Abstract
Context: The leaves and roots of the Taraxacum officinale F. (Asteraceae) is widely used as traditional medicinal herb in Eastern Asian countries.
Objective: In the present study, the antidepressant-like effects of the water extract of T. officinale (WETO) leaves and roots were investigated in mice using forced swimming test (FST), tail suspension test (TST) and open field test (OFT).
Materials and methods: Effects of acute (1-day) and chronic treatments (14-days) with WETO (50, 100 and 200 mg/kg) on the behavioral changes in FST, TST and OFT, and the serum corticotrophin releasing factor (CRF), adrenocorticotropic hormone (ACTH) and corticosterone concentration were assessed in mice.
Results: Chronic treatment (14-days) with WETO at the doses of 50, 100 and 200 mg/kg significantly decreased the immobility time in both FST (92.6, 85.1 and 77.4 s) and TST (84.8, 72.1 and 56.9 s). Acute treatment (1-day) with WETO at a dose of 200 mg/kg also markedly decreased the immobility time in both FST (81.7 s) and TST (73.2 s). However, all treatments did not affect the locomotor activity in the OFT. Moreover, FST induced a significant increase in serum CRF (5.8 ng/ml), ACTH (104.7 pg/ml) and corticosterone levels (37.3 ng/ml). Chronic treatment (14-days) with WETO decreased the serum CRF (200 mg/kg: 3.9 ng/ml) and corticosterone (50 mg/kg: 29.9 ng/ml; 100 mg/kg: 22.5 ng/ml; 200 mg/kg: 19.8 ng/ml) levels.
Discussion and conclusion: These results clearly demonstrated the antidepressant effects of WETO in animal models of behavioral despair and suggested the mechanism involved in the neuroendocrine system.
Introduction
Depression is increasingly becoming a global public health problem and can result in many serious consequences (McKenna et al., Citation2005). Although tremendous progress had been made in the development of antidepressants during the past several decades, the therapeutic efficacy is still unsatisfactory because of the numerous accompanying side effects, including constipation, insomnia, orthostatic hypotension, headache, sexual dysfunction, and anxiety (Papakostas, Citation2010). Therefore, the task of discovering and developing safe and effective drugs for depression is important and urgent at the present day.
Growing evidence indicates that natural products and natural product-derived compounds exhibited a remarkable antidepressant-like effect (Mao et al., Citation2008; Li et al., Citation2009; Yi et al., Citation2010; Zhu et al., Citation2012). These findings strongly present a huge potential source for antidepressant drugs. Taraxacum officinale F. (Asteraceae), often known as dandelion, a widely grown plant in China, Korea and Japan, and has been used as a traditional Chinese medicine in the treatment of infectious diseases. Nowadays, numerous biological activities of dandelion have been found, such as anti-tumor (Ovadje et al., Citation2012), anti-oxidant (Choi et al., Citation2010; Colle et al., Citation2012), anti-inflammatory (Koh et al., Citation2010), and hepato-protective (Park et al., Citation2010). Moreover, T. officinale decreased the immobility time of mice exposed to forced swimming test (FST) and exhibited a potent anti-fatigue effect (Lee et al., Citation2012). FST is a common animal model to screen potential antidepressant drugs, and drugs which can reduce the immobility time in FST are more likely to be a candidate for antidepressants. In addition, it has been reported that dandelion possesses protective effects against lead-induced brain damage (Gargouri et al., Citation2012). Based on these findings, we hypothesize that T. officinale might possess an antidepressant effect. The purpose of this study was to examine the antidepressant effect of the water extract of T. officinale (WETO) in mice exposed to FST and tail suspension test (TST), and explore its possible mechanisms.
Materials and methods
Plant materials and preparation of extract
The leaves and roots of T. officinale were purchased from a traditional drug store, Zhang Zhongjing Pharmacy (Zhengzhou, Henan province, P.R. China), and identified by Associate Prof. Han-Bing Li, Henan University of Traditional Chinese Medicine in March 2012. A voucher specimen (HAC/LI-12017) was deposited in our laboratory. The plant materials (500 g) were decocted in boiling water (2 l) for 2 h, and the decoctions were collected. The protocol was repeated two times, and the decoctions were combined and filtered by a syringe filter (0.45 µm). Finally, the percolates were concentrated under reduced pressure and powdered (27.8 g) using a lyophilizer.
Animals
Male ICR mice (20 ± 2 g) were obtained from the Laboratory Animal Centre of Henan province, P.R. China. All mice were housed 10 per cage (48 × 32 × 20 cm) under standard conditions (12 h light/dark cycle; light on 8 a.m. 22 ± 2 °C; 50 ± 10% relative humidity) with free access to water and food, and were allowed to acclimate one week before the experiments. The protocols were performed in accordance with the published guidelines of the China Council on Animal Care, and approved by the Committee of Animal care of Henan university of Traditional Chinese Medicine. All efforts were made to minimize the suffering of animals.
Drug treatment
Mice were randomly divided into five groups (n = 10), i.e., control group, fluoxetine group (20 mg/kg) and three WETO treatment groups (50, 100 and 200 mg/kg). WETO and fluoxetine were dissolved in distilled water, and administered by oral gavage in a volume of 10 ml/kg at 9:00 am and lasted for 1 or 14 consecutive days. The control group received an equal volume of distilled water. The dose selection was based on our results of pre-experiment. The FST and TST were conducted 1 h after the last administration, and the OFT was performed 24 h after the last administration. The mice were used only once in each behavioral test. In particular, a normal control group without swimming or treatments was added in the FST.
Open field test
To explore the locomotor activity of all mice, OFT was performed according to the procedure of Yi et al. (Citation2011). Briefly, the field was made of a black box (40 × 40 × 30 cm), and the floor was divided into 25 equal squares (8 × 8 cm) by white stripes. After 24 h of the last administration, each mouse was individually placed in the central region of floor and the test sessions were recorded by a video camera for 3 min. The number of crossings and rearings were counted.
Forced swimming test
The FST was conducted as described by Porsolt et al. (Citation1977) with some modification. Each mouse was individually placed in a glass cylinder (30 cm in height, 15 cm in diameter) with 10 cm high of water. The water maintains the temperature at 25 ± 1 °C. The test lasted for 6 min, and the duration of immobility in final 4 min was recorded by a video camera. The immobility time was counted only when they were floating in the water without struggling and making only slight movements necessary to keep their head above the water.
Tail suspension test
As described by Steru et al. (Citation1985), with some modification, each mouse was suspended by tail on the edge of a shelf with the head 5 cm above the floor. The duration of immobility in final 4 min of the total 6 min was recorded by a video camera. The immobility time were counted only when they hung passively and were completely motionless.
Blood sampling and serum hormone assay
Mice were sacrificed immediately after the FST. To avoid the fluctuations of hormone levels due to circadian rhythm, the blood samples were collected at 10:00 – 11:00 am and centrifuged at 4 °C. The obtained serum was kept at −80 °C until analysis. Serum corticosterone, ACTH and CRF levels were measured using commercially available ELISA kits (R&D Systems, Inc., Minneapolis, MN).
Statistical analyses
All data were expressed as mean ± S.E.M. and analyzed by one-way ANOVA followed by Dunnett’s test. A value of p < 0.05 was considered statistically significance. The figures were drawn by Graphpad Prism 4 (GraphPad Software, Inc., La Jolla, CA).
Results
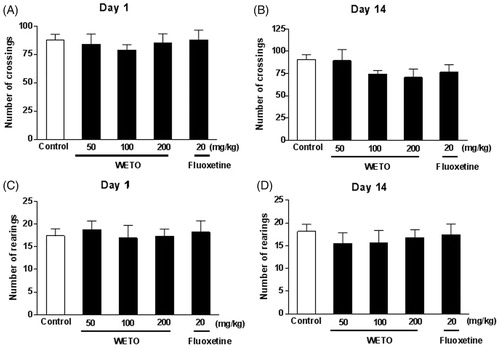
Effects of WETO on the locomotor activity in the OFT
To avoid the possibility of false positive results of WETO in FST and TST, the locomotor activity of mice was tested in the OFT. As shown in , treatment with WETO at doses of 50, 100 and 200 mg/kg and fluoxetine at 20 mg/kg for 1 day and 14 consecutive days had no significant effect on the number of crossing and rearing in mice.
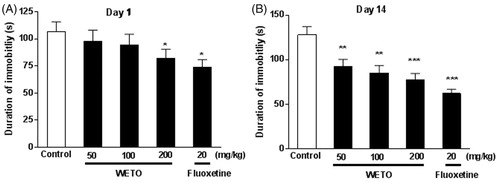
Effects of WETO on the immobility time in the FST
The effects of WETO and fluoxetine on the immobility time of mice in FST are shown in . One-way ANOVA revealed a significant effect of the treatment on days 1 and 14 (p < 0.05, p < 0.01). Post hoc analysis showed a significant decrease in the duration of immobility elicited by the 14 day treatment with WETO at 50, 100 and 200 mg/kg (p < 0.05, p < 0.01, p < 0.001, respectively). After one-day treatment, WETO (200 mg/kg) produced a significant reduction in the duration of immobility (p < 0.05), however, WETO (50 and 100 mg/kg) only exhibited a slight effects. In addition, fluoxetine (20 mg/kg) significantly decreased the immobility time on days 1 and 14 (p < 0.05, p < 0.001).
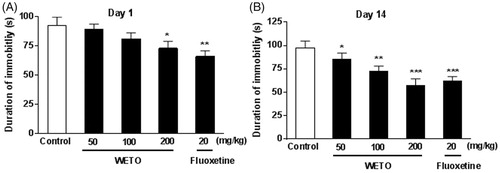
Effects of WETO on the immobility time in the TST
The effects of WETO and fluoxetine on the immobility time of mice in TST are shown in . A significant treatment effect was observed on days 1 and 14 (p < 0.05, p < 0.01). Post hoc analysis indicated WETO at 50 and 100 mg/kg significantly decreased the immobility time on day 14 (p < 0.05, p < 0.01). Moreover, WETO at 200 mg/kg (p < 0.05, p < 0.001) and fluoxetine at 20 mg/kg (p < 0.01, p < 0.001) caused a significant decline in the immobility time on days 1 and 14.
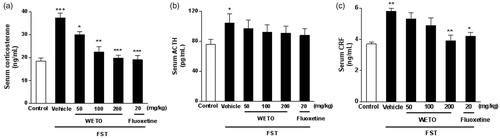
Effects of WETO on serum hormones levels in FST mice
The effects of WETO and fluoxetine on serum hormones levels of mice in FST are shown in . FST induced a remarkable elevation of serum corticosterone, ACTH, and CRF levels compared with the normal control group (p < 0.001, p < 0.05, p < 0.01). Administration with WETO (200 mg/kg) for 14 days significantly decreased the serum corticosterone and CRF levels (p < 0.001, p < 0.01). Moreover, WETO at doses of 50 and 100 mg/kg also prevented the increase of serum corticosterone levels induced by the FST (p < 0.05, p < 0.01). Additionally, fluoxetine (20 mg/kg) obviously decreased serum corticosterone and CRF levels (p < 0.001, p < 0.05). Both WETO and fluoxetine had no significant effect on serum ACTH.
Discussion
“Behavioral despair” models, such as FST and TST, provide a fast and efficient way to screen the potential antidepressant drugs (Porsolt et al., Citation1977; Steru et al., Citation1985). At the beginning of the test, mice usually struggled against the condition and some of them gradually show despair and gave up the struggle. Increased immobility time represents increased despair and depression (Corbett et al., Citation1999). Therefore, the duration of immobility is considered as the core indicator of FST and TST to evaluate the antidepressant effects. Many clinical antidepressant drugs, such as selective serotonin reuptake inhibitors (SSRIs), tricyclics and monoamine oxidase inhibitors (MAOIs), can effectively reduce the immobility time and increase the activity (Cryan et al., Citation2005; Porsolt et al., Citation1977; Steru et al., Citation1985). The present study investigated the acute and chronic effects of WETO on the duration of immobility of mice exposed to FST and TST. Our results showed that a 14-day treatment with WETO at doses of 50, 100 and 200 mg/kg significantly reduced the duration of immobility in both FST and TST, suggesting that WETO has antidepressant-like effects. Moreover, acute treatment (1 day) of WETO at a dose of 200 mg/kg, but not 50 and 100 mg/kg, also exhibited a similar effect. These results showed that chronic treatment with WETO can maintain an effective blood concentration to produce antidepressant activity, but the larger dose is needed to produce similar effect following acute treatment.
Although the predictive validity of the FST and TST in the rodent model of depression has been confirmed in numerous studies, there are several defects in the antidepressant screening (Bourin et al., Citation2001). Some central excitatory drugs can stimulate the activity, and produce a false positive effect. To avoid the possibility of false positive result, the effect of WETO on the locomotor activity was observed in the OFT. Our findings showed that WETO did not evoke any significant changes on the number of crossings and rearings, which suggested that the reduced immobility time of mice in both FST and TST induced by WETO has nothing to do with psycho-stimulant effects.
It is well known that the dysfunction of hypothalamic-pituitary-adrenal (HPA) axis involved in the pathophysiology of depression (Risbrough & Stein, Citation2006). HPA axis is an important neuroendocrine axis, and is responsible for the physiological response to various stressors (Boyle et al., Citation2006; Szafarczyk et al., Citation1993). The sustained activation of the HPA axis can lead to increased levels of glucocorticoids. Excess glucocorticoids impair hippocampal function and neurogenesis (Stranahan et al., Citation2008; Zhang et al., Citation2008), which further cause depression-related diseases. Depressed subjects have markedly higher glucocorticoids levels (mainly cortisol in human and corticosterone in rodents) (Manthey et al., Citation2011), which imply the dysregulation of HPA axis. Reduction of glucocorticoids and normalization of the HPA axis function are able to alleviate the degree of depression (Mello et al., Citation2007; Tyrka et al., Citation2006). Several studies indicated the HPA axis activity was significantly inhibited by antidepressants in both clinical trials and animal experiments (Espallergues et al., Citation2012; Piwowarska et al., Citation2012; Surget et al., Citation2011). In the present study, FST induced a markedly increase in serum CRF, ACTH and corticosterone levels, which further confirmed the swimming stress was a stronger activator of the HPA axis. These results were consistent with the reports of others (Chen et al., Citation2012; Idayu et al., Citation2011). In addition, our data indicated that chronic treatment with WETO and fluoxetine significantly reversed the FST-induced increase in serum CRF and corticosterone levels, but not ACTH. Specially, a dose–effect relationship of WETO in serum corticosterone levels was also observed. These findings suggested that the antidepressant effects of WETO might be partially mediated via regulation of neuroendocrine system.
Conclusions
The present study demonstrated that the treatment with WETO can decrease the duration of immobility in mice exposed to FST and TST, without affecting the locomotor activity in the OFT. Moreover, WETO also prevented the elevation of serum corticosterone induced by FST. These findings strongly suggested that WETO possesses potential antidepressant-like activity, and the mechanism might be at least partially involved in the neuroendocrine system. However, further work will be required to examine the exact mechanism of WETO based on the other model of depression.
Declaration of interest
The authors report no declarations of interest. The project was supported by grants from the National Natural Science Foundation of China (No. 81303278), Natural Science Foundation of Education Department of Henan Province of China (No. 12B310004) and Henan University of Traditional Chinese Medicine to Yu-Cheng Li (BSJJ2010-24).
References
- Bourin M, Fiocco AJ, Clenet F. (2001). How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16:9–21.
- Boyle MP, Kolber BJ, Vogt SK, et al. (2006). Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 26:1971–8
- Chen L, Chen M, Wang F, et al. (2012). Antidepressant-like effects of shuyusan in rats exposed to chronic stress: Effects on hypothalamic-pituitary-adrenal function. Evid Based Complement Alternat Med 2012:940846. doi: 10.1155/2012/940846
- Choi UK, Lee OH, Yim JH, et al. (2010). Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int J Mol Sci 11:67–78
- Colle D, Arantes LP, Gubert P, et al. (2012). Antioxidant properties of Taraxacum officinale leaf extract are involved in the protective effect against hepatoxicity induced by acetaminophen in mice. J Med Food 15:549–56
- Corbett R, Zhou LL, Sorensen SM, Mondadori C. (1999). Animal models of negative symptoms: M100907 antagonizes PCP-induced immobility in a forced swim test in mice. Neuropsychopharmacology 21:S211–18
- Cryan JF, Valentino RJ, Lucki I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–69
- Espallergues J, Mamiya T, Vallée M, et al. (2012). The antidepressant-like effects of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane in mice is related to changes in neuroactive steroid and monoamine levels. Neuropharmacology 62:492–502
- Gargouri M, Ghorbel-Koubaa F, Bonenfant-Magné M, et al. (2012). Spirulina or dandelion-enriched diet of mothers alleviates lead-induced damages in brain and cerebellum of newborn rats. Food Chem Toxicol 50:2303–10
- Idayu NF, Hidayat MT, Moklas MA, et al. (2011). Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 18:402–7
- Koh YJ, Cha DS, Ko JS, et al. (2010). Anti-inflammatory effect of Taraxacum officinale leaves on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J Med Food 13:870–8
- Lee BR, Lee JH, An HJ. (2012). Effects of Taraxacum officinale on fatigue and immunological parameters in mice. Molecules 17:13253–65
- Li YC, Wang FM, Pan Y, et al. (2009). Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog Neuropsychopharmacol Biol Psychiatry 33:435–49
- Manthey L, Leeds C, Giltay EJ, et al. (2011). Antidepressant use and salivary cortisol in depressive and anxiety disorders. Eur Neuropsychopharmacol 21:691–9
- Mao Q, Huang Z, Ip S, Che C. (2008). Antidepressant-like effect of ethanol extract from Paeonia lactiflora in mice. Phytother Res 22:1496–9
- McKenna MT, Michaud CM, Murray CJ, Marks JS. (2005). Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med 28:415–23
- Mello AF, Juruena MF, Pariante CM, et al. (2007). Depression and stress: Is there an endophenotype? Rev Bras Psiquiatr 29:S13–18.
- Ovadje P, Hamm C, Pandey S. (2012). Efficient induction of extrinsic cell death by dandelion root extract in human chronic myelomonocytic leukemia (CMML) cells. PLoS One 7:e30604
- Papakostas GI. (2010). The efficacy, tolerability, and safety of contemporary antidepressants. J Clin Psychiatry 71:e03 . doi: 10.4088/JCP.9058se1c.03gry
- Park CM, Youn HJ, Chang HK, Song YS. (2010). TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl(4)-induced hepatic damage through the modulation of NF-kappaB and its regulatory mediators. Food Chem Toxicol 48:1255–61
- Piwowarska J, Chimiak A, Matsumoto H, et al. (2012). Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatry Res 198:407–11
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–36
- Risbrough VB, Stein MB. (2006). Role of corticotropin releasing factor in anxiety disorder: A translational research perspective. Horm Behav 50:550–61
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–70
- Stranahan AM, Arumugam TV, Cutler RG, et al. (2008). Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11:309–17
- Surget A, Tanti A, Leonardo ED, et al. (2011). Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16:1177–88
- Szafarczyk A, Ixart G, Gaillet S, et al. (1993). Stress. Neurophysiologic studies. Encephale 19:137–42
- Tyrka AR, Mello AF, Mello MF, et al. (2006). Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology 31:1036–45
- Yi LT, Li CF, Zhan X, et al. (2010). Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Prog Neuropsychopharmacol Biol Psychiatry 34:1223–8
- Yi LT, Xu HL, Feng J, et al. (2011). Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol Behav 102:1–6
- Zhang WJ, Tan YF, Yue JT, et al. (2008). Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand 117:205–10
- Zhu WL, Shi HS, Wei YM, et al. (2012). Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol Res 65:74–80