Abstract
Context: The hexane extracts of Dictyota ciliolata Sonder ex Kützing (Dictyotaceae), Padina sanctae-crucis Børgesen (Dictyotaceae), and Turbinaria tricostata E.S. Barton (Sargassaceae) were found to exhibit cytotoxic and antiproliferative activities in vitro. Bioactive compounds responsible for these activities have not been studied in detail for these species and phytochemical studies are very limited.
Objective: Isolate, evaluate, and elucidate the bioactive constituents of D. ciliolata, P. sanctae-crucis, and T. tricostata.
Materials and methods: Bioassay-guided cytotoxicity fractionations using the Hep-2 cell line of the hexane extracts from these brown algae were analyzed using various chromatographic techniques. Cytotoxic and antiproliferative activities of all isolated compounds were also evaluated on a panel of cell lines (KB, Hep-2, MCF-7, and SiHa). Furthermore, their selectivity index, the ratio of cytotoxicity on normal cells to cancer cells, was evaluated using the HEK-293 cell line.
Results: Four compounds were isolated from studied species: two sterol, fucosterol (1) and 24ξ-hydroperoxy-24-vinylcholesterol (2); and two diterpenes, pachydictyol A (3) and dictyol B acetate (4). The major bioactive components of the hexane extracts of T. tricostata and P. sanctae-crucis were compounds 1 and 2 (with CC50 varying around 3.1–25.6 µg/mL) on cell lines tested. Whereas compounds 1, 3, and 4 showed cytotoxic activity against cancer cell lines (CC50 varying between 14.8 and 41.2 µg/mL) and were major bioactive constituents of hexane extract of D. ciliolata. Compounds 1 and 4 showed antiproliferative activity on MCF-7 (IC50 = 43.3 µg/mL for compound 1 and 38.3 µg/mL for compound 2) and SiHa (IC50 = 43.3 µg/mL for compound 1 and 38.3 µg/mL for compound 2) cell lines.
Conclusion: This study is the first investigation on the bioactive components of D. ciliolata, P. sanctae-crucis, and T. tricostata. Although compounds 1–3 were described previously, the pharmacological activity of compound 4 is presented here for the first time.
Introduction
The search for marine natural products has gained great interest for the pharmaceutical industry, which regards them as a new source of bioactive compounds with medicinal potential. In this context, marine algae have been selected for bioprospecting studies due to the great diversity of available species, its ability to produce secondary metabolites with various pharmacological activities, and their ease to be raised as industrial crops (Hu et al., Citation2011). To date, more than 2400 natural products with a wide diversity of chemical structures have been isolated mostly from tropical marine algae (Faulkner, Citation2002). In particular, several species of macroalgae from the Yucatan Peninsula have been the subject of bioprospecting studies by our group (Freile-Pelegrín et al., Citation2008; León-Deniz et al., Citation2009; Moo-Puc et al., Citation2009; Zubia et al., Citation2007). This is also true for Dictyota ciliolata Sonder ex Kützing (Dictyotaceae), Padina sanctae-crucis Børgesen (Dictyotaceae), and Turbinaria tricostata E.S. Barton (Sargassaceae) that have recently reported to have promising cytotoxic activity (Caamal-Fuentes et al., Citation2013). However, investigations on the elucidation of the bioactive constituents of these algae have not been completed by our group; except for Lobophora variegata (J.V. Lamouroux) Womersley ex E.C. Oliveira (Dictyotaceae) (Cantillo-Ciau et al., Citation2010).
In this study, we searched for compounds with biological activity from three brown macroalgae of the Yucatan Peninsula and evaluated their cytotoxic and antiproliferative activities. Bioassay-guided fractionation of the hexane extracts of D. ciliolata, P. sanctae-crucis, and T. tricostata led to the isolation of two sterol fucosterol (1) and 24ξ-hydroperoxy-24-vinylcholesterol (2), together with two diterpenes pachydictyol A (3) and dictyol B acetate (4). In addition, their selectivity was evaluated using a normal cell line (HEK-293).
Materials and methods
General experimental procedures
NMR spectra (1H and 13C) were acquired on a Varian 600 spectrometer, and TMS was used as an internal standard. Column chromatography was carried out on silica gel 60 (70–230 mesh, Merck, Darmstadt, Germany) and silica gel 60H for vacuum liquid chromatography (Merck, Darmstadt, Germany). Preparative thin layer chromatography (PTLC) was performed on percolated silica gel GF254 (1 mm) (Sigma, St. Louis, MO); TLC was performed on percolated silica gel 60 F254 (0.25 mm) (Merck, Darmstadt, Germany). Spots on TLC were visualized under UV light and by spraying with phosphomolybdic acid reagent followed by heating.
Plant material
Dictyota ciliolata, P. sanctae-crucis, and T. tricostata were collected at the Caribbean coast of the Yucatan peninsula (20°46′07″N 86°57′14″W) in the winter of 2012. The seaweeds were centrifuged on site to remove excess seawater using a commercial portable centrifuge and stored in plastic bags on ice during transport to the laboratory. Once in the laboratory, samples were washed thoroughly with fresh water to remove salts, sand, and epiphytes, and stored at −20 °C. Voucher specimens for all species were identified according to Wynne (Citation2005) at the MEXU Herbarium by José Luis Godinez Ortega (Curator IB-UNAM).
Extraction and bioassay-guided fractionation
The milled algae (100 g) were mixed using ethanol (EtOH) (80% v/v) extraction at room temperature for 12 h. The EtOH supernatants were filtered and evaporated under vacuum by means of a rotary evaporator to obtain a dried extract. The EtOH extract was suspended in methanol (MeOH) adjusted to (1:3) with water and extracted using solvents of increasing polarity: hexane (Hx), dichloromethane (DCM), and ethyl acetate (EA). The active hexane fractions of D. ciliolata (3 g), P. sanctae-crucis (1 g), and T. tricostata (1.2 g) were subjected to liquid chromatographic column vacuum using Hx (100%), Hx–DCM (1:1), DCM (100%), and mixture DCM-EA as a mobile phase obtaining seven fractions of D. ciliolata (1D-7D), eight fractions of P. sanctae-crucis (1P-8P), and 10 fractions of T. tricostata (1T-10T).
The fractions that showed activity using the cytotoxic assay on Hep-2 cell line were used for purification and elucidation. The active fractions from T. tricostata (from 4T to 6T) were pooled together based on their similarity by TLC analysis, subsequently subjected to a chromatographic column by gravity (CCG), and eluted with Hx/acetone (An) (7:3) to obtain fucosterol (1) (10 mg) and 24ξ-hydroperoxy-24-vinylcholesterol (2) (5 mg). The active fractions from P. sanctae-crucis (3P, 4P, and 5P) were pooled together using the same criteria as above and eluted on a CCG with DCM/An (9:1) to yield fucosterol (5.5 mg) and 24ξ-hydroperoxy-24-vinylcholesterol (3.4 mg). D. ciliolata fractions 2D, 3D, and 5D were subjected to further purification. The fraction 2D was purified using a CCG and eluted with an isocratic system of Hx/An [9:1] to obtain pachydictyol A (3) (10 mg). The fraction 3D was purified by CCG and eluted with an isocratic system of Hx:An [8:2] to yield dictyol B acetate (4) (5 mg), whereas the fraction 5D was purified and isolated using PTLC (DCM/MeOH, 9:1) to obtain fucosterol (5 mg).
Identification
Fucosterol (1): 1H NMR (600 MHz, CDCl3) δH (ppm): 5.35 (1H, d, J = 5.4, H-6), 5.18 (1H, q, J = 6.6 Hz, H-28), 3.52 (1H, m, H-3), 1.57 (3H, d, J = 6.9 Hz, H-29), 1.01 (3H, s, H-19), 0.99 (3H, d, J = 6.6 Hz, H-21), 0.98 (3H, d, J = 6.9 Hz, H-27), 0.97 (6H, d, J = 6.9, H-26, 27), 0.69 (3H, s, H-18); 13C NMR (150 MHz, CDCl3) δC (ppm): 147.0 (C-24), 140.7 (C-5), 121.7 (C-6), 115.5 (C-28), 71.8 (C-3), 56.7 (C-14), 55.7 (C-17), 50.1 (C-9), 42.4 (C-4), 42.3 (C-13), 39.8 (C-12), 37.3 (C-1), 36.5 (C-10), 36.4 (C-8), 35.2 (C-22), 34.8 (C-20), 31.9 (C-2), 31.9 (C-25), 31.7 (C-7), 28.2 (C-16), 25.7 (C-23), 24.3 (C-15), 22.2 (C-27), 22.1 (C-26), 21.1 (C-11), 19.4 (C-19), 18.7 (C-21), 13.2 (C-29), 11.9 (C-18) (compared with Sheu et al. (Citation1997).
24ξ-Hydroperoxy-24-vinylcholesterol (2): 1H NMR (600 MHz, CDCl3) δH (ppm): 7.00 (OOH), 5.74 (1H, ddd, J = 18.6, 12, 3.6 Hz, H-28), 5.35 (1H, d, J = 4.8 Hz, H-6), 5.28 (1H, dd, J = 12.6, 1.8 Hz,H-29), 5.16 (1H, dd, J = 18.6, 1.8 Hz, H-29), 3.52 (1H, m, H-3), 1.01 (3H, s, H-19), 0.96 (3H, d, J = 6.3 Hz, H-21), 0.88 (3H, d, J = 6.3 Hz, H-27), 0.86 (3H, d, J = 6.6 Hz, H-26), 0.69 (3H, s, H-18); 13C NMR (150 MHz, CDCl3) δC (ppm): 140.7 (C-5), 137.1 (C-28), 121.7 (C-6), 116.3 (C-29), 89.1 (C-24), 71.8 (C-3), 56.7 (C-14), 55.9 (C-17), 50.1 (C-9), 42.4 (C-13), 42.3 (C-4), 39.7 (C-12), 37.2 (C-1), 36.5 (C-10), 36.2 (C-20), 31.9 (C-7), 31.9 (C-8), 31.7 (C-2), 30.9 (C-25), 28.8 (C-22), 28.4 (C-16), 28.3 (C-23), 24.3 (C-15), 21.1 (C-11), 19.4 (C-19), 18.9 (C-21), 17.7 (C-27), 16.7 (C-26), 11.9 (C-18) [compared with Moghadam et al., Citation2013; Sheu et al., Citation1997).
Pachydictyol A (3): 1H NMR (600 MHz, CDCl3) δH (ppm): 5.33 (1H, m, H-3), 5.11 (1H, m, H-14), 4.73 (2H, br, H-18), 3.90 (1H, d, J = 7.6 Hz, H-6), 1.79 (3H, s, H-17), 1.67 (3H, s, H-16), 1.60 (3H, s, H-20), 0.94 (3H, d, J = 6.0 Hz, H-19); 13C NMR (150 MHz, CDCl3) δC (ppm): 152.5 (C-10), 141.4 (C-4), 131.5 (C-15), 124.5 (C-14), 124.0 (C-3), 107.1 (C-18), 40.6 (C-9), 75.1 (C-6), 60.5 (C-5), 47.8 (C-7), 46.1 (C-1), 33.9 (C-12), 35.1 (C-11), 34.8 (C-2), 23.5 (C-8), 25.8 (C-16), 25.5 (C-13), 17.5 (C-20), 17.7 (C-19), 15.7 (C-17) (compared with Gedara et al., Citation2003).
Dictyol B acetate (4): 1H NMR (600 MHz, CDCl3) δH (ppm): 5.33 (1H, d, J = 1.2 Hz, H-3), 5.14 (1H, m, H-14), 4.97 (1H, br, H-18), 4.92 (1H, br, H-18′), 3.92 (1H, dd, J = 8.4, 3.6 H-6), 2.14 (3H, s, COOCH3) 1.79 (3H, s, H-17), 1.68 (3H, s, H-16), 1.60 (3H, s, H-20), 1.02 (3H, d, J = 6.0 Hz, H-19); 13C NMR (150 MHz, CDCl3) δC (ppm): 170.1 (COOCH3), 149.5 (C-10), 140.8 (C-4), 131.8 (C-15), 124.5 (C-14), 123.9 (C-3), 104.7 (C-18), 77.3 (C-9), 74.5 (C-6), 61.0 (C-5), 43.8 (C-7), 43.0 (C-1), 35.0 (C-12), 34.7 (C-11), 33.7 (C-2), 30.2 (C-8), 25.8 (C-16), 25.5 (C-13), 21.3 (COOCH3), 17.8 (C-20), 17.4 (C-19), 15.7 (C-17) (compared with Vázquez et al., Citation1988).
Cytotoxicity and antiproliferative assay
All isolated compounds were tested on the following cell lines: oral carcinoma (KB), epithelial carcinoma of the larynx (Hep-2), breast adenocarcinoma (MCF-7), cervix adenocarcinoma (SiHa), and a human cell embryonic kidney cell line (HEK-293) which were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Cells were routinely cultured in sterile Costar T-25 flasks containing Dulbecco's modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum, 100 U/mL penicillin G, and 100 μg/mL streptomycin at 37 °C in an 5% CO2 atmosphere (95% humidity). At 70–80% confluence, cells were detached from the cultured flask by 0.05% trypsin-EDTA (Gibco) treatment and a suspension of 5 × 104 cells/mL of viable cells was seeded and incubated in a 96-well microtitre plate (Costar).
Cytotoxic activity was determined using the MTT assay (Moo-Puc et al., Citation2009; Rahman et al., Citation2001). When cells reached 90% confluence in a microtitre plate, the medium was replaced and cells were incubated with compounds at various concentrations. After 72 h of incubation, 10 μL of MTT solution (5 mg/mL) were added to each well and incubated at 37 °C for 4 h. The medium was aspirated, and the formazan product was solubilized with acidified isopropanol (0.4 N HCl). The amount of MTT-formazan is directly proportional to the number of living cells and was determined by measuring the optical density (OD) at 590 nm using a GloMax®microplate reader (Promega, San Luis Obispo, CA). In contrast, the inhibition of cell proliferation on the cell lines was evaluated by the sulforhodamine B (SRB) method (Moo-Puc et al., Citation2009; Skehan et al., Citation1990). Briefly, cells reached 70% confluence in a microtitre plate, the medium was replaced with DMEM 10% SFB and compounds at various concentrations. After 48 h, the medium was discarded and cells were fixed by adding 50 μL of 10% trichloroacetic acid (TCA, Aldrich Chemical, Steinheim, Germany). The cells were then incubated at 4 °C for 30 min, TCA was drained off, and the plates were left to dry. Then, 50 μL of SRB stain (10 mg 1% acetic acid, Sigma, St. Louis, MO) were added to each well for 30 min. Finally, the plates were washed four times with 1% acetic acid (100 μL). The OD was measured at 540 nm using a GloMax® microplate reader (Promega, Madison, WI). In addition, the selectivity of compounds was assessed using the ratio between the activity of the normal line (HEK-293) and cancer cell lines. All results were representative of three experiments and were reported as mean. In addition, the selectivity index of compounds was assessed using the ratio between the activity of the normal line (HEK-293) and each cancer cell line tested. The cytotoxic and antiproliferative activities of tested compounds were calculated as the percentage of inhibition of cell proliferation or percentage of cells killed. The activity is shown as IC50 value, which is the concentration that results in 50% inhibition of cell growth and as CC50 value, which is the concentration that killed 50% of the cells. Both values were calculated towards non-linear regression using the software GraphPad Prism 4 (GraphPad Software, San Diego, CA). Docetaxel was used as a positive control.
Results and discussion
Bioassay-guided fractionation of the active hexane extract from the brown algae D. ciliolata, P. sanctae-crucis, and T. tricostata, using cytotoxic assay on Hep-2 cell lines led to the isolation of four compounds (): fucosterol (1) which has been previously isolated in Turbinaria conoides (Sheu et al., Citation1997) and tested for its cytotoxicity against breast and colon carcinoma cell line (Khanavi et al., Citation2012), 24ξ-hydroperoxy-24-vinylcholesterol (2) reported for Turbinaria conoides (Sheu et al., Citation1997), T. ornata (Sheu et al., Citation1999), Padina pavonica (Ktari et al., Citation1999), and Nizamuddinia zanardinii (Moghadam et al., Citation2013), pachydictyol A (3) reported also for Dictyota dichotoma (Gedara et al., Citation2003), and dictyol B acetate (4) which has been found in genus Dictyota ciliolata and D. menstrualis (Cronin et al., Citation1995). The cytotoxic and antiproliferative potential from all isolated compounds was as follows: the principal cytotoxic constituent from D. ciliolata was compound 1, which was active against Hep-2 and SiHa cell lines (); compound 3 that demonstrated reduced activity against all cancer cell lines tested; whereas compound 4 showed cytotoxic activity only against Hep-2. Interestingly, compound 1 from D. ciliolata also showed a high selectivity index towards Hep-2 (). The antiproliferative assay demonstrated that only compounds 1 and 4 showed antiproliferative activity against breast (MCF-7) and cervix (SiHa) cancer cell lines (). Although a previous study reported the presence of compounds 3 and 4 on other species of Dictyota, including D. ciliolata (Cronin et al., Citation1995; Freitas et al., Citation2007), this study is the first report on the anticancer potential of compound 4. This is the first chemical study reported for P. sanctae-crucis and T. tricostata. In particular, the cytotoxic principles from P. sanctae-crucis and T. tricostata were compound 1 and their hydroperoxide (2). This last compound 2 had the highest cytotoxic activity together with a high selectivity index (SI = 16.2) on KB cell lines. Additionally, it was moderately active towards Hep-2, MCF-7 and SiHa cell lines with lower selectivity indices.
Figure 1. Structure of isolated compounds fucosterol (1), 24ξ-hydroperoxy-24-vinylcholesterol (2), pachydictyol (3), and dictyol B acetate (4).
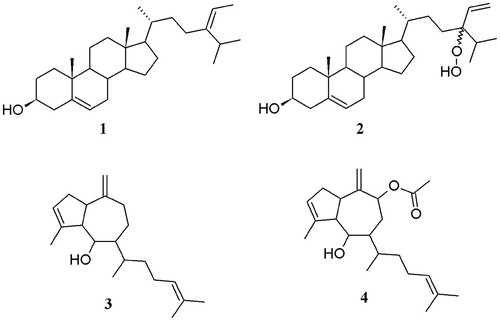
Table 1. Cytotoxic activity CC50 (µg/mL) of isolated compounds on cell lines tested. In parentheses 95% confidence intervals are indicated.
Table 2. Antiproliferative activity IC50 (µg/mL) of isolated compounds on cell lines tested. In parentheses 95% confidence intervals are indicated.
It is known that compound 2 is a potent cytotoxic principle from other species of brown algae (Moghadam et al., Citation2013; Permeh et al., Citation2011; Teixeira et al., Citation2006) and has been also reported for Padina pavonica (Ktari et al., Citation1999), T. conoides and T. ornata (Sheu et al., Citation1997, Citation1999). A recent study by Moghadam et al. (Citation2013) suggests that the mechanism by which this compound exerts its cytotoxic effect is apoptosis, but its mechanism of action is still unknown. The same lack of studies stands for compounds 1, 3, and 4 which mechanism of action for their cytotoxicity it is also unknown, and may be highlighted for further studies.
Conclusion
In this bio-guided study on the cytotoxic and antiproliferative components from D. ciliolata, P. sanctae-crucis, and T. tricostata, four compounds were isolated. To our knowledge, for P. sanctae-crucis and T. tricostata, this is the first chemical study reported in the literature for compounds 1 and 2, whereas the pharmacological activity of compound 4 is presented for the first time.
Declaration of interest
The authors declare no conflict of interest. This study was financed by SEP-CONACYT project 83869-Z.
Acknowledgements
Technical support from C. Chávez Quintal and G. González García from LANNBIO is greatly acknowledged. The first author acknowledges receipt of a postdoctoral fellowship from CONACYT PNPC (N° 000309).
References
- Caamal-Fuentes E, Chale-Dzul J, Moo-Puc R, et al. (2013). Bioprospecting of brown seaweed (Ochrophyta) from the Yucatan Peninsula: Cytotoxic, antiproliferative, and antiprotozoal activities. Avaliable from: http://www.Link.springer.com/article/10.1007/s10811-013-0129-x [last accessed 1 Nov 2013]
- Cantillo-Ciau Z, Moo-Puc R, Quijano L, et al. (2010). The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar Drugs 16:1292–304
- Cronin G, Lindquist NL, Hay ME, et al. (1995). Effects of storage and extraction procedures on yields of lipophilic metabolites from the brown seaweeds Dictyota ciliolata and D. menstrualis. Mar Ecol Prog Ser 119:265–73
- Faulkner DJ. (2002). Marine natural products. Nat Prod Rep 19:1–49
- Freile-Pelegrín Y, Robledo D, Chan-Bacab MJ, et al. (2008). Antileishmanial properties of tropical marine algae extracts. Fitoterapia 79:374–7
- Freitas OSP, Oliveira AS, De-Paula JC, et al. (2007). Chemical variation in the diterpenes from the Brazilian brown alga Dictyota mertensii (Dictyotaceae, Phaeophyta). Nat Prod Commun 2:13–15
- Gedara SR, Abdel-Halim OB, el-Sharkawy SH, et al. (2003). Cytotoxic hydroazulene diterpenes from the brown alga Dictyota dichotoma. Z Naturforsch C 58:17–22
- Geran RI, Greenberg NH, Macdonald MM, et al. (1972). Protocols for screening chemical agents and natural product against animal tumours and other biological system (3rd ed.). Cancer Chemother Rep 3:1–88
- Hu GP, Yuan J, Sun L, et al. (2011). Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar Drugs 9:514–25
- Khanavi M, Gheidarloo R, Sadati N, et al. (2012). Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacog Mag 29:60–4
- Ktari L, Guyot M. (1999). A cytotoxic oxysterol from the marine alga Padina pavonica (L.) Thivy. J Appl Phycol 11:511–13
- León-Deniz LV, Dumonteil E, Moo-Puc R, et al. (2009). Antitrypanosomal in vitro activity of tropical marine algae extracts. Pharm Biol 47:864–71
- Moghadam MH, Firouzi J, Saeidnia S, et al. (2013). A cytotoxic hydroperoxy sterol from the brown alga, Nizamuddinia zanardinii. Daru. Avaliable from: http://www.darujps.com/content/21/1/24. doi:10.1186/2008-2231-21-24 [last accessed 1 Nov 2013]
- Moo-Puc R, Robledo D, Freile-Pelegrín Y. (2009). In vitro cytotoxic and antiproliferative activities of marine macroalgae from Yucatán, Mexico. Cienc Mar 35:345–58
- Permeh P, Saeidnia S, Mashinchian-Moradi A, Gohari AR. (2011). Sterols from Sargassum oligocystum, a brown algae from the Persian Gulf, and their bioactivity. Nat Prod Res 26:774–7
- Rahman A, Choudhary MI, Thomsen WJ. (2001). Bioassay Techniques for Drug Development. Netherlands: Taylor & Francis Group
- Sheu JH, Wang GH, Sung PJ, Duh CY. (1999). New cytotoxic oxygenated fucosterols from the brown alga Turbinaria conoides. J Nat Prod 62:224–7
- Sheu JH, Wang GH, Sung PJ, et al. (1997). Cytotoxic sterols from the formosan brown alga Turbinaria ornata. Planta Med 63:571–2
- Skehan P, Storeng R, Scudiero D, et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–12
- Teixeira VL, Barbosa JP, Rocha FD, et al. (2006). Hydroperoxysterols from Dictyopteris justii and Spatoglossum schroederi. Nat Prod Commun 4:293–7
- Vázquez J, Chang M, Nakanishi K, et al. (1988). Structure of hydroazulenoid diterpenes from a marine alga and their absolute configuration based on circular dichroism. J Org Chem 53:4797–800
- Wynne MJ. (2005). A checklist of benthic marine algae of the tropical and subtropical Western Atlantic: Second revision. Nova Hewigia Beih 129:1–152
- Zubia M, Robledo D, Freile-Pelegrín Y. (2007). Antioxidant activities in marine macroalgae from the coasts of Quintana Roo and Yucatan, Mexico. J Appl Phycol 19:449–58

