gastric secretion. J Ethnopharmacol 5:79–89
- Satish BN, Swami Vrushabendra BM, Kumar GK, Gobinda B. (2010). Review on Careya arborea Roxb. IJRAP 1:306–15
- Shay H, Komarov SA, Fels SS, et al. (1945). A simple method for the uniform production of gastric ulceration. Gastroenterology 5:43–61
- Zou Y, Lu Y, Wei D. (2004). Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem 52:5032–9