Abstract
Context: Pharmacological interest of lichens lies in their capacity to produce bioactive secondary metabolites, being most of them phenolic compounds with reactive hydroxyl groups that confer antioxidant potential through various mechanisms. Increasing incidence and impact of oxidative stress-related diseases (i.e., neurodegenerative disorders) has encouraged the search of new pharmacological strategies to face them. Lichens appear to be a promising source of phenolic compounds in the discovery of natural products exerting antioxidant activity.
Objective: The present review thoroughly discusses the available knowledge on antioxidant properties of lichens, including both in vitro and in vivo studies and the parameters assessed so far on lichen constituents.
Methods: Literature survey was performed by using as main databases PubMed, Google Scholar, Scopus, Science Direct, and Recent Literature on Lichens. We reviewed 98 highlighted research articles without date restriction.
Results: Current report collects data related to antioxidant activities of more than 75 lichen species (from 18 botanical families) and 65 isolated metabolites. Much information comes from in vitro investigations, such as chemical assays evaluating radical scavenging properties, lipid peroxidation inhibition, and reducing power of lichen species and compounds; similarly, research on cellular substrates and animal models generally measures antioxidant enzymes levels and other antioxidant markers, such as glutathione levels or tissue peroxidation.
Conclusion: Since consistent evidence demonstrated the contribution of oxidative stress on the development and progression of several human diseases, reviewed data suggest that some lichen compounds are worthy of further investigation and better understanding of their antioxidant and neuroprotective potentials.
Introduction
A lichen is a stable, ecologically obligate, self-supporting mutualism between an exhabitant fungus (the mycobiont) and one or more extracellularly located inhabitants, which can be either unicellular or filamentous photoautotrophic partners (the photobiont: alga or cyanobacterium) (Hawksworth & Honegger, Citation1994). Lichens have been used with medicinal purposes since the ancient times. For instance, Usnea barbata (L.) Weber ex F.H. Wigg (Parmeliaceae) and other Usnea species were used to treat hair-related diseases, Lobaria pulmonaria (L.) Hoffm. (Lobariaceae) and Parmelia sulcata Taylor (Parmeliaceae) for pulmonary and cranial diseases, respectively, yellow-orange colored Xanthoria parietina (L.) Th. Fr. (Teloschistaceae) for jaundice, Peltigera aphthosa (L.) Willd. (Peltigeraceae) for aphta, Parmelia saxatilis (L.) Ach. (Parmeliaceae) for epilepsy, etc. (Brodo et al., Citation2001; Malhotra et al., Citation2008).
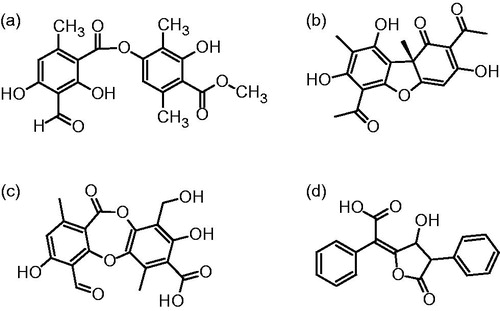
Pharmaceutical importance of lichens lies in their capacity to produce a great variety of secondary metabolites, many of which only appear in these lichenised fungi. Phenolic compounds are the most relevant secondary metabolites of lichen samples, and the best studied metabolites can be principally classified as depsides, depsidones, dibenzofurans, and pulvinic acid derivatives (Huneck, Citation1999). Chemical structures of some representative compounds of each group are shown in . Systematic study of pharmacological properties of lichen compounds has recently started and they have attracted much attention in recent investigations because of their antiviral, antibiotic, antitumor, allergenic, and plant growth inhibitory activities (Dias & Urban, Citation2009; Einarsdóttir et al., Citation2010; Esimone et al., Citation2007; Honda & Vilegas, Citation1999; Nishitoba et al., Citation1987).
Figure 1. Chemical structures of depside atranorin (a), dibezofuran usnic acid (b), depsidone protocetraric acid (c), and pulvinic acid (d).
In the last years, there is an increasing interest in new bioactive natural products for the prevention and treatment of various human diseases, with remarkable attention to neurodegenerative diseases and compounds exerting neuroprotective potential and oxidative stress reversion (Gonzalez-Burgos et al., Citation2013; Wang et al., Citation2013). This is due to the fact that compounds of natural origin normally have beneficial effects on the human organism with lower incidence of unwanted effects. Regarding this point, lichens are the subject of many research teams (Karakus et al., Citation2009; Kosanić et al., Citation2012b).
The present review aims to summarize and discuss the available information about the antioxidant properties of lichens and their isolated secondary metabolites in order to facilitate and guide future research on these natural products.
Oxidative stress
Free radicals (including reactive oxygen species, such as the hydroxyl radical, superoxide anion, hydrogen peroxide, and reactive nitrogen species, such as nitric oxide) play important roles in many chemical processes of the cells under physiological conditions, but they are also associated with pathology and cell damage. Oxidative stress is defined as an imbalance between biochemical processes leading to the production of reactive oxygen species (ROS) and those responsible for the removal of ROS, the antioxidant cascade. In that situation, free radicals will be able attack nucleic acids and proteins, as well as unsaturated fatty acids in the cell membrane; several human chronic diseases (such as neurodegenerative diseases) are related to this problem (Molnár & Farkas, Citation2010; Sayre et al., Citation2008).
On the contrary, antioxidant agents inhibit and prevent those ROS that can cause degenerative diseases. Since many synthetic antioxidants have shown toxic and/or mutagenic effects (Grice, Citation1986; Wichi, Citation1988), the scientific attention shifted towards the discovery of naturally occurring antioxidants. With this regard, numerous plant constituents have been shown to exert antioxidant activity, being flavonoids and other phenolic compounds such as hydroxycinnamic derivatives, catechins, theaflavins, curcumins and terpenoids remarkable among them (Gonzalez-Burgos et al., Citation2012; Sundararajan et al., Citation2006). They are mostly phenolic compounds containing reactive hydroxyl groups and their antioxidant properties might be based on their ability to scavenge ROS, chelate metal ions (i.e., iron and copper), stabilize unpaired electrons, and modulate the endogenous enzymatic and non-enzymatic antioxidant defense systems.
There is unquestionable evidence for some participation of oxidative stress in all neurodegenerative diseases (such Alzheimer’s, Parkinson’s and Huntington’s diseases or amyotrophic lateral sclerosis among them). It therefore suggests possibilities of intervention into etiology or disease progression through individual or combined use of antioxidants capable to enhance endogenous enzymatic or non-enzymatic defense processes (Sayre et al., Citation2008). Thus, since natural antioxidants are preferred over synthetic ones and there is solid basis in thinking of a potential neuroprotective activity for phenolic compounds, investigation of the antioxidant potential of lichen metabolites becomes an interesting strategy for the prevention or treatment of various oxidative stress-related diseases.
Detailed mechanisms and pathways involved in the antioxidant activity of lichens still need further investigation. But classification of these issues known so far could help to understand the pharmacology of lichens secondary metabolites, and their possibilities in the treatment of neurodegenerative disorders, thus promoting the development of neuroprotective natural products.
Literature search
Current report is intended to discuss past and current research on antioxidant properties of lichens and their secondary metabolites. With this aim, an extensive review of scientific literature was carried out by considering all highlighted research articles and other reviews on the issue, without date or language restriction. Five main databases (PubMed, Google Scholar, Scopus, Science Direct, and Recent Literature on Lichens) were used as information sources through the inclusion of the search terms “lichens”, “lichen metabolites”, “antioxidant activities”, “oxidative stress”, and their combinations. As a result, a total of 98 bibliographic references are included in the present work.
Antioxidant properties of lichens
Some lichen extracts and metabolites have already been reported for antioxidant properties due to their phenolic content; for instance, the antioxidant activities of some depsides and depsidones isolated from several lichen species have been demonstrated (de Paz et al., Citation2010; Hidalgo et al., Citation1994; Jayaprakasha & Rao, Citation2000), as well as the in vitro properties of some lichen extracts (Gülçin et al., Citation2002; Stojanović et al., Citation2010). Even so, both studies on intracellular ROS modulation by lichen metabolites/extracts and their in vivo effects have been recently started.
actually includes the antioxidant activity as revealed by in vitro assays of more than 75 species divided into 18 botanical families, among which Parmeliaceae family is the best studied, as it is one of the most rife and widespread. Some of the reflected studies are macrostudies in which authors evaluated the same antioxidant parameter in numerous species. Similarly, in , we gather all available data related to in vitro antioxidant activity of isolated lichen metabolites, referring to more than 65 compounds. In general, antioxidant activity has been mainly evaluated based on some chemical assays, such as DPPH free radical scavenging activity, superoxide anion radical scavenging activity, reducing power, and lipid peroxidation inhibition. Methanol arises as one of the most used solvents for an efficient extraction of lichens bioactive compounds with antioxidant activities and, therefore, many antioxidant activity assays have been performed on methanol extracts (Stojanović et al., Citation2010; Kosanić & Ranković, Citation2011; Zambare & Christopher, Citation2012).
Table 1. Antioxidant activities of lichen extracts.
Table 2. Antioxidant activities of isolated compounds from lichens.
In the last part of our review, we focus on antioxidant responses of these natural products to oxidative stress occurring at intracellular level and in vivo trials. Therefore, we collect the more recent investigations on antioxidant activity of lichens species (both purified metabolites and extracts) on in vitro cellular substrates () and in vivo animal models ().
Table 3. Evaluations of antioxidant parameters on cellular substrates.
Table 4. In vivo evaluations of antioxidant activities.
Apart from all species and assays shown in the tables, some authors have measured similar parameters by other methods (e.g., other radical scavenging properties) and also different parameters related to the antioxidant potential of the aforementioned lichens and compounds. Regarding other radical scavenging properties, Papadopolou et al. (Citation2007) evaluated the hydroxyl radical scavenging activity of β-orcinol metabolites of the lichen Hypotrachyna revoluta (Flörke) Hale (Parmeliaceae); this OH.-radical scavenging activity has also been measured in Toninia candida (Weber) Th.Fr. (Ramalinaceae) (Manojlovic et al., Citation2012b), Usnea ghattensis G. Awasthi (Parmeliaceae) (Verma et al., Citation2008a), and Umbilicaria cylindrica (L.) Delise ex Duby (Umbilicariaceae) (Manojlovic et al., Citation2012c). Nitric oxide radical scavenging activity was assayed on Usnea complanata (Müll. Arg.), Motyka (Parmeliaceae) (Behera et al., Citation2012), Usnea ghattensis (Verma et al., Citation2008b), psoromic and usnic acids (Behera et al., Citation2012), and on other 14 lichen-purified metabolites (Thadhani et al., Citation2011). Moreover, hydrogen peroxide scavenging activity was investigated on Parmelia saxatilis (Özen & Kinalioglu, Citation2008) and the TEAC value (Trolox equivalent antioxidant capacity) has been determined using the ABTS radical assay in several polar lichen species (Paudel et al., Citation2008; Singh et al., Citation2011), as well as in Usnea ghattensis (Behera et al., Citation2005a; Verma et al., Citation2008a), Laurera benguelensis Zahlbr. (Zahlbr.) (Manojlovic et al., Citation2010), and ramalin compound (Paudel et al., Citation2011).
Behera et al. (Citation2003, Citation2006a) assessed the xanthine oxidase inhibitory capacity for many species of the family Graphidaceae; the ferrous ion-chelating activity was investigated in Umbilicaria cylindrica (Manojlovic et al., Citation2012c) and Toninia candida (Manojlovic et al., Citation2012b); and the tyrosinase inhibitory activity has been studied in some lichens and isolated compounds (Behera et al., Citation2006b; Kim & Cho, Citation2007; Paudel et al., Citation2011).
Finally, it is remarkable that the work conducted by Lopes et al. (Citation2008) assayed the radical scavenging properties for many semisynthetic derivatives from the lecanoric acid obtained from a Parmotrema tinctorum (Delise ex Nyl.) Hale (Parmeliaceae) specimen and treated with alcohols. They obtained the three most active compounds at scavenging DPPH radical that were orsellinic acid, orcinol and resorcinol, and the lowest activity was displayed by methyl orsellinate.
Conclusions
The present review reports the biological activity of more than 75 different lichen species, as well as more than 65 purified metabolites, isolated from these or other species. The study of their antioxidant activities has recently been started and they have been determined by various chemical in vitro assays as first approach, with some of them showing interesting results. Further knowledge of this potential implies deeper research on their activities in order to understand the implied mechanisms. Thus, in this report, we also reflect the few available data about in vitro antioxidant activities of some lichen species and purified metabolites on cellular substrates and in vivo on animal models.
Concerning antioxidation, the most interesting compounds are polyphenols. The antioxidant properties of polyphenols are due to the presence of their many phenolic hydroxyl groups, which confer high potential for scavenging free radicals (Dai & Mumper, Citation2010; Sawa et al., Citation1999). For instance, phenolic compounds are able to donate hydrogen to reactive radicals and break the chain reaction of lipid oxidation at the initiation step (Gülçin et al., Citation2004).
Then, the strong antioxidant activity shown by some lichen extracts or metabolites, and assessed by different systems, can be attributed to their high total polyphenolic contents (specially depsides, depsidones, dibenzofurans, etc.), since a positive correlation between phenolic composition and antioxidant activity has been proved for most of them (Kosanić et al., Citation2011; Manojlovic et al. Citation2012c); at least, it suggests that polyphenols might be the major antioxidant compounds in studied lichens. Nevertheless, there have been other studies in which results did not show any positive correlation between antioxidant activity of certain lichens and total phenolic contents (Odabasoglu et al., Citation2004; Stojanović et al., Citation2010). This fact implies that other minor compounds should not be ignored but antioxidant activity might be as well attributed to the presence of non-phenolic compounds, antagonistic or synergistic interactions between constituents, and even distinct antioxidant activities of individual phenolics.
In the previous tables, the great diversity of lichens and their substances is shown, and one might deduce that the increasing interest in the study of its pharmacological properties is promoting further phylogenetic studies in an evolutionary context. Based on molecular data mainly, they are leading to a more complex classification of lichen families and species (Crespo et al., Citation2010). Moreover, phylogenetic analysis of biosynthetic genes can facilitate the discovery of novel compounds, novel genes, and, therefore, unknown producers of pharmaceutical relevant compounds, including antioxidants: the greatest challenges would be to find the biosynthetic gene of interest or assign function to each of the biosynthetic genes found in a lichen genome (Schmitt & Barker, Citation2009). Considering the difficulties still found for the in vitro culture of lichens and different culture conditions result in different antioxidant activities of lichen extracts (due to the production of different amount and type of secondary metabolites depending on culture characteristics) (Behera et al., Citation2005b), a global approach to the lichen metabolomic features seems to be crucial for the development of new and viable biotechnological processes. These will allow production of suitable amounts of unique isolated antioxidant compounds from lichens (Boustie & Grube, Citation2005).
Through this review of literature, we can conclude that lichens are a potential source of natural antioxidants but, at the same time, there is still a need for a deeper research in order to establish their possibilities and a better understanding of their mechanisms of action. This goal can be achieved by the better isolation of purified metabolites and more studies on appropriate cell lines and in vivo, in order to identify molecular targets, active compounds, and structure–activity correlations.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by a Doctoral Grant from the Spanish Ministry of Education, Culture and Sports (FPU programme), awarded to Carlos Fernández Moriano (No. FPU12/03824).
References
- Alpsoy L, Orhan F, Nardemir G, et al. (2015). Antigenotoxic potencies of a lichen species, Evernia prunastri. Toxicol Ind Health 31:153–61
- Aslan A, Agar G, Alpsoy L, et al. (2011). Protective role of methanol extracts of two lichens on oxidative and genotoxic damage caused by AFB1 in human lymphocytes in vitro. Toxicol Ind Health 28:505–12
- Atalay F, Halici MB, Mavi A, et al. (2011) Antioxidant phenolics from Lobaria pulmonaria (L.) Hoffm. and Usnea longissima Ach. lichen species. Turk J Chem 35:647–66
- Bayir Y, Odabasoglu F, Cakir A, et al. (2006). The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine 13:584–90
- Behera BC, Adawadkar B, Makhija U. (2003). Inhibitory activity of xanthine oxidase and superoxide-scavenging activity in some taxa of the lichen family Graphidaceae. Phytomedicine 10:536–43
- Behera BC, Adawadkar B, Makhija U. (2006a). Tissue-culture of selected species of the Graphis lichen and their biological activities. Fitoterapia 77:208–15
- Behera BC, Adawadkar B, Makhija U. (2006b). Tyrosinase-inhibitory activity in some species of the lichen family Graphidaceae. J Herb Pharmacother 6:55–69
- Behera BC, Mahadik N, Morey M. (2012). Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm Biol 50:968–79
- Behera BC, Verma N, Sonone A, Makhija U. (2005a). Antioxidant and antibacterial activities of lichen Usnea ghattensis in vitro. Biotechnol Lett 27:991–5
- Behera BC, Verma N, Sonone A, Makhija U. (2005b). Evaluation of antioxidant potential of the cultured mycobiont of a lichen Usnea ghattensis. Phytothet Res 19:58–64
- Behera BC, Verma N, Sonone A, Makhija U. (2006c). Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT 39:80–5
- Bhattarai HD, Paudel B, Hong SG, et al. (2008). Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. J Nat Med 62:481–4
- Boustie J, Grube M. (2005). Lichens: A promising source of bioactive secondary metabolites. Plant Genet Resour 3:273–87
- Brisdelli F, Perilli M, Sellitri D, et al. (2013). Cytotoxic activity and antioxidant capacity of purified lichen metabolites: An in vitro study. Phytother Res 27:431–7
- Brodo MI, Sharnon SD, Sharnon S. (2001). Lichens of North America. New Haven, London: Yale University Press
- Buçukoglu TZ, Albayrak S, Halici MG, Tay T. (2012). Antimicrobial and antioxidant activities of extracts and lichen acids obtained from some Umbilicaria species from Central Anatolia, Turkey. J Food Process Pres 37:1103–10
- Cernescu I, Tarţău L, Macavei A, Lupuşoru CE. (2011). Experimental research on the effects of a Cetraria islandica extract on oxidative stress in laboratory animals. Rev Med Chir Soc Med Nat Iasi 115:899–904
- Crespo A, Kauff F, Divakar PK, et al. (2010). Phylogenetic generic classification of parmelioid lichens (Parmeliaceae, Ascomycota) based on molecular, morphological and chemical evidence. Taxon 59:1735–53
- Dai J, Mumper RJ. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–52
- de Barros Alves GM, de Sousa Maia MB, de Souza Franco E, et al. (2014). Expectorant and antioxidant activities of purified fumarprotocetraric acid from Cladonia verticillaris lichen in mice. Pulm Pharmacol Ther 27:139–43
- de Paz G, Raggio J, Gómez-Serranillos MP, Palomino OM, et al. (2010). HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J Pharm Biomed Anal 53:165–71
- Dias DA, Urban S. (2009). Phytochemical investigation of the Australian lichens Ramalina glaucescens and Xanthoria parietina. Nat Prod Commun 4:959–64
- Einarsdóttir E, Groeneweg J, Björnsdóttir GG, et al. (2010). Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med 76:969–74
- Esimone CO, Ofokansi KC, Adikwu MU, et al. (2007). In vitro evaluation of the antiviral activity of extracts from the lichen Parmelia perlata (L.) Ach. against three RNA viruses. J Infect Dev Ctries 1:315–20
- Gonzalez-Burgos E, Carretero ME, Gomez-Serranillos MP. (2012). Diterpenoids isolated from Sideritis species protect astrocytes against oxidative stress via Nrf2. J Nat Prod 75:1750–8
- Gonzalez-Burgos E, Carretero ME, Gómez-Serranillos MP. (2013). Kaurane diterpenes from Sideritis spp. exert a cytoprotective effect against oxidative injury that is associated with modulation of the Nrf2 system. Phytochemistry 93:116–23
- Grice HC. (1986). Enhanced tumour development by butylated hydroxytoluene (BHT) in the liver, lung and gastro-intestinal tract. Food Chem Toxicol 24:1127–30
- Gülçin I, Beydemir S, Alici HA, et al. (2004). In vitro antioxidant properties of morphine. Pharmacol Res 49:59–66
- Gülçin I, Oktay M, Küfrevioglu I, Aslan A. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Etnopharmacol 79:325–9
- Güllüce M, Aslanc A, Sokmend M, et al. (2006). Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine 13:515–21
- Güvenç A, Akkol EK, Süntar I, et al. (2012). Biological activities of Pseudevernia furfuracea (L.) Zopf extracts and isolation of the active compounds. J Etnopharmacol 144:726–34
- Halici M, Odabasoglu F, Suleyman H, et al. (2005). Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 12:656–62
- Hara K, Endo M, Kawakami H, et al. (2011). Anti-oxidation activity of ethanol extracts from natural thalli of lichens. Mycosystema 30:950–54
- Hawksworth DL, Honegger R. (1994). The lichen thallus: A symbiotic phenotype of nutritionally specialized fungi and its response to gall producers. In: Williams MAJ, ed. Plant Galls. Special vol. 49. Oxford: The Systematics Association, 77–98
- Hidalgo ME, Fernández E, Quilhot W, Lissi E. (1994). Antioxidant activity of depsides and depsidones. Phytochemistry 37:1585–7
- Honda NK, Vilegas W. (1999). The chemistry of lichens. Quimica Nova 22:110–25
- Huneck S. (1999). The significance of lichens and their metabolites. Naturwissenschaften 86:559–70
- Jayaprakasha GK, Rao LJ. (2000). Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z Naturforsch 55:1018–22
- Karakus B, Odabasoglu F, Cakir A, et al. (2009). The effects of methanol extract of Lobaria pulmonaria, a lichen species, on indometacin-induced gastric mucosal damage, oxidative stress and neutrophil infiltration. Phytother Res 23:635–9
- Kim MS, Cho HB. (2007). Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol 45:578–82
- Kinoshita K, Togawa T, Hiraishi A, et al. (2010). Antioxidant activity of red pigments from the lichens Lethariella sernanderi, L. cashmeriana, and L. sinensis. J Nat Med 64:85–8
- Kosanic M, Manojlovic N, Jankovic S, et al. (2013). Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem Toxicol 53:112–18
- Kosanić M, Ranković B, Stanojković T. (2012a). Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J Sci Food Agric 92:1909–16
- Kosanić M, Ranković B, Stanojković TJ. (2012b). Antioxidant, antimicrobial, and anticancer activity of 3 Umbilicaria species. Food Sci 71:20–5
- Kosanić M, Ranković B, Vukojević J. (2011). Antioxidant properties of some lichen species. J Food Sci Technol 48:584–90
- Kosanić M, Ranković B. (2011). Lichens as possible sources of antioxidants. Pak. J Pharm Sci 24:165–70
- Kotan E, Alpsoy L, Anar M, et al. (2011). Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol Ind Health 27:599–605
- Lohézic-Le Dévéhat F, Tomasi S, Elix JA, et al. (2007). Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities. J Nat Prod 70:1218—20
- Lopes TI, Coelho R, Yoshida N, Honda NK. (2008). Radical-scavenging activity of Orsellinates. Chem Pharm Bull 56:1551–4
- Luo H, Ren M, Lim K, et al. (2006). Antioxidative activity of lichen Thamnolia vermicularis in vitro. Mycobiology 34:124–7
- Luo H, Yamamoto Y, Kim JA, et al. (2009). Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar Biol 32:1033–40
- Luo H, Yamamoto Y, Liu Y, et al. (2010). The in vitro antioxidant properties of Chinese highland lichens. J Microbiol Biotechnol 20:1524–8
- Malhotra S, Subban R, Singh A. (2008). Lichens – Role in traditional medicine and drug discovery. Internet J Altern Med 5:1–5
- Manojlovic NT, Ranković B, Kosanić M, et al. (2012a). Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 19:1166–72
- Manojlovic NT, Vasiljevic PJ, Gritsanapan W, et al. (2010). Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res 43:169–76
- Manojlovic NT, Vasiljevic PJ, Maskovic PZ. (2012b). Chemical composition and antioxidant activity of lichen Toninia candida. Rev Bras Farmacogn 22:291–8
- Manojlovic NT, Vasiljevic PJ, Maskovic PZ, et al. (2012c). Chemical composition, antioxidant, and antimicrobial activities of lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid Based Complement Alternat Med 2012:452431
- Melo MG, dos Santos JP, Serafini MR, et al. (2011). Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol In Vitro 25:462–8
- Mitrović T, Stamenković S, Cvetković V, et al. (2011). Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 12:5428–48
- Molnár K, Farkas E. (2010). Current results on biological activities of lichen secondary metabolites: A review. Z Naturforsch 65c:157–73
- Nardemir G, Yanmis D, Alpsoy L, et al. (2013). Genotoxic, antigenotoxic and antioxidant properties of metanol extracts obtained from Peltigera horizontalis and Peltigera praetextata. Toxicol Ind Health. [Epub ahead of print]. doi: 10.1177/0748233713480207
- Nishitoba Y, Nishimura I, Nishiyama T, Mizutani J. (1987). Lichen acids, plant growth inhibitors from Usnea longissima. Phytochemistry 26:3181–5
- Odabasoglu F, Aslan A, Cakir A, et al. (2004). Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res 18:938–41
- Odabasoglu F, Aslan A, Cakir A, et al. (2005). Antioxidant activity, reducing power and total phenolic content of some lichen species. Fitoterapia 76:216–19
- Odabasoglu F, Cakir A, Suleyman H, et al. (2006). Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol 103:59–65
- Özen T, Kinalioglu K. (2008). Determination of antioxidant activity of various extracts of Parmelia saxatilis. Biologia 63:211–16
- Papadopolou P, Tzakou O, Vagias C, et al. (2007). β-Orcinol metabolites from the lichen Hypotrachyna revolute. Molecules 12:997–1005
- Paudel B, Bhattarai HD, Koh HY, et al. (2011). Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 18:1285–90
- Paudel B, Bhattarai HD, Lee JS, et al. (2008). Antioxidant activity of polar lichens from King George Island (Antarctica). Polar Biol 31:605–8
- Prashith Kekuda TR, Vinayaka KS, Praveen Kumar SV, Sudharshan SJ. (2009). Antioxidant and antibacterial activity of lichen extracts, honey and their combination. J Pharm Res 2:1875–78
- Praveen Kumar SV, Prashith Kekuda TR, Vinayaka KS, et al. (2010). Studies on antibacterial, anthelmintic and antioxidant activities of a Macrolichen Parmotrema pseudotinctorum (des. Abb.) Hale (Parmeliaceae) from Bhadra wildlife sanctuary, Karnataka. Int J Pharm Tech Res 2:1207–14
- Rabelo TK, Zeidán-Chuliá F, Vasques L, et al. (2012). Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol In Vitro 26:304–14
- Racine PH, Hartmann VE, Tollard D′Audiffret Y. (1980). Antioxidant properties of wax from Yougoslavian oakmoss (Evernia prunastri). Int J Cosmet Sci 2:305–13
- Ranković B, Kosanić M, Stanojković T. (2011). Antioxidant, antimicrobial and anticancer activity of the lichens Cladonia furcata, Lecanora atra and Lecanora muralis. BMC Complement Altern Med 11:97
- Ranković B, Kosanić M, Stanojković T, et al. (2012). Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int J Mol Sci 13:14707–22
- Ranković B, Ranković D, Kosanić M, Marić D. (2010a). Antioxidant and antimicrobial properties of the lichens Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Cent Eur J Biol 5:649–55
- Ranković B, Ranković D, Marić D. (2010b). Antioxidant and antimicrobial activity of some lichen species. Microbiology 79:809–15
- Sawa T, Nakao M, Akaike T, et al. (1999). Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem 47:397–402
- Sayre LM, Perry G, Smith MA. (2008). Oxidative stress and neurotoxicity. Chem Ress Toxicol 21:172–88
- Schmitt I, Barker FK. (2009). Phylogenetic methods in natural product research. Nat Prod Rep 26:1585–602
- Silva JA, Bomfim RR, Estevam Cdos S, et al. (2010). Pharmacological properties of lichen Cladonia clathrata. Pharm Biol 48:745–52
- Singh SM, Singh P, Ravindra R. (2011). Screening of antioxidant potential of Arctic lichens. Polar Biol 34:1775–82
- Stojanović G, Stojanović I, Stankov-Jovanović V, et al. (2010). Reducing power and radical scavenging activity of four Parmeliaceae species. Cent Eur J Biol 5:808–13
- Sundararajan R, Ahamad NH, Venkatesan K, et al. (2006). Cytisus scoparius link – A natural antioxidant. BMC Complem Altern Med 6:8
- Takenaka Y, Tanahashi T, Nagakura N, Hamada N. (2000). Production of xanthones with free radical scavenging properties, emodin and sclerotiorin by the cultured lichen mycobionts of Pyrenula japonica. Z Naturforsch 55c:910–14
- Tanas S, Odabasoglu F, Halici Z, et al. (2010). Evaluation of anti-inflammatory and antioxidant activities of Peltigera rufescens lichen species in acute and chronic inflammation models. J Nat Med 64:42–9
- Thadhani VM, Choudhary MI, Ali S, et al. (2011). Antioxidant activity of some lichen metabolites. Nat Prod Res 25:1827–37
- Toledo-Marante FJ, García Castellano A, Estevez Rosas F, et al. (2003). Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. J Chem Ecol 29:2049–71
- Turkez H, Aydin E, Aslan A. (2011). Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 64:679–86
- Valencia-Islas N, Zambrano A, Rojas JL. (2007). Ozone reactivity and free radical scavenging behavior of phenolic secondary metabolites in lichens exposed to chronic oxidant air pollution from Mexico City. J Chem Ecol 33:1619–34
- Verma N, Behera BC, Makhija U. (2008a). Antioxidant and hepatoprotective activity of a lichen Usnea ghattensis in vitro. Appl Biochem Biotechnol 151:167–81
- Verma N, Behera BC, Sonone A, Makhija U. (2008b). Lipid peroxidation and tyrosinase inhibition by lichen symbionts grown in vitro. African J Biochem Res 2:225–31
- Vinayaka KS, Praveen Kumar SV, Prashith Kekuda TR, et al. (2009). Proximate composition, antioxidant, anthelmintic and insecticidal activity of a macrolichen Ramalina conduplicans Vain. (Ramalinaceae). European J App Sci 1:40–6
- Wang JY, Yang JY, Wang F, et al. (2013). Neuroprotective effect of pseudoginsenoside-f11 on a rat model of Parkinson's disease induced by 6-hydroxydopamine. Evid Based Complement Alternat Med 2013:152798
- Wichi, HP. (1988) Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–23
- Yücel O, Odabasoglu F, Güllüce M, et al. (2007). Antioxidant and antimicrobial properties of a lichen species Cladonia rangiformis growing in Turkey. Turkish J Pharm Sci 4:101–9
- Zambare VP, Christopher LP. (2012). Biopharmaceutical potential of lichens. Pharm Biol 50:778–98

