Abstract
Context: Dyslipidemia is one of the major risk factors for cardiovascular disease in diabetes mellitus (DM). The availability of multiple lipid-lowering drugs and supplements provides new opportunities for patients to regulate lipid levels.
Objective: The present study was designed to evaluate the effect of Butea monosperma Lam. (Fabaceae) bark extract in diabetes-induced dyslipidemia.
Materials and methods: A daily dose of B. monosperma bark extract (BMBE, 500 mg/kg body weight) was given orally to streptozotocin (STZ)-induced diabetic rats for 60 d. Several indices such as blood glucose, insulin, glycosylated hemoglobin, TC, TG, high-density lipoprotein-cholesterol (HDL-C), apo A1, apo B, activities of lipogenic enzymes in tissues, liver function tests, and histopathology of liver were analyzed to assess the modulation of STZ-induced diabetic dyslipidemia by B. monosperma bark.
Results: BMBE significantly reduced blood glucose (40.79%) and increased plasma insulin (37.5%) levels in diabetic rats. Altered levels of serum lipids, lipoproteins, and activities of lipogenic enzymes in tissues were partially restored upon the administration of BMBE in diabetic rats. Liver function tests and histopathological examination revealed that consumption of BMBE at a dose of 500 mg/kg body weight had no toxic effects in experimental rats.
Conclusion: The findings suggest that BMBE supplementation could ameliorate dyslipidemia in DM.
Introduction
Diabetes mellitus (DM) is a multifaceted, dynamic expression of pathological disequilibria, resulting in various micro- and macro-vascular complications. It is characterized by the presence of hyperglycemia due to defective insulin secretion, defective insulin action, or both. The number of people suffering from diabetes is estimated to be 151 million and this number is projected to double in the next decade (Amos et al., Citation1997). Diabetes is associated with profound alterations in the plasma lipid and lipoprotein profile and with an increased risk of coronary heart disease (Betteridge, Citation1997). Dyslipidemia in DM is characterized by increased levels of triglycerides, very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and decreased levels of high-density lipoprotein (HDL) cholesterol. Dyslipidemia further raises the risk of atherosclerosis in people with diabetes. Epidemiologic studies have demonstrated that DM is an independent risk factor for cardiovascular disease and it amplifies the effects of other common risk factors, such as smoking, hypertension, and hypercholesterolemia. The mortality associated with vascular diseases in people with DM is significantly higher than the mortality in non-diabetic individuals (Kannel & McGee, Citation1979).
Current diabetes pharmacotherapy gives sharp and good control of blood glucose levels, but efficiency becomes strictly reduced after long-term use (Wagman & Nuss, Citation2001). The management of diabetes without any side effects is still a challenge to the medical system. Herbal drugs are prescribed widely because of their effectiveness, fewer side effects, and relatively low cost. More than 1200 plant species are used worldwide in diabetes phytotherapy. In addition to regulation of blood glucose levels, several hypoglycemic plants have the potential effect in ameliorating lipid metabolic abnormalities of DM (Thompson & Ernst, Citation2003). Thus, the study of plant hypoglycemic and hypolipidemic activities may give a new pharmacological approach in the treatment of DM.
Butea monosperma Lam. (Fabaceae), commonly known as “Flame of the forest” (Patil et al., Citation2006), is extensively used in Ayurvedic medicine and has become a cynosure of modern medicine. Each and every part of the plant, namely bark, root, stem, fruit, leaves, and flowers, are useful in one way or the other. It is used as a tonic, astringent, aphrodisiac, and diuretic. Its reported pharmacological properties include free radical scavenging, hypolipidemic, antidiabetic, anti-inflammatory, anticonvulsive, fertility inhibiting, hepatoprotective, wound healing, and antimicrobial activities (Divya & Mini, Citation2011; Mengi & Deshpande, Citation1999; Somani et al., Citation2006). A literature survey revealed that the bark of B. monosperma is traditionally used for the treatment of diabetes. However, there are no available reports on the hypolipidemic activity of bark of B. monosperma in diabetic conditions. Therefore, in the present study, we evaluated the effect of B. monosperma bark in diabetes-induced dyslipidemia.
Materials and methods
Plant material
Butea monosperma bark was collected during February–March 2012 from Kottoor, Trivandrum, India. The plant material was identified and authenticated by Dr. Valsala Devi, Department of Botany, University of Kerala, India. A voucher specimen (Voucher no. KUBH 5803) has been deposited at the herbarium of Department of Botany, University of Kerala, India.
Preparation of extract
The bark of B. monosperma was shade-dried and crushed to moderately coarse powder. The powder was extracted with ethanol at room temperature by cold maceration method. The resulting extract was filtered using Whatman No. 1 filter paper. The filtrate was evaporated in vacuum to obtain the residue (13.8%, w/w).
Preliminary phytochemical analysis
The ethanol extract of B. monosperma bark was screened for phytochemical constituents using standard procedures of analysis (Sofowora, Citation1993; Trease & Evans, Citation2002).
Experimental animals
Healthy male Wistar Albino rats (weighing 220–240 g) were used for the experiment. The animals were housed in standard conditions of temperature (21 ± 2 °C), humidity (55 ± 10%), and a 12-h light/dark cycle. The rats were fed with standard pellet diet and water ad libitum.
Induction of diabetes
Rats were made diabetic by a single intraperitoneal injection of streptozotocin (STZ, 40 mg/kg body weight) in freshly prepared citrate buffer (0.1 M, pH 4.5). They were given 5% glucose in drinking water for the first 24 h to encounter any initial hypoglycemia. Diabetes in STZ-induced rats was confirmed by measuring blood glucose after 3 d. The animals with blood glucose above 250 mg/dL were considered to be diabetic and used for the study.
Experimental design
Rats were divided into four groups of six animals as follows:
Group I: normal control
Group II: normal rats treated with BMBE (500 mg/kg bw)
Group III: diabetic control
Group IV: diabetic rats treated with BMBE (500 mg/kg bw)
The test drug was fed orally for 60 d. After the experimental period, rats were sacrificed; blood and tissues were collected in ice cold containers for various biochemical estimations. Animal experiments were approved by Institutional Animal Ethics Committee [IAEC-KU-10/2010-2011-BC-SM (5)].
Biochemical parameters
Blood glucose, glycosylated hemoglobin, total cholesterol (TC), triglycerides (TG), HDL-cholesterol (HDL-C), apolipoprotein A1 (Apo A1), apolipoprotein B (Apo B) levels, activities of serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), acid phosphatase (ACP), and alkaline phosphatase (ALP) were determined using AGAPPE diagnostics kits (Agappe Diagnostics Limited, Ernakulam, Kerala). Plasma insulin level was assayed with an ELISA kit (SPI-BIO, Bertin) using rat insulin as standard. The specific activities of enzymes, namely HMG CoA reductase (EC 1.1.1.34), malic enzyme (EC 1.1.1.40), isocitrate dehydrogenase (EC 1.1.1.41), and glucose 6-phosphate dehydrogenase (EC 1.1.1.49), were estimated with standard colorimetric and photometric techniques (Kornberg & Horecker, Citation1955; Ochoa, Citation1955a,Citationb; Rao & Ramakrishnan, Citation1975). The protein in the enzyme extract was estimated by the method of Lowry et al. (Citation1951).
Histopathological analysis
After the experimental period, the liver of the animals of all groups was separated and small pieces were fixed in 10% formaldehyde. Dehydration and clearing of the tissues were performed. The paraffin sections were stained with hematoxylin and eosin for histological studies and examined under light microscope.
Statistical analyses
The data were expressed as mean ± standard deviation (SD). All statistical analyses were performed by using one-way ANOVA with standard statistical software package of social science (SPSS) version 11.5 (SPSS Inc., Chicago, IL). Differences of p value <0.05 were considered statistically significant.
Results
Preliminary phytochemical analysis
Preliminary phytochemical analysis of the ethanol extract of B. monosperma bark revealed the presence of tannins, saponins, steroids, terpenoids, phenols, and flavonoids.
Body weight and serum glucose
Significant decrease in the body weight was observed in the diabetic controls from the fourth day after diabetes induction. However, the body weights of BMBE-treated diabetic animals were towards the normal range. Fasting blood glucose levels of normal healthy rats were in the normal range. STZ elevated the blood glucose levels, which were partially restored (40.79%) upon the administration of BMBE ().
Table 1. Effect of Butea monosperma bark on body weight, serum glucose, plasma insulin, and glycosylated hemoglobin in normal and diabetic rats.
Plasma insulin and glycosylated hemoglobin
The insulin level in diabetic-control rats decreased significantly compared to normal rats. Administration of BMBE significantly increased the insulin level (37.5%) compared to untreated diabetic rats. The rats exposed to STZ developed diabetes as evident from the significant elevation in glycosylated hemoglobin as compared to the normal control. The administration of BMBE reduced the glycosylated hemoglobin level compared to untreated diabetic rats ().
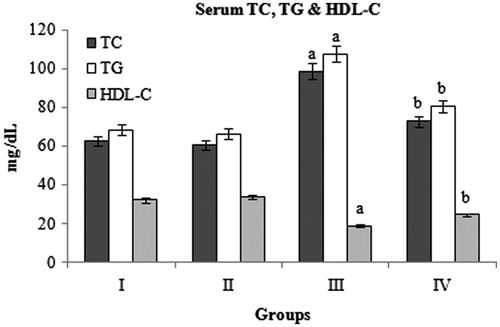
Serum TC, TG, and HDL-C
Diabetic rats showed significant increase in the levels of serum TC and TG and decrease in HDL-C compared to normal control rats. BMBE treatment significantly decreased the TC (26.3%) and TG (25.4%) and increased the HDL-C (31.1%) levels compared to diabetic control rats. The normal rats treated with BMBE did not show any significant change compared to normal untreated rats ().
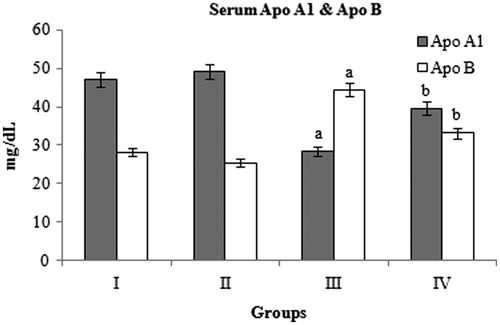
Serum Apo A1 and Apo B
Diabetic control rats showed significant decrease in Apo A1 and increase in Apo B levels compared with normal control rats. BMBE treatment significantly increased the Apo A1 (39.8%) and decreased the Apo B (25.4%) levels compared to diabetic control rats ().
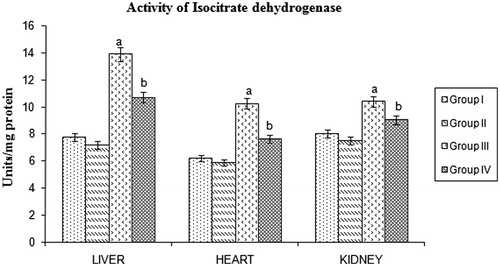
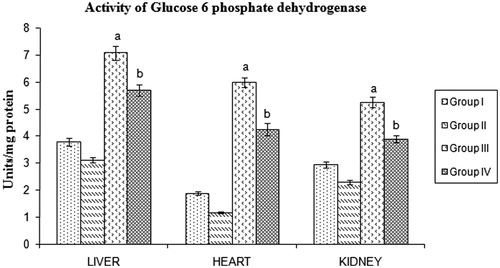
HMG CoA reductase, malic enzyme, isocitrate dehydrogenase and glucose 6-phosphate dehydrogenase in tissues
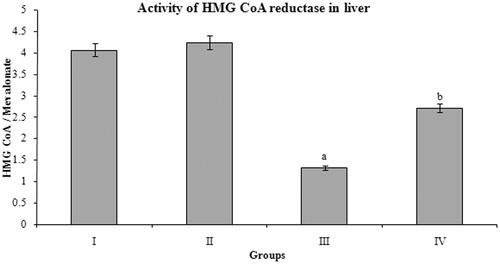
The BMBE-treated diabetic rats showed significant increase in the HMG CoA/mevalonate ratio compared to diabetic-control rats. Oral administration of BMBE to normal rats has no significant effect when compared to normal controls ().
Figure 3. Effect of Butea monosperma bark on HMG CoA reductase in normal and diabetic rats. The data are expressed as mean ± SD. ap < 0.05 compared to the normal control group; bp < 0.05 compared to the diabetic control group.
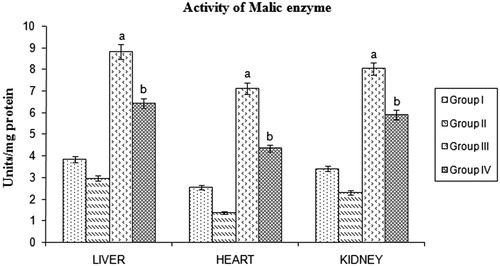
STZ-induced diabetic rats showed a significant increase in malic enzyme, isocitrate dehydrogenase, and glucose 6-phosphate dehydrogenase activities. Significant reduction of these lipogenic enzymes were found in BMBE-treated diabetic rats when compared with diabetic untreated rats ().
Figure 4. Effect of Butea monosperma bark on malic enzyme in normal and diabetic rats. The data are expressed as mean ±SD. ap < 0.05 compared to the normal control group; bp < 0.05 compared to the diabetic control group.
Liver function tests
STZ administration increased liver function biomarkers significantly in comparison with normal control rats. BMBE treatment decreased the activities of SGOT (26.3%), SGPT (24.6%), ACP (22.9%), and ALP (18.3%) in diabetic rats ().
Table 2. Effect of Butea monosperma bark on liver function tests in normal and diabetic rats.
Histopathology of liver
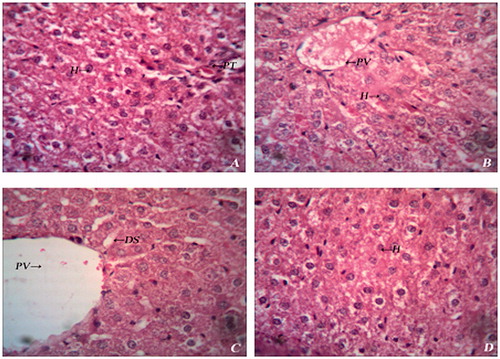
Histopathological examination of liver sections revealed that, the normal liver architecture was disturbed by STZ intoxicity. Sections obtained from BMBE-treated group showed normal cell architecture, although less visible changes were observed which further corroborate the hepatoprotective activity ().
Figure 7. Histopathology of liver of experimental rats. (A) Group I – Normal histology showing hepatocytes (H) and portal triad (PT); (B) Group II – Normal histology showing hepatocytes (H) and portal vein (PV); (C) Group III – Diabetic liver showing dialated sinusoids (DS) and haemorrhage; (D) Group IV – Diabetic liver showing mild degeneration of hepatocytes (H).
Discussion
Hyperlipidemia has been ranked as one of the greatest risk factors contributing to the prevalence and severity of atherosclerosis, stroke, and coronary heart diseases (Saravanan et al., Citation2003). Cardiovascular disease, cerebrovascular disease, and peripheral vascular disease resulting from atherosclerosis are leading causes of morbidity and mortality among adults with diabetes, and there is unequivocal evidence that atherosclerosis is well-established in adolescence with dyslipidemia being the major risk factor. The known lipid lowering drugs, such as fibrates, statins, and bile acid sequestrants, have many side effects in patients (Chattopadhyaya et al., Citation1996). A sustained reduction in hyperglycemia will decrease the risk of developing microvascular diseases and reduce their complications (Kim et al., Citation2006). Thus, there is a considerable interest on the development of dual acting drugs from natural products in the recent years.
Dehydration and loss of body weight have been reported to be associated with diabetes (Pupim et al., Citation2005). Results of the present study show a significantly decreased body weight in STZ-induced diabetic rats when compared to normal rats. While inclusion of BMBE in STZ-induced diabetic rats significantly improved their body weight. This could be due to better control of the hyperglycemic state in diabetic rats by BMBE.
At low dose, STZ (50 mg/kg body weight) partially destructs the β cells, resulting in insufficient insulin secretion causing type 2 diabetes (Gomes et al., Citation2001). Plasma insulin levels were increased in diabetic rats by BMBE treatment for 60 d, indicating their action on pancreatic β cells. In the normal BMBE-treated rats, no significant effect was observed, i.e., BMBE exerts hypoglycemic effect by stimulating glucose-dependent insulin secretion from pancreatic β cells. This is an interesting observation, as the continuous use of the extract or the accidental overdose of this drug will not result in hypoglycemic shock. In this way, this drug will be better than insulin or sulfonyl urea drugs, which causes severe hypoglycemia when taken in excessive doses (Ferner & Neil, Citation1988).
The measurement of glycosylated hemoglobin is one of the well-established means of monitoring glycemic control in patients with diabetes mellitus. Administration of BMBE in diabetic rats resulted in decreased levels of glycosylated hemoglobin showing its hypoglycemic effect. Glycosylated hemoglobin level was noted as showing positive correlation with total cholesterol, LDL cholesterol, triglycerides, and VLDL-cholesterol in DM patients (Ercyias et al., Citation2004). Our study also showed a significant correlation between glycosylated hemoglobin and lipid profile. Administration of BMBE in diabetic rats resulted in decreased levels of glycosylated hemoglobin, showing its hypolipidemic effect in diabetes.
High total cholesterol level is one of the major risk factors for coronary heart diseases, constituting the main cause of morbi-mortality in DM (Bhandari et al., Citation2005; Tan et al., Citation2005). Hypertriglyceridemia is a common finding in patients with DM and is responsible for vascular complications. Hypercholesterolemia and hypertriglyceridemia in STZ-induced diabetic rats are mainly due to insulin deficiency leading to increased lipolysis (Shirwaikar et al., Citation2004). It has been reported that the treatment of diabetes with insulin served to lower plasma triglyceride levels by regulating lipoprotein lipase and hydrolysing triglycerides (Babu & Srivivasan, Citation1997). The administration of BMBE significantly decreased serum cholesterol and triglycerides in diabetic rats. So the cholesterol and triglyceride lowering properties of BMBE may be attributed to hypocholesteromic compounds that may act as inhibitors or activators for some enzymes, which participate in cholesterol metabolism and also its potentiality to release insulin in diabetic rats.
Apolipoprotein A1 and apolipoprotein B are the major protein constituents of HDL and LDL, respectively. It has been suggested that apo A1 and apo B are the sensitive biomarkers of cardiovascular disease. Reduced plasma HDL due to the inhibition of apo A1 synthesis may contribute to the increased incidence of coronary heart disease in diabetes (Haffner et al., Citation1998; Mooradian et al., Citation1997; Solymoss et al., Citation1995). Epidemiological studies suggest that apo A1 and HDL have cardioprotective properties, presumably because of their roles in reverse cholesterol transport (RCT) (Franceschini et al., Citation1991). In DM, alterations in RCT may be responsible for the high prevalence of atherosclerosis (Dirican et al., Citation2003). Thus, in diabetic rats, BMBE treatment seem to improve RCT and consequently to reduce cardiovascular risk.
The major rate-limiting step in the biosynthesis of cholesterol is the conversion of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) to mevalonate, which is catalyzed by the enzyme HMG CoA reductase. The ratio of HMG CoA to mevalonate is inversely proportional to HMG CoA reductase activity, i.e., an increase in the ratio indicates a decrease in the enzyme activity. Drugs that inhibit HMG CoA reductase are used to lower serum cholesterol as a means of reducing the risk for cardiovascular disease ( Farmer,
Citation1998 ) . Statins are a class of drugs used to lower the cholesterol levels by inhibiting the enzyme HMG-CoA reductase, which play a central role in the production of cholesterol in the liver. The cholesterol lowering activity of BMBE may be same as statins by inhibiting HMG CoA reductase.Malic enzyme, isocitrate dehydrogenase, and glucose 6-phosphate dehydrogenase are involved in the lipogenesis by supplying NADPH, an essential factor for fatty acids and cholesterol biosynthesis. Alterations in the redox potential have been suggested to cause fatty liver due to the inhibition of fatty acid oxidation and tricarboxylic acid (TCA) cycle in addition to stimulating lipogenesis (Koh et al., Citation2004). BMBE treatment significantly reduced lipogenesis by reducing the activities of the lipogenic enzymes, namely malic enzyme, isocitrate dehydrogenase, and glucose 6-phosphate dehydrogenase. Decrease in the activity of lipogenic enzymes correlates with lower level of triglycerides (Sambaiah & Satyanarayana, Citation1982).
Barneo et al. (Citation1990) showed that STZ-induced diabetes in rats produced alterations in hepatic functions. This alteration in hepatic function may be because of increased activity and mRNA levels of arginase as reported by Salimuddin et al. (Citation1999). Modulation of serum aminotransferases and phosphatases in diabetic rats towards normal levels and histopathological analysis of liver shows that BMBE offers protection and helps to maintain the structural integrity of hepatic cells.
Previous reports and our preliminary phytochemical screening revealed the presence of tannic acid, gallic acid, pyrocatechin, glycosides, saponins, and steroids in the bark of B. monosperma (Sharma & Deshwal, Citation2011). Tannins have shown to decrease blood sugar in experimental animal models (Suba et al., Citation2004). It is well-established that saponins are useful in the treatment of diabetes, phytosterols have beneficial effects on hyperlipidemia, and polyphenols and flavonoids have potential antioxidant properties (George et al., Citation2002; Scalbert et al., Citation2005). Therefore, it could be possible that the presence of these compounds in B. monosperma bark may be responsible for the marked improvement in lipid metabolism in diabetic rats.
In conclusion, administration of BMBE to diabetic animals has been shown to possess hypoglycemic and hypolipidemic activities by enhancing insulin secretion and partially restoring the activities of key enzymes of lipid metabolism. Therefore, the potential exists to explore the utilization of B. monosperma bark in the development of nutraceuticals and functional foods for the management of diabetes and related disorders. Further pharmacological and biochemical investigations are underway to elucidate the mechanism of hypolipidemic activity of B. monosperma bark in the diabetic condition.
Acknowledgements
The authors are thankful to the University Grants Commission, New Delhi, India, for the financial assistance to carry out the work efficiently.
References
- Amos AF, McCarty DJ, Zimmet P. (1997). The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet Med 14:S1–85
- Babu PS, Srivivasan K. (1997). Hypolipidemic action of curcumin, the active principle of turmeric in streptozotocin induced diabetic rats. Mol Cell Biochem 166:169–75
- Barneo L, Esteban MM, Garcia Pravia C, et al. (1990). Normalization of altered liver function tests after islet transplantation in diabetic rats. Diabet Metab 16:284–9
- Betteridge J. (1997). Lipid disorders in diabetes mellitus. In: Pickup JC, Williams G, eds. Textbook of Diabetes. London: Blackwell Science, 1–35
- Bhandari U, Kanojia R, Pillai KK. (2005). Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol 97:227–30
- Chattopadhyaya R, Pathak D, Jindal DP. (1996). Antihyperlipidemic agents. A review. Indian Drugs 33:85–97
- Dirican M, Serdar Z, Sarandol E, Gur ES. (2003). Lecithin: Cholesterol acyltransferase activity and cholesteryl ester transfer rate in patients with diabetes mellitus. Turk J Med Sci 33:95–101
- Divya BT, Mini S. (2011). In vitro radical scavenging activity of different extracts of Butea monosperma bark. Int J Curr Pharm Res 3:114–16
- Ercyias F, Taneli F, Arslan B, Uslu Y. (2004). Glycemic control, oxidative stress and lipid profile in children with type 1 diabetes mellitus. Arch Med Res 35:134–40
- Farmer JA. (1998). Aggressive lipid therapy in the statin era. Prog Cardiovasc Dis 41:71–94
- Ferner RE, Neil HAW. (1988). Sulphonylureas and hypoglycemia. Br Med J 296:949–50
- Franceschini G, Maderna P, Sirtori CR. (1991). Reverse cholesterol transport: Physiology and pharmacology. Atherosclerosis 88:99–107
- George F, Zohar K, Harinder PS, Makkar Klaus B. (2002). The biological action of saponins in animal systems: A review. Br J Nutr 88:587–605
- Gomes A, Vedasiromoni JR, Das M, et al. (2001). Antihyperglycemic effect of black tea (Camellia sinensis) in rat. J Ethnopharmacol 27:243–75
- Haffner SM, Lehtos S, Ronnemaa T, et al. (1998). Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–34
- Kannel WB, McGee DL. (1979). Diabetes and cardiovascular disease. The Framingham study. JAMA 241:2035–8
- Kim SH, Hyun SH, Choung SY. (2006). Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol 104:119–23
- Koh HJ, Lee SM, Son BG, et al. (2004). Cytosolic NAD+ dependent isocitrate dehydrogenase plays a role in lipid metabolism. Biol Chem 279:19968–74
- Kornberg A, Horecker CB. (1955). Glucose-6-phosphate dehydrogenase. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. New York: Academic Press, 323–7
- Lowry OH, Roesborough MJ, Farr AL, Randall RJ. (1951). Protein measurement with Folin’s phenol reagent. J Biol Chem 193:265–75
- Mengi SA, Deshpande SG. (1999). Anti-inflammatory activity of Butea frondosa leaves. Fitoterapia 70:521–2
- Mooradian AD, Wong NCW, Shah GN. (1997). Apolipoprotein A1 expression in young and aged rats is modulated by dietary carbohydrates. Metabolism 46:1132–6
- Ochoa S. (1955a). Malic enzyme. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. New York: Academic Press, 739–53
- Ochoa S. (1955b). Isocitric dehydrogenase system (TPN) from pig heart. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. New York: Academic Press, 699–700
- Patil MV, Pawar S, Patil DA. (2006). Ethnobotany of Butea monosperma (Lam.) Kuntze in North Maharashtra, India. Nat Prod Rad 5:323–5
- Pupim LB, Heimburger Q, Qureshi AR, et al. (2005). Accelerated lean body mass loss in incident chronic dialysis patients with diabetic mellitus. Kidney Int 68:2368–74
- Rao AV, Ramakrishnan S. (1975). Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem 21:1523–5
- Salimuddin, Upadhyaya KC, Baquer NZ. (1999). Effects of vanadate on expression of liver arginase in experimental diabetic rats. IUBMB 48:237–40
- Sambaiah K, Satyanarayana MN. (1982). Influence of red pepper and capsaicin on body composition and lipogenesis in rats. J Biosci 4:425–30
- Saravanan R, Rajendra Prasad N, Pugalandi KV. (2003). Effect of Piper beetle leaf extract on alcoholic toxicity in the rat brain. J Med Food 6:261–5
- Scalbert A, Johnson IT, Saltmarsh M. (2005). Polyphenols: Antioxidants and beyond. Am J Clin Nutr 81:S215–17
- Sharma AK, Deshwal N. (2011). An overview: On phytochemical and pharmacological studies of Butea monosperma. Int J Pharm Tech Res 3:864–71
- Shirwaikar A, Rajendra K, Kumar CD, Bodla R. (2004). Antidiabetic activity of aqueous leaf extracts of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J Ethnopharmacol 91:171–5
- Sofowora H. (1993). Screening plants for bioactive agents. In: Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd, Sunshine House, 134–56
- Solymoss BC, Marcil M, Chasur M, et al. (1995). Fasting hyperinsulinism, insulin resistance syndrome, and coronary heart disease in men and women. Am J Cardiol 76:1152–6
- Somani R, Kasture S, Singhai AK. (2006). Antidiabetic potential of Butea monosperma in rats. Fitoterapia 77:86–90
- Suba V, Murugesan T, Rao RB, et al. (2004). Antidiabetic potential of Barleria lupulina extract in rats. Fitoterapia 75:1–4
- Tan BK, Tan CH, Pushparaj PN. (2005). Anti-diabetic activity of the semi-purified fractions of Averrhoa bilimbi in high fat diet fed streptozotocin-induced diabetic rats. Life Sci 76:2827–39
- Thompson Coon JS, Ernst E. (2003). Herbs for serum cholesterol reduction: A systematic view. J Fam Pract 52:468–78
- Trease GE, Evans WC. (2002). Pharmacology. London: Saunders Publishers, 42–4, 221–9, 246–9, 303–6, 331–2, 391–3
- Wagman AS, Nuss JM. (2001). Current therapies and emerging targets for the treatment of diabetes. Curr Pharm Des 7:417–50