Abstract
Context: Tamoxifen (TAM) is widely used for treatment of hormone-dependent breast cancer; however, it may be accompanied with hepatic injury. Allicin is the most abundant thiosulfinate molecule from garlic with the potential to provide beneficial effects on various diseases.
Objective: To elucidate the effect of commercially available allicin on both antitumor activity and liver injury of TAM.
Materials and methods: The cytotoxicity of TAM and/or allicin was evaluated in vitro using cultured Ehrlich ascites carcinoma (EAC) cells and in vivo against murine tumor (solid) model of EAC. TAM induced liver injury in rats by intraperitoneally (i.p.) injection at a dose of 45 mg/kg, for 7 successive days.
Results: TAM at a dose of 3 µM (IC50) significantly decreased percent survival of EAC to 52%. TAM combination with allicin (5 or 10 µM) showed a significant cytotoxic effect compared with the TAM-treated group as manifested by a decrease in percent survival of EAC to 35% and 29%, respectively. Allicin (10 mg/kg, orally) enhanced the efficacy of TAM (1 mg/kg, i.p.) in mice as manifested by a significant increase in solid tumor growth inhibition by 82% compared with 70% in the TAM group. In rats, TAM intoxication resulted in a significant decline in SOD, GSH, and total protein with significant elevation in TBARS, ALT and AST, ALP, LDH, total bilirubin, γGT, and TNF-α levels. These changes are abrogated by allicin treatment.
Discussion and conclusion: The results suggest the beneficial role of allicin as an adjuvant to TAM in cancer treatment by alleviating liver injury.
Introduction
Tamoxifen citrate, 2-[4-[(Z)-1,2-diphenylbut-1-enyl] phenoxy]-N,N dimethylethanamine, is a non-steroidal antiestrogen drug that is used in the treatment and prevention of all stages of hormone-dependent breast cancer (Desai et al., Citation2002; Jordan, Citation2003). It reduces the level of estrogen and estrogen receptor with no change in progesterone contents (Liu et al., Citation2004). It was revealed that TAM in high dose is a known liver carcinogen in rats (Ahotupa et al., Citation1994; Caballero et al., Citation2001) which is due to oxygen radical overproduction that occurs during TAM metabolism. This strong hepatocarcinogenic effect of TAM in rats raises issues bearing on the prophylactic chronic administration to healthy women. Garlic has long been used for treating various diseases; its consumption has been related to reduce cancer risk (Galeone et al., Citation2006). Allicin (diallyl thiosulfinate), the major pharmacological component of garlic (Rivlin, Citation2001), has attracted attention of the international medical field gradually due to its potential for disease prevention and treatment. It is formed by the action of the enzyme alliinase on alliin in crushed fresh garlic cloves. It possesses antioxidant activity and is shown to cause a variety of actions potentially useful for human health. Allicin exhibits hypolipidemic, antiplatelet, antibacterial, and antifungal effects. It has been reported that allicin inhibits various cancer cells demonstrating anticancer and chemopreventive activities (Hirsch et al., Citation2000; Jakubikova & Sedlak, Citation2006). The present study aimed at studying the impact of allicin supplementation on chemotherapeutic efficacy and its hepatoprotective properties when used as an adjuvant to TAM.
Materials and methods
Animals
Female Swiss albino mice weighing 20–30 g were used to study the cytotoxic effect of TAM alone and in combination with allicin. Twenty-four adult female albino rats weighing 150–200 g were used in all the experiments. They were obtained from Urology and Nephrology Center of Mansoura University, Mansoura, Egypt. The animals were maintained under standard conditions of temperature 24 ± 1 °C and 55 ± 5% relative humidity with regular 12 h light/12 h dark cycles. They were allowed free access to standard laboratory food and water. The experiments were conducted in accordance with the ethical guidelines for investigations in laboratory animals and were approved by the Ethical Committee of Faculty of Pharmacy, Mansoura University, Egypt.
Drugs and chemicals
Tamoxifen citrate (Nolvadex®) was purchased from AstraZeneca UK Limited (Macclesfield, Cheshire, UK). Allicin was obtained as pharmaceutical drug (Allimax capsule containing 100% stabilized allicin powder). Ellman’s reagent, pyrogallol, reduced glutathione, 1,1,3,3-tetramethoxypropane, and tris (hydroxymethyl) amino-methane were purchased from Sigma Aldrich Chemical Co (St. Louis, MO). n-Butanol was purchased from El-Nasr Chemical Co. (Abou-Zaabal, Cairo, Egypt). Thiobarbituric acid (TBA) was purchased from Fluka (Chemie, Switzerland). Trichloroacetic acid (TCA) was purchased from Winlab (Leicestershire, UK). All other chemicals used in this study were of high purity and purchased locally.
Ehrlich ascites carcinoma cells
Ehrlich ascites carcinoma cells (EAC) were established in the Netherlands Cancer Institute. The Ehrlich tumor line was maintained in the laboratory of Faculty of Pharmacy, Mansoura University, Egypt, in female Swiss albino mice by serial intraperitoneal passage at 7–10 d intervals.
Cytotoxicity study
In vitro experiment
Cell culture
EAC cells suspended in RPMI 1640 medium supplemented with 10% fetal calf serum were cultured in cell culture sterile tubes at a density of 2 × 105 cells/ml/tube. The tubes were incubated in a humidified atmosphere containing 5% CO2 at 37 °C for 24 h. The Trypan blue dye exclusion test was used to determine the rate of cellular growth inhibition. The dye stains the dead cells only while leaving viable cells unstained (Weisenthal et al., Citation1983).
In vitro cytotoxic assay
Ascitic fluid from the intraperitoneal cavity of the donor animal was aseptically aspirated, 7–8 d after EAC cells inoculation and washed three times with N-2-hydroxyethyl-piperazine-N-2-ethanesulfonic acid buffered Hanks’ balanced salt solution. EAC cells were counted under a microscope using a hemocytometer and resuspended in normal saline so that each 0.1 ml contained 2 × 105 cells (Badary et al., Citation2000). EAC cells were incubated in RPMI 1640 medium for 24 h in cell culture tubes, each tube contained 0.1 ml cells and 0.9 ml medium (final concentration of the cells = 2 × 105 cells/ml). After 24 h incubation, the tubes were centrifuged at 67 g and cells were separated. EAC cells were resuspended in RPMI 1640 medium and drugs were added so that the content of each tube was 0.8 ml medium, 0.1 ml cells, and 0.1 ml drug. The final drug concentrations of TAM were 1, 2, 4, 8, 16, and 32 µM. The cytotoxicity dose–response curve for TAM was constructed in order to determine the concentration that inhibits 50% of cell survival (IC50). EAC cells were incubated with 3 µM TAM (IC50), allicin, 5 and 10 µM or the combination for 24 h. After incubation of cells with drugs, cells were separated, washed with phosphate buffered saline, and resuspended in a drug-free medium. EAC cells were stained with Trypan blue dye and the percent survival of cells was determined by the Trypan blue dye exclusion method. Cytotoxicity was determined three times and the mean was recorded. Control experiments in which EAC cells were incubated in a drug-free medium were also conducted. Percent survival of cells = (T/C) × 100 was calculated, where T and C represent the number of viable cells in a unit volume of the test drug tube and the control tube, respectively.
In vivo experiment
Ascitic fluid was withdrawn under aseptic conditions from tumor-bearing mice by needle aspiration from the peritoneal cavity, 7 d after EAC cells inoculation, and washed three times with normal saline by centrifugation at 67 g. EAC cells obtained after washing were tested for viability using Trypan blue. The cells were examined microscopically using a hemocytometer, suspended in normal saline so that each 0.1 ml contained 5 × 105 viable EAC cells. The cells were counted under the microscope.
Solid tumors were induced in mice by subcutaneous inoculation of 0.1 ml containing 5 × 105 viable tumor cells into the right thigh of the lower limb of mice (Raja Naresh et al., Citation1996). Tumor growth was determined by a digital caliper measurement of the largest diameter and its perpendicular (Schirner et al., Citation1998)
where a is the largest diameter and b is its perpendicular.
When the primary tumor reached a size of 50–100 mm3, 40 mice were grouped into four groups (10 mice each). Group (1) received normal saline (EAC-bearing control, 5 ml/kg). Group (2) received TAM (1 mg/kg) I.P. Group (3) received allicin (10 mg/kg, orally). Group (4) received TAM and allicin. Treatment continued for 21 consecutive days. Tumor size at day 0 (5 d after tumor inoculation) and after treatment (day 21) was measured. Antitumor activity was calculated by the determination of ΔT (change of tumor size between day 0 and day 21 in the treatment group) and ΔC (change of tumor size between day 0 and day 21 in the control). The degree of tumor growth inhibition can be obtained from ΔT/ΔC × 100 (Schirner et al., Citation1998).
Liver injury
The animals were divided at random into four groups of six rats each. The first group, received normal saline (2.5 ml/kg) for 17 d. The second group, TAM-intoxicated rats: rats were treated with TAM at a dose of 45 mg/kg/day, i.p., for 7 successive days (Hard et al., Citation1993). The third group, allicin-treated rats: rats were orally administered 50 mg allicin/kg/day for 17 successive days. The fourth group, TAM-allicin rats: rats were orally administered 50 mg allicin/kg body weight daily for 10 successive days before and 7 d with TAM intoxication.
At the end of the experiment, animals were subjected to light ether anesthesia and blood samples were collected in centrifuge tubes and centrifuged to obtain serum. Animals were killed by cervical dislocation, the abdomen was excised, and the liver was removed immediately by dissection, washed in ice-cold isotonic saline and blotted between two filter papers. A 10% (w/v) liver homogenate was prepared in ice-cold 0.1 M potassium phosphate buffer, pH 7.
Estimation of liver injury
Serum was used to assess hepatic profile enzymes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (γGT), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) according to the standard procedures given along with the kits purchased. Serum total bilirubin and total protein levels were also determined. Kits from BioMed-Diagnostics, Egy-Chem Co., Cairo, Egypt, were used. Fresh aliquots from liver homogenate were used to estimate thiobarbituric acid reactive substance (TBARS), reduced glutathione (GSH), superoxide dismutase (SOD), and tumor necrosis factor-alpha (TNF-α).
Estimation of antioxidant profile
TBARS was measured as malondialdehyde (MDA), the end product of lipid peroxidation, according to the method of Ohkawa et al. (Citation1979). MDA solution (made freshly by the hydrolysis of 1,1,3,3-tetramethoxypropane) was used as the standard. The absorbance was determined at 532 nm spectrophotometrically and the concentrations were expressed as nmol/g wet tissue. The level of acid-soluble thiols, mainly GSH, in the liver was assayed colorimetrically, based on its reaction with Ellman’s reagent [5,5′-dithio-bis(2-nitrobenzoic acid)] according to the method earlier described by Ellman (Citation1959) after protein precipitation with TCA. The absorbance was measured at 412 nm and the concentrations were expressed as µmol/g wet tissue. The enzymatic activity of SOD was assessed according to Marklund (Citation1985). SOD activity was expressed as U/g wet tissue. One unit of SOD activity is defined as the amount of the enzyme causing 50% inhibition of auto-oxidation of pyrogallol.
Determination of TNF-α
Enzyme-linked immunosorbent assay (ELISA) was used to detect TNF-α level in rat liver homogenate according to the manufacturer’s manual (Quantikine R&D system Inc, Minneapolis, MN). This assay employs the quantitative sandwich enzyme immunoassay technique. A polyclonal antibody specific for rat TNF-α has been pre-coated onto a microplate. Standards, controls, and samples are pipetted into the wells and any rat TNF-α present is bound by the immobilized antibody. A biotinylated polyclonal antibody specific for rat to TNF-α is added which binds to rat TNF-α captured by the first antibody. Following incubation, unbound biotinylated TNF-α antibody is removed during a wash step. Streptavidin conjugated to horseradish peroxidase (HRP) is added and binds to biotin-conjugated anti-rat TNF-α antibody. Following incubation, unbound streptavidin–HRP is removed during a wash step. A substrate solution reactive with HRP is added to the wells. The enzyme reaction yields a blue product that turns yellow when the stop solution is added. The intensity of the color measured is proportional to the amount of rat TNF-α bound in the initial step. TNF-α concentration was calculated from standard curve and expressed as pg/g of liver.
Statistical analysis
Data are expressed as mean ± S.E.M. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparisons test, in addition to linear regression analysis for the best fitting line of all standard points (Daniel, Citation1991). Statistical calculations were carried out using the Instat-2 computer program (GraphPad Software Inc. V2.04, San Diego, CA).
Results
Effect of allicin on the cytotoxic effect of TAM on cultured EAC cells
The IC50 of TAM was found to be about 3 μM. Allicin (10 µM) showed a significant cytotoxic effect when compared with the control group but non-significant from the TAM-treated group. Combination of TAM with allicin (5 or 10 µM) showed a significant cytotoxic effect (p < 0.01, p < 0.001, respectively) compared with the TAM-treated group ().
Table 1. Effect of allicin (5 and 10 µM) on the cytotoxic effect of TAM (3 µM) on cultured EAC cells.
Effect of TAM and/or allicin on tumor size in mice
Implantation of EAC cells resulted in a solid palpable tumor mass that developed after 5 d from inoculation (day 0). The size of tumor progressively increased with time and reached about five-fold its initial mass after additional 21 d (day 21). This is considered 100% tumor growth.
Treatment with TAM significantly decreased the relative tumor size compared with the control group, showing 30% tumor growth (i.e., 70% tumor growth inhibition). Mice treated with allicin showed 45% tumor growth (i.e., 55% tumor growth inhibition). Administration of TAM with allicin showed an increase in tumor growth inhibition (82%) compared with the TAM-treated group ().
Table 2. Effect of TAM (1 mg/kg) and/or allicin (10 mg/kg) on tumor size in mice after 21 d treatment.
Effect of TAM and/or allicin on serum biochemical measurements in rats
TAM-intoxication produced significant elevation of serum liver enzymes, AST, ALT, γGT, LDH, and ALP () and total bilirubin () compared with normal control rats. All these mentioned changes were significantly reduced as compared with TAM-treated rats upon administration of allicin to TAM-treated rats. A significant decrease in serum total protein level was observed in the TAM group, which was ameliorated by allicin administration ().
Table 3. Effect of oral administration of allicin on serum hepatic profile enzymes; (ALT, AST, γGT, LDH, and ALP) of TAM-treated rats.
Table 4. Effect of oral administration of allicin on serum total bilirubin, and total protein of TAM-treated rats.
Effect of TAM and/or allicin on liver content of TBARS, GSH, SOD and TNF-α in rats
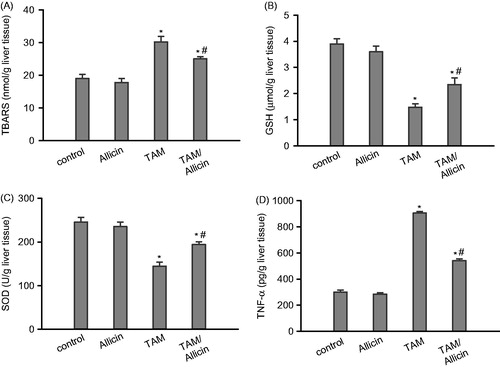
TAM administration produced a significant increase in liver lipid peroxides formation expressed as TBARS by 59% (30.2 ± 1.7), as compared with normal control rats (19 ± 1.3). This elevation was attenuated after administration of allicin to TAM intoxicated rats by 17% (25 ± 0.7) compared with TAM-intoxicated rats (). TAM administration produced a significant decrease in liver GSH level by 62% (1.48 ± 0.13), compared with normal control rats (3.9 ± 0.2). The GSH level was significantly elevated upon administration of allicin to TAM-intoxicated rats by 58% (2.35 ± 0.25) as compared with TAM treated rats (). TAM administration produced significant decrease in liver SOD level by 41% (144.3 ± 9.9), when compared with normal control rats (245.7 ± 10.7). The SOD level was significantly raised after the administration of allicin to TAM intoxicated rats by 34% (194 ± 6.7) as compared with TAM-treated rats (). TAM administration produced a significant increase in liver TNF-α (905 ± 13.2), which significantly (p < 0.05) decreased (540 ± 14.6) upon allicin administration ().
Figure 1. Effect of oral administration of allicin on (A) lipid peroxides formation expressed as TBARS, (B) GSH, (C) SOD, and (D) TNF-α of TAM-intoxicated rat liver homogenate. Data are expressed as mean ± SEM, n = 6. p < 0.05 compared with the control group (*) and compared with the TAM-treated group (#). A one-way ANOVA followed by Tukey–Kramer’s multiple comparisons post hoc test were used.
Discussion
The beneficial effects of garlic have been reported. They include antibacterial, antiparasitic, anticancer, immunomodulatory, hypocholesterolemic, and hypotensive effects (Tattelman, Citation2005). Allicin, one of the garlic constituents, was implicated to mediate its biological activity. The purpose of the present study was to demonstrate the possible beneficial effect of allicin on the anticancer activity and liver toxicity of TAM.
In the present study, it was found that EAC cells are sensitive to TAM in a concentration-dependent manner. Allicin produced a significant cytotoxic effect against EAC-cells when compared with the control group. A combination between TAM and allicin significantly produced a synergistic cytotoxic effect on cultured EAC cells.
This study has been extended to explore if allicin could alter the antitumor effect of TAM in vivo and thus, modify its therapeutic response by using a mouse model of solid tumor. The antitumor activity of TAM was evidenced by the reduction in the relative tumor size compared with untreated tumor-bearing control animals. This observation is in agreement with the previously reported cytotoxicity of TAM on MCF-7 and MDA-MB-231 human breast cancer cells (Al-Akoum et al., Citation2007), OC2 human oral cancer cells (Chu et al., Citation2007), human epidermal keratinocyte cell line (Bhatia et al., Citation2010), human chronic myeloid leukemia (K562) cell line (Pedroso et al., Citation2013). Allicin produced a reduction in relative tumor size supporting the well-established antitumor effect of allicin against a variety of tumors in preclinical models (Padilla-Camberos et al., Citation2010; Patya et al., Citation2004). It was suggested that the antitumor effect of allicin is related to its immune-stimulatory properties (Patya et al., Citation2004) and/or induced apoptosis (Padilla-Camberos et al., Citation2010). Combination between TAM and allicin increased the percent inhibition of the growth of solid tumor in mice confirming the synergistic effect between the drugs. This study was further extended to address whether or not allicin would have effects on TAM-induced liver injury. TAM in toxic doses led to oxidative liver damage, as it has been shown to produce liver injury in rats (Ahotupa et al., Citation1994; El-Beshbishy, Citation2007). The results of this study demonstrated that TAM administration resulted in significant elevation of sALT, sAST, LDH, ALP, and γGT activities () and serum total bilirubin (). These findings were in accordance with El-Beshbishy et al. (Citation2010). In contrast, the total protein was decreased as compared with the control untreated group. All these biochemical changes were significantly improved after allicin administration to TAM-intoxicated rats. Lipid peroxidation is one of the major characteristics that can be included as an oxidative damage marker (Ahotupa et al., Citation1994). In accordance with the data obtained from this study, TAM administration resulted in significant elevation in TBARS production. The level of TBARS was significantly decreased compared to TAM intoxicated group () upon administration of allicin. To further substantiate the antioxidant activity of allicin, the activity of SOD enzyme was assessed. In this study, hepatic SOD was significantly reduced in TAM-treated rats. Oxidative stress noticed after TAM intoxication was associated with decreased hepatic GSH concomitant with increased peroxidation (Oge et al., Citation2003). SOD decreased after initiation of lipid peroxidation process (El-Beshbishy, Citation2007) which was in accordance with results achieved from this study. Decreased activity of hepatic SOD of TAM-intoxicated rats may be due to oxidative stress-induced inactivation and/or exhaustion (Kakkar et al., Citation1997). The impaired regeneration of protective and antioxidants such as GSH also contribute to oxidative stress (Lee et al., Citation2007). The level of hepatic antioxidant enzymes was significantly improved upon the treatment of TAM-intoxicated rats with allicin.
Tamoxifen is one of the drugs capable of inducing macrovascular steatosis and steatohepatitis. This may be attributed to the drug ability to impair mitochondrial respiratory chain thus causing not only fatty acid oxidation impairment and steatosis but also enhanced reactive oxygen species (ROS) production. ROS overproduction may be a key event in the pathogenesis of drug-induced steatohepatitis by inducing lipid peroxidation (favored by lipid accumulation), and possibly by triggering the generation of pro-apoptotic (TNF-α) and pro-fibrotic (TGF-β) cytokines by Kupffer cells and other inflammatory cells (Labbe et al., Citation2008). In the current study, treatment of rats with TAM resulted in a three-fold increase in TNF-α level. In accordance with our results, it was reported that TAM administration resulted in a significant increase in TNF-α levels in rats (Albukhari et al., Citation2009; El-Beshbishy et al., Citation2010). Pretreatment with allicin significantly inhibited the rise in the TNF-α levels. This is consistent with the known anti-inflammatory activity of allicin (Hasan et al., Citation2006; Su et al., Citation2008). Allicin was also discovered to enhance the antioxidant status by lowering the level of reactive oxygen species and stimulating the production of glutathione (Chan et al., Citation2013).
In conclusion, data achieved from this study revealed that the pre-treatment with allicin potentiates the antitumor effect of TAM and protects against its hepatic injury by preventing oxidative stress and lipid peroxidation, enhancing antioxidant enzyme activities and inhibiting hepatic inflammation. Further studies to elucidate the detailed mechanism of action are in progress.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
References
- Al-Akoum M, Dodin S, Akoum A. (2007). Synergistic cytotoxic effects of tamoxifen and black cohosh on MCF-7 and MDA-MB-231 human breast cancer cells: An in vitro study. Can J Physiol Pharmacol 85:1153–9
- Albukhari AA, Gashlan HM, El-Beshbishy HA, et al. (2009). Caffeic acid phenethyl ester protects against tamoxifen-induced hepatotoxicity in rats. Food Chem Toxicol 47:1689–95
- Ahotupa M, Hirsimaki P, Parssinen R, Mantyla E. (1994). Alterations of drug metabolizing and antioxidant enzyme activities during tamoxifen-induced hepatocarcinogenesis in rats. Carcinogenesis 15:863–8
- Badary OA, Sharaby SA, Kenawy SA, et al. (2000). Evaluation of cisplatin combined with ondansetron in Ehrlich ascites carcinoma in vitro and in vivo. Tumori 86:153–6
- Bhatia A, Singh B, Bhushan S, Katare OP. (2010). Tamoxifen-encapsulated vesicular systems: Cytotoxicity evaluation in human epidermal keratinocyte cell line. Drug Dev Ind Pharm 36:350–4
- Caballero F, Gerez E, Oliveri L, et al. (2001). On the promoting action of tamoxifen in a model of hepatocarcinogenesis induced by p-dimethylaminobenzene in CF1 mice. Int J Biochem Cell Biol 33:681–90
- Chan JY, Yuen AC, Chan RY, Chan SW. (2013). A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother Res 27:637–46
- Chu ST, Huang CC, Huang CJ, et al. (2007). Tamoxifen-induced [Ca2+]i rises and Ca2+-independent cell death in human oral cancer cells. J Recept Signal Transduct Res 27:353–67
- Daniel WW. (1991). Hypothesis testing. In: Biostatistics: A Foundation for Analysis in the Health Sciences, 5th ed. New York, Chichester, Brisbane, Toronto, Singapore: John Wiley & Sons, 191
- Desai PB, Nallani SC, Sane RS, et al. (2002). Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos 30:608–12
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- El-Beshbishy HA. (2007). Lipoic acid attenuates DNA fragmentation, oxidative stress and liver injury induced by tamoxifen in rats. Asian J Trad Med 2:175–88
- El-Beshbishy HA, Mohamadin AM, Nagy AA, Abdel-Naim AB. (2010). Amelioration of tamoxifen-induced liver injury in rats by grape seed extract, black seed extract and curcumin. Indian J Exp Biol 48:280–8
- Galeone C, Pelucchi C, Levi F, et al. (2006). Onion and garlic use and human cancer. Am J Clin Nutr 84:1027–32
- Hard GC, Iatropoulos MJ, Jordan K, et al. (1993). Major difference in the hepatocarcinogenicity and DNA adduct forming ability between toremifene and tamoxifen in female Crl:CD(BR) rats. Cancer Res 53:4534–41
- Hasan N, Yusuf N, Toossi Z, Islam N. (2006). Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett 580:2517–22
- Hirsch K, Danilenko M, Giat J, et al. (2000). Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer 38:245–54
- Jakubikova J, Sedlak J. (2006). Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma 53:191–9
- Jordan VC. (2003). Tamoxifen: A most unlikely pioneering medicine. Natl Rev Drug Discov 2:205–13
- Kakkar R, Mantha S, Radhi J, et al. (1997). Antioxidant defense system in diabetic kidney: A time course study. Life Sci 60:667–79
- Labbe G, Pessayre D, Fromenty B. (2008). Drug-induced liver injury through mitochondrial dysfunction: Mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol 22:335–53
- Lee CP, Shih PH, Hsu CL, Yen GC. (2007). Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol 45:888–95
- Liu Z, Shi HY, Nawaz Z, Zhang M. (2004). Tamoxifen induces the expression of maspin through estrogen receptor alpha. Cancer Lett 209:55–65
- Marklund SL. (1985). Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res 148:129–34
- Oge A, Sezer ED, Ozgonul M, et al. (2003). The effects of estrogen and raloxifene treatment on the antioxidant enzymes and nitrite–nitrate levels in brain cortex of ovarictomized rats. Neuroscience 338:217–20
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Padilla-Camberos E, Zaitseva G, Padilla C, Puebla AM. (2010). Antitumoral activity of allicin in murine lymphoma L5178Y. Asian Pac J Cancer Prev 11:1241–4
- Patya M, Zahalka M, Vanichkin A, et al. (2004). Allicin stimulates lymphocytes and elicits an antitumor effect: A possible role of p21ras. Int Immunol 16:275–81
- Pedroso LS, Fávero GM, de Camargo LE, et al. (2013). Effect of the o-methyl catechols apocynin, curcumin and vanillin on the cytotoxicity activity of tamoxifen. J Enzyme Inhib Med Chem 28:734–40
- Raja Naresh RA, Ndupa N, Uma Devi P. (1996). Effect of macrophage activation on niosome encapsulated bleomycin in tumor-bearing mice. Ind J Pharamcol 28:175–80
- Rivlin RS. (2001). Historical perspective on the use of garlic. J Nutr 131:951–4S
- Schirner M, Hoffmann J, Menrad A, Schneider MR. (1998). Antiangiogenic chemotherapeutic agents: Characterization in comparison to their tumor growth inhibition in human renal cell carcinoma models. Clin Cancer Res 4:1331–6
- Su QS, Tian Y, Zhang JG, Zhang H. (2008). Effects of allicin supplementation on plasma markers of exercise-induced muscle damage, IL-6 and antioxidant capacity. Eur J Appl Physiol 103:275–83
- Tattelman E. (2005). Health effects of garlic. Am Fam Phys 72:103–6
- Weisenthal LM, Dill PL, Kunick NB, Lippman ME. (1983). Comparison of dye exclusion assays with a cologenic assay in the determination of drug-induced cytotoxicity. Cancer Res 43:258–64

