Abstract
Context: Sophocarpine, a tetracyclic quinolizidine alkaloid, is one of the most abundant active ingredients in Sophora alopecuroides Linn. (Kudouzi). Sophocarpine injection was found to have significant antiviral effects against coxsackievirus B3 and therapeutic effects for viral myocarditis in the clinic.
Objective: This study assessed the effects of sophocapine on overload-induced cardiac fibrosis and investigated potential mechanisms.
Materials and methods: Adult male Sprague–Dawley rats were subjected to a suprarenal abdominal aorta constriction (AC) or sham to induce sustained pressure overload. Six weeks later, rats were randomly assigned to receive sophocapine (10, 20, and 40 mg/kg, gavage) or vehicle treatment for an additional 6 weeks. Six weeks after treatment, cardiac dysfunction, cardiac coefficient, cardiac fibrosis, hydroxyproline concentration, and inflammation mediators were examined.
Results: When compared with the model group, the left ventricular weight/body weight decreased by 25.4% and 39.0% in 20 and 40 mg/kg sophocarpine groups, respectively. The beneficial effects were associated with amelioration of left ventricular systolic pressure (LVSP) and left ventricular enddiastolic pressure (LVEDP). Moreover, pressure overload-induced cardiac fibrosis was attenuated in sophocarpine treated groups. Importantly, sophocarpine (20 and 40 mg/kg) decreased pro-inflammatory cytokine levels (IL-6, 14.6% and 18.5%; IL-1β, 23.1% and 32.6%), collagen content (27.7% and 50.1%), as well as matrix metalloproteinases-2, 9 (MMP-2, 9) expression (MMP-2, 11.8% and 18.5%; MMP-9, 16.2% and 21.1%). Sophocarpine (40 mg/kg) inhibited IκB-α phosphorylation (19.0%).
Conclusion: These findings indicated that sophocarpine potentially had antifibrotic effects. The mechanism might be due to modulation of the balance between pro-inflammatory cytokine expression and collagen content level as well as MMPs expression via the NF-κB signaling pathway.
Introduction
A direct relation has been found between the decrease of cardiac mortality and the regression of cardiac hypertrophy (Verdecchia et al., Citation2001). Pressure overload-induced myocardial remodeling is characterized by cardiac hypertrophy and fibrosis, which increases myocardial stiffness and inducts to diastolic dysfunction even to death. However, a convincing and potent antifibrogenic therapeutic modality by targeting myocardial local inflammation activation is still unavailable. Recent studies have indicated that an increase of myocardial proinflammatory mediator (i.e., interleukin-6, IL-6) levels and inflammatory cells infiltration preceded the occurrence of cardiac fibrosis in response to chronic pressure overload stress (Kudo et al., Citation2009; Levick et al., Citation2009, Citation2010).
Sophora alopecuroides Linn. (compound Kudouzi injection) has been used mainly for the treatment of inflammation, fever, pain, and edema. Sophocarpine (), the most abundant active ingredient in Kudouzi, is a tetracyclic quinolizidine alkaloid. Of note, sophocarpine injection (called Kangke injection) has been shown to have obviously therapeutic effects for viral myocarditis in clinical trials. Importantly, the administration of sophocarpine significantly improved cardiac function and reduced infarct size in ischemia reperfusion injury (I/R) rat heart in vivo. Furthermore, sophocarpine ameliorated the contents of inflammatory mediators, neutrophil infiltration, and myeloperoxidase activity (Li et al., Citation2011). Recently, studies have shown that sophocarpine could inhibit the levels of IL-6 and TNF-α in murine macrophages and prevent cachexia-related symptoms induced by colon 26 adenocarcinoma in mice (Zhang et al., Citation2008). Chen et al. (Citation2005) have also suggested that sophocarpine could improve the end-systolic pressure and the end-diastolic volume, and play a pivotal role in maintaining healthy cardiac function.
In light of previous studies, we hypothesized that sophocarpine could protect heart against pressure overload-induced cardiac fibrosis. To verify our hypothesis, we used a rat model of cardiac hypertrophy to investigate the cardioprotective properties of sophocarpine on hypertensive ventricular remodeling and the potential mechanisms involved.
Materials and methods
Plant materials
Dried and fresh Kudouzi was obtained from China’s Ning-Xia province on 11 August 2011 and was identified by Prof. L. Li. A voucher specimen was deposited in the Herbarium of our laboratory. The test substance, sophocarpine, was isolated from the dry seeds of Kudouzi according to previous studies (Gao et al., Citation2009; Xiao et al., Citation1999).
Surgical preparation of animals
Sprague–Dawley male rats, 250 ± 15 g, were housed individually in cages under hygienic conditions and placed in a controlled environment with a 12-h light/dark cycle at 23 ± 3°C and 40–70% humidity, and were quarantined for 7 d before the experiment. The animals were allowed free access to food and water. All animal experiments were approved by the Ethics Committee for Animal Care and Use. After anesthetization with an intraperitoneally administered sodium pentobarbital (35 mg/kg, i.p.), a suprarenal abdominal aorta constriction (AC) was performed by using a 4–0 suture tied twice around the suprarenal aorta and a 21-gauge needle to yield a 0.8 mm internal diameter as previously described (Hao et al., Citation2007; Kuwahara et al., Citation2004; Yu et al., Citation2004; Zhao et al., Citation2009). The sham-operated animals underwent the same procedure except for AC. Six weeks post-operation, rats were randomly assigned to AC group (model), AC + sophocarpine treatment at doses of 10, 20, and 40 mg/kg for an additional 6 weeks. Rats were treated by gavage with sophocarpine or the same volume of distilled water (sham and model).
Measurements of hemodynamics and cardiac coefficient
Six weeks after treatment with sophocarpine, rats were anesthetized with sodium pentobarbital (35 mg/kg, 10 ml/kg, i.p.) and hemodynamic measurements were recorded. In brief, a Millar vessel was inserted into the left ventricular cavity of rat via the right common carotid artery. The pressure was transduced and amplified by a pressure transducer. Left ventricular systolic pressure (LVSP) and left ventricular enddiastolic pressure (LVEDP) were recorded and programmed by using a biotic signal collection and processing system (BIOPAC, MP150, Goleta, CA). After the hemodynamic examination, the weight of left ventricle was measured after rinsing blood. Left ventricular mass index (LVMI) was expressed as the left ventricular weight/body weight.
Measurement of IL-1β, IL-6, MMP-2, and MMP-9 concentrations by ELISA
After measuring hemodynamic parameters, blood samples were centrifuged at 1000 × g for 15 min at 4 °C and the plasma was quickly stored at −70 °C for assay. The levels of IL-1β, IL-6, MMP-2, and MMP-9 were measured using a commercially available ELISA kits (BIOTANG Inc., Waltham, MA) according to the manufacturer's instructions. Each sample was analyzed in duplicate and the mean value was calculated.
Measurement of collagen content
In addition, hydroxyproline, an indicator of total collagen content, was determined as described previously (Cleutjens et al., Citation1995). Briefly, tissues were weighted and homogenized in 0.1 mol/L NaCl and 5 mmol/L NaHCO3, washed five times with the same solution, and hydrolyzed with 6 N HCl for 16 h at 110 °C. Samples were filtered and vacuum-dried, then dissolved in distilled water. The resulting chromophore was quantitated spectrophotometrically at 560 nm against a hydroxyproline standard curve. Hydroxyproline concentration was calculated by linear regression analysis and expressed as mg/g dry weight.
Assessment of cardiac fibrosis with Masson’s trichrome staining
For the assessment of cardiac fibrosis, the sections of heart were stained with Masson’s trichrome staining in paraffin-embedded sections (4–5 μm thick). Briefly, the samples were deparaffinized in histoclear and rehydrated using sequential passage through 100–70% ethanol for 6 min, and then were washed with distilled water. The slides were stained with Masson’s trichrome stain for 5 min. The sections were washed in distilled water and then stained with phosphomolybdic for 5 min, in aniline blue solution for 5 min, and then differentiated for 60 s. After a final wash in distilled water, the sections were dehydrated through 95% and 100% alcohol. Moreover, the heart tissue sections were digitally imaged in high-pixel resolution according to the method previously described (Shibata et al., Citation2007).
Western blot analysis
The peri-infarcted zone of the left ventricle and the same zone of left ventricle in the sham group were used for the western blot analysis as described previously (Zhao et al., Citation2009). Actin was used as a standard of protein-loading control for normalizing bands. The final results were calculated as the ratio of intensity of the sham group.
Statistical analysis
All data were expressed as mean ± SD and analyzed with a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Statistical analyses were performed by using the SPSS 11.5 software (SPSS Inc., Chicago, IL), p < 0.05 was considered statistically significant.
Results
Effects of sophocarpine on hemodynamics and cardiac coefficient
Following 6 weeks after cardiac hypertrophy, all animal body weights were the same (data not shown). However, the left ventricular weight/body weight in model animals was higher than that of sham animals, indicating cardiac hypertrophy (p < 0.01). In contrast, sophocarpine (20 and 40 mg/kg) obviously ameliorated these parameters (p < 0.05, p < 0.01) (). also depicts the hemodynamic values recorded in experimented animals. After AC, LVSP was decreased, while LVEDP was increased when compared with the sham group (p < 0.01). Early treatment with sophocarpine (20 and 40 mg/kg) significantly improved LVSP and LVEDP (p < 0.05, p < 0.01).
Table 1. Effects of sophocarpine on hemodynamics and cardiac coefficient.
Effects of sophocarpine on the expression of pro-inflammatory cytokines
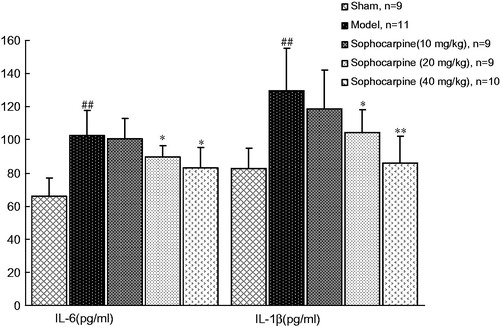
In the current study, two representative pro-inflammatory cytokines, i.e., IL-6 and IL-1β, were measured. In model animals, circulating IL-6 (102.29 ± 16.46 versus 65.98 ± 10.88 pg/mL) and IL-1β (126.08 ± 25.03 versus 82.84 ±12.34 pg/mL) were higher than that in sham-operated animals (p < 0.01). However, sophocarpine treatment significantly decreased the levels of two pro-inflammatory cytokines (IL-6, 87.36 ± 7.97 and 83.49 ± 13.34 at doses of 20 and 40 mg/kg, respectively; IL-1β, 97.02 ± 7.18 and 85.00 ± 17.21 at dose of 20 and 40 mg/kg, respectively. p < 0.05 and p < 0.01), suggesting an anti-inflammatory effect of sophocarpine treatment ().
Figure 2. Effects of sophocarpine on the levels of plasma pro-inflammatory cytokines. Values are expressed as the mean ± SD, n = 9–11 animals. Significance was determined by ANOVA followed by Tukey’s test. ##p < 0.01 compared with the sham group; *p < 0.05 and **p < 0.01 compared with the model group.
Effects of sophocarpine on collagen content level
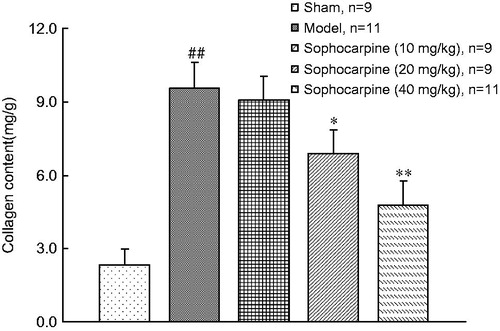
Both in experimental and clinical conditions, markers of collagen turnover were regarded as a reliable tool for monitoring from a distance cardiac tissue repair and fibrosis. We then tested the effects of sophocarpine on collagen content level in animals. At 6 weeks following cardiac hypertrophy, the collagen content was greater in AC groups compared with the sham group. However, chronic continuous treatment with sophocarpine (20 and 40 mg/kg) significantly ameliorated this parameter (p < 0.05, p < 0.01) ().
Effects of sophocarpine on cardiac fibrosis
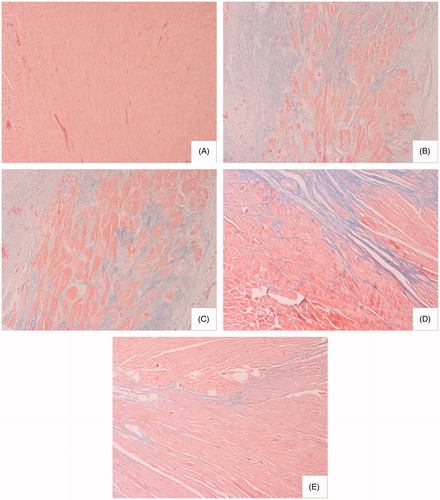
The effects of sophocarpine on the extent of injury were examined in Masson’s trichrome-stained sections from vehicle- and sophocarpine-treated hearts after ligation or sham surgery. A significant increase in fibrosis (collagen staining in blue color) was evident in AC rats compared with sham-operated animals. However, sophocarpine markedly attenuated those pathological changes ().
Figure 4. Effect of sophocarpine on cardiac fibrosis (Masson’s trichrome staining, × 200): (A) sham-operated rats (n = 9), (B) model rats (n = 11), (C) rats treated with sophocarpine 10 mg/kg (n = 9), (D) rats treated with sophocarpine 20 mg/kg (n = 9), and (E) rats treated with sophocarpine 40 mg/kg (n = 10).
Effects of sophocarpine on MMP-2 and MMP-9 content
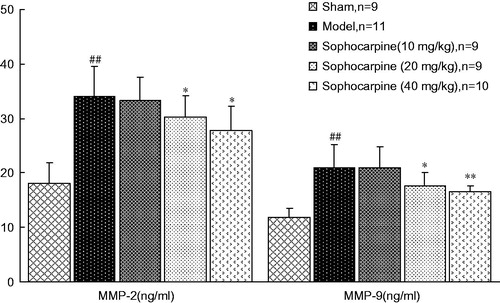
As shown in , the content of MMP-2 and MMP-9 in serum was also elevated in the model group (34.10 ± 5.49 and 21.01 ± 4.17 ng/mL, respectively) when compared with the sham-operated group (18.14 ± 3.71 and 11.83 ± 1.76 ng/mL, respectively) at the end of the 6th week. Treatment with sophocarpine reduced two MMPs (MMP-2, 30.19 ± 3.98 and 27.83 ± 4.45 at a dose of 20 and 40 mg/kg, respectively; MMP-9, 17.60 ± 2.41 and 16.56 ± 1.06 at a dose of 20 and 40 mg/kg, respectively, ng/mL).
Effects of sophocarpine on p-IKBα expression
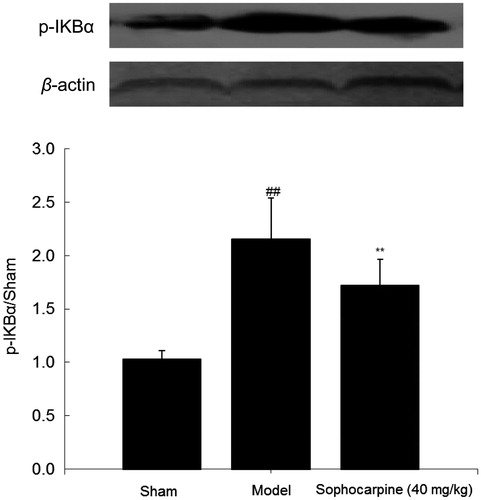
Six weeks later, the expression of p-IKBα was examined by the western blot analysis, and the result indicated that this parameter was significantly higher in the model group than in the sham group (2.15 ± 0.38 versus 1.03 ± 0.08, p < 0.01); while sophocarpine dramatically ameliorated this change (1.72 ± 0.24, p < 0.01), indicating that test substance might block IKBα phosphorylation ().
Discussion
There is growing evidence that anti-inflammatory therapy may be a promising strategy against cardiac fibrosis (Taqueti et al., Citation2006; Weber, Citation2004). In the current study, we provided the first evidence that sophocarpine had the potential to attenuate cardiac fibrosis and improve cardiac diastolic function in pressure-overloaded heart. Based on the obtained results, further investigations were performed by focusing on the potential mechanisms involved. The results indicated that sophocarpine treatment significantly decreased pro-inflammatory cytokine expression, inhibited inflammatory lesions, and reduced collagen content. Collectively, the results suggested that inhibiting myocardial inflammation activation and pro-fibrogenic factor production might be critical mechanisms for anti-fibrogenic action of sophocarpine in pressure-overloaded heart.
To test the effects of sophocarpine on chronic pressure overload-induced cardiac remodeling, rats were subjected to AC or sham operation for 6 weeks. Consistent with the previous study (Sakamoto et al., Citation2013), we presently observed that chronic pressure overload not only elevated cardiac coefficient but also decreased LVSP and increased LVEDP, indicating that chronic pressure overload caused significant pathological cardiac remodeling. At the end of the study, Masson's trichrome staining suggested significant increase in left ventricles myocardial fibrosis in response to AC compared with sham-operated rats. However, chronic continuous treatment with sophocarpine (20 and 40 mg/kg) could significantly decrease cardiac coefficient and LVEDP, but improve LVSP, these were associated with the amelioration of lesions.
Cardiac tissue consists mainly of cardiomyoctyes, which are surrounded by extracellular matrix. The major component of the extracellular matrix is collagen fibers. These collagen fibers form a collagen network that provides cardiac strength during contractile forces (de Jong et al., Citation2012). It is well known that collagen content and the progression of fibrosis formation play pivotal roles in appropriate prognosis and therapy of cardiac patients. The hydroxylation of proline within the collagen molecule results in hydroxyproline, and this modified amino acid stabilizes the triple helical structure in the mature collagen. Notably, hydroxyproline is highly specific for collagen, and hydroxyproline measurement accurately reflects the amount of collagen in the tissue (Ferreira et al., Citation2011; Tschöpe et al., Citation2004). To further support our findings, hydroxyproline was calculated, and the results demonstrated that the collagen content was greater in the AC group as compared with the sham group. However, sophocarpine significantly blocked this change. These findings suggested that sophocarpine could limit cardiac fibrosis in a rat model of AC, and this perfect ability might be attributed to the inhibition of collagen synthesis.
Since inflammation activation plays a role in the process of cardiac remodeling process, next we tested whether the anti-fibrogenic effect of sophocarpine is associated with the modulation of circulating pro-inflammatory cytokine levels. We measured two representative pro-inflammatory cytokines, i.e., IL-6 and IL-1β. The results indicated that AC-elevated IL-6 and IL-1β expression, suggesting a sustained status of inflammation activation was induced by pressure-overloaded stress. Then, sophocarpine treatment significantly decreases the levels of these pro-inflammatory cytokines in AC-treated rats compared with the vehicle treatment. IL-6 infusions cause concentric left ventricular hypertrophy and obviously increase collagen deposition and ventricular stiffness (Nian et al., Citation2004). IL-6 can directly attenuate myocardial contractility and induce the development of myocyte hypertrophy, collagen deposition, and fibrosis (Kuusisto et al., Citation2012). IL-1β, as a pro-inflammatory cytokine, is correlated with myocardial collagen deposition, cardiomyocyte apoptosis, and inflammation (Gullestad & Aukrust, Citation2005; Kuusisto et al., Citation2012). Based on the above-mentioned favorable regulatory role of sophocarpine on IL-6 and IL-1β levels, the results potently suggested that inhibiting pro-inflammatory cytokines might be a key mechanism of antifibrotic effects of sophocarpine in pressure-overloaded heart.
MMPs belong to a family of structurally related zinc-containing endopeptidases, which play pivotal roles in inflammatory diseases including hypertension-associated cardiac fibrosis (López et al., Citation2004). Importantly, MMP-2 and MMP-9 are recognized as major contributors, increased release of MMP-2 and MMP-9 has been seen in the coronary blood of patients with myocardial infarction (Yasuda et al., Citation2007). In the current study, sophocarpine could obviously decrease MMP-2 and MMP-9 in AC rat, suggesting that it might mediate the balance of MMPs. It is well known that NF-κB plays an important role in the regulation of MMP-2 and MMP-9, especially in the development of cardiac dysfunction and myocardial interstitial fibrosis (Gu et al., Citation2012). Under normal physiological conditions, NF-κB forms a cytoplasmic complex with its inhibitors, the IkBs. Once the IkBs become phosphorylated, NF-κB is released and translocates to the nucleus where its target genes are then activated (Israf et al., Citation2007). Our study results indicated that sophocarpine not only decreased MMP-2 and MMP-9 levels but also inhibited p-IKBα expression in AC rats. These data might explain in part the inhibition of test substance on the progressive cardiac dysfunction and myocardial interstitial fibrosis.
In conclusion, sophocarpine had a potential antifibrotic effect, the mechanism might be due to the modulation of the balance between pro-inflammatory cytokine levels and collagen content as well as MMPs expression. Of note, NF-κB signaling pathway may play an important role in these beneficial effects of sophocarpine. These findings revealed that the administration of sophocarpine could attenuate AC-induced cardiac fibrosis in animal model, and helped to gain some insight into therapeutic potential of sophocarpine in pressure overload-induced myocardial injure.
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Chen SX, Hang YN, Zhang MZ, et al. (2005). Hemodynamic study of sophocarpine in dogs. Chinese J Integr Med Cardio/Cerebrovascular Dis 10:875–7
- Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. (1995). Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 147:325–38
- de Jong S, van Veen TA, de Bakker JM, van Rijen HV. (2012). Monitoring cardiac fibrosis: A technical challenge. Neth Heart J 20:44–8
- Ferreira AJ, Shenoy V, Qi Y, et al. (2011). Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol 96:287–94
- Gao YL, Li GS, Li CM, et al. (2009). Anti-nociceptive and anti-inflammatory activity of sophocarpine. J Ethnopharmacol 125:324–9
- Gu Y, Wang X, Wang X, et al. (2012). Artemisinin attenuates post-infarct myocardial remodeling by down-regulating the NF-κB pathway. Tohoku J Exp Med 227:161–70
- Gullestad L, Aukrust P. (2005). Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol 95:17–23C; discussion 38–40C
- Hao J, Kim CH, Ha TS, Ahn HY. (2007). Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats. J Vet Sci 8:121–9
- Israf DA, Khaizurin TA, Lajis NH, Khozirah S. (2007). Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-κB nuclear translocation and Iκ-B phosphorylation in RAW264.7 macrophage cells. Mol Immunol 44:673–9
- Kudo H, Kai H, Kajimoto H, et al. (2009). Exaggerated blood pressure variability superimposed on hypertension aggravates cardiac remodeling in rats via angiotensin II system-mediated chronic inflammation. Hypertension 54:832–8
- Kuusisto J, Kärjä V, Sipola P, et al. (2012). Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 98:1007–13
- Kuwahara F, Kai H, Tokuda K, et al. (2004). Hypertensive myocardial fibrosis and diastolic dysfunction: Another model of inflammation? Hypertension 43:739–45
- Levick SP, McLarty JL, Murray DB, et al. (2009). Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension 53:1041–7
- Levick SP, Murray DB, Janicki JS, Brower GL. (2010). Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension 55:270–6
- Li C, Gao Y, Tian J, et al. (2011). Sophocarpine administration preserves myocardial function from ischemia-reperfusion in rats via NF-κB inactivation. J Ethnopharmacol 135:620–5
- López B, González A, Díez J. (2004). Role of matrix metalloproteinases in hypertension-associated cardiac fibrosis. Curr Opin Nephrol Hypertens 13:197–204
- Nian M, Lee P, Khaper N, Liu P. (2004). Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94:1543–53
- Sakamoto A, Hongo M, Furuta K, et al. (2013). Pioglitazone ameliorates systolic and diastolic cardiac dysfunction in rat model of angiotensin II-induced hypertension. Int J Cardiol 167:409–15
- Shibata R, Izumiya Y, Sato K, et al. (2007). Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol 42:1065–74
- Taqueti VR, Mitchell RN, Lichtman AH. (2006). Protecting the pump: Controlling myocardial inflammatory responses. Annu Rev Physiol 68:67–95
- Tschöpe C, Walther T, Königer J, et al. (2004). Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J 18:828–35
- Verdecchia P, Carini G, Circo A, et al.; MAVI (MAssa Ventricolare sinistra nell'Ipertensione) Study Group. (2001). Left ventricular mass and cardiovascular morbidity in essential hypertension: The MAVI study. J Am Coll Cardiol 38:1829–35
- Weber KT. (2004). From inflammation to fibrosis: A stiff stretch of highway. Hypertension 43:716–19
- Xiao P, Kubo H, Komiya H, et al. (1999). Lupin alkaloids from seeds of Sophora viccifolia. Phytochemistry 50:189–93
- Yasuda S, Miyazaki S, Kinoshita H, et al. (2007). Enhanced cardiac production of matrix metalloproteinase-2 and -9 and its attenuation associated with pravastatin treatment in patients with acute myocardial infarction. Clin Sci (Lond) 112:43–9
- Yu XJ, He ZY, Wang XY. (2004). Dynamic changes of myocardial fibrosis in two hypertensive models of rats and the relationship between pressure overload and RAS. J Chin Microcircul 8:209–13
- Zhang Y, Wang S, Li Y, et al. (2008). Sophocarpine and matrine inhibit the production of TNF and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon 26 adenocarcinoma in mice. Int Immunopharmacol 8:1767–72
- Zhao LJ, Jiao DD, Zhao ZM. (2009). The establishment of rat myocardial fibrosis model by abdominal aortic banding. J Chin Microcircul 13:402–4