Abstract
Context: Broussonetia papyrifera (L.) Vent. (Moraceae), a traditional Chinese medicinal herb, has been extensively applied for many years to treat various diseases. Recently, a number of compounds with biological and pharmacological activities have been extracted from the plant and used as chemotherapeutic candidates to treat a range of diseases such as cancer.
Objective: The current study was designed to isolate the alkaloid compounds from ethyl acetate extraction of Broussonetia papyrifera fruits, and to evaluate the cytotoxic activity of total alkaloids as well as individual isoquinoline alkaloids from B. papyrifera fruits.
Methods: Alkaloid compounds were isolated from the ethyl acetate extraction by silica gel column chromatography methods using CHCl3/MeOH as eluents. The compounds’ structures were determined by detailed analysis of NMR, MS spectral data, and chemical methodology. Cytotoxic activity was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) methods against human A375, Hela, BEL-7402 cancer cells, and non-cancer cells.
Results: Two isoquinonline alkaloids were isolated and characterized as N-norchelerythrine and dihydrosanguinarine. The total alkaloids and seven individual alkaloids had higher activities on BEL-7402 and Hela cell lines with low IC50 values 6.61–47.41 and 5.97–40.17 μg/mL (<50 μg/mL). Nitidine, broussonpapyrine, and chelerythrine had strong toxic on non-cancer cells with IC50 value 18.01, 19.91, and 22.31 μg/mL, respectively.
Discussion: N-Norchelerythrine and dihydrosanguinarine were isolated from this plant for the first time. Our data implicated that seven isoquinoline alkaloids had cytotoxity with structure–activity relationships, which provided fundamental information for further modification of their anticancer effect.
Introduction
Broussonetia papyrifera (L.) Vent. (Moraceae), an effective medical plant, is widely distributed in China, has traditionally been applied for treatment of various diseases. The extracts of the plant of B. papyrifera have been reported for antifungal, antihepatotoxic, antioxidant, and inhibition of lens aldose reductase activities (Lee et al., Citation2001; Pang et al., Citation2006), respectively. The fruits of B. papyrifera called Chushizi as an herbal remedy in the traditional Chinese medicine (Pharmacopoeia commission of PRC, Citation2010) have been reported for treatment of lung cancer (Xu et al., Citation1990). However, the individual compounds related to the activity remain poorly understood.
We have previously extracted the total alkaloids from the fruits of the plant, which exhibited anticancer activities in vitro (Pang et al., Citation2007a,Citationb). Five isoquinoline alkaloids from n-butanol extraction of the fruits, namely broussonpapyrine, nitidine, oxyavicine, liriodenine, and chelerythrine have been found previously (Pang et al., Citation2007a,Citationb, Citation2009). Herein, we report another two individual compounds isolated from the fruits. The structures of the two compounds were determined as isoquinoline alkaloids by detailed analysis of NMR, MS spectral data, and chemical methods. The total alkaloids and each of the seven purified compounds were investigated for their cytotoxic activity against A375, Hela, and BEL-7402 cancer cells using normal human skin epidermal cells as control. Our data included their structure–activity relationships.
Materials and methods
Raw materials
Broussonetia papyrifera fruits were collected from Bozhou, Anhui Province, China, in autumn, 2008. The specimens were identified by Prof. Xu Xian-xiang, Huaqiao University of China. Voucher specimens (voucher no. 20070822) were deposited at School of Biomedical Sciences, Huaqiao University of China. The fresh fruits were washed, dried, and the grey white membranous persistent calyx and impurities were removed. Crude materials (2.5 kg) were obtained and stored in a vacuum dryer for further use.
Extraction of total alkaloids from the fruits
The total alkaloids were extracted from the collected dried fruits. The solvents (petroleum ether, ethyl acetate, ethanol, chloroform, ammonia, and sulfuric acid) used for the extraction were all technical grade which were purchased from Shanghai Chemical Reagent Factory, Shanghai, China. The 2.5 kg raw materials were ground and defatted with three times of 10 L petroleum ether macerations. The duration was 2 h for each time. The remainder powder was collected after evaporation of the track petroleum ether in a fume cupboard. The total alkaloids (10.56 g) were obtained by the acid alcohol extraction – alkali precipitation method (Pang et al., Citation2007).
Individual compounds purification
The total alkaloids were extracted using ethyl acetate, the extraction was chromatographed over a column of silica gel (300–400 mesh) using CHCl3/MeOH (the concentration gradients 1:0, 20:1, 10:1, 5:1, 1:1, 1:5, 1:10, 1:20, and 0:1) as eluents. Two compounds, compound 1 (26 mg) and 2 (18 mg), were obtained from the fractions (15:1) and (1:1), respectively.
Structural determination
The structures of the compounds were identified by NMR analysis, the spectra data recorded on a Bruker AM-500 spectrometer. The molecular weight of each of the compounds was determined by a Varian Mat-212 mass spectrometer. The melting point value was detected by MP50 melting point apparatus.
Human skin epidermal cells (original cells) preparation
Normal adolescent male circumcision foreskin tissue (NAMCFT) was obtained from the operation room of the urology department, Haixia Hospital, Fujian, China, approved by the hospital ethics committee (approval no. 20110907). (1) The NAMCFT specimens were immersed in 75% ethanol for about 10 min, and then washed with HEPES buffer (containing streptomycin 0.1 mg/mL and penicillin 100 IU/mL) for 6–18 times to get rid of the residual blood and blood clots. (2) The subcutaneous adipose tissue, dermis, and blood clots were removed from the NAMCFT before washing 5–6 times again with HEPES buffer. (3) The specimens were then cut into squares (2 mm × 2 mm) and placed on a sterile Petri dish containing 0.25% dispase 5 mL. The epidermis squares were placed with outside facing down and kept at 4 °C for 16 h before incubation (5% CO2, 100% humidity, 37 °C) for 1 h. (4) Adding fetal bovine serum (FBS) (Hyclone, Logan, UT) in a final concentration of 0.5–1.0 mL 10% to termination of digestion.
Cell culture
Cell lines A375 (human skin carcinoma), BEL-7402 (human hepatoma), and Hela (human cervix carcinoma) were obtained from Shanghai Cancer Institute, Shanghai, China. The human skin epidermal cells were tested in parallel to check the compounds’ effects on normal cells. Cells were maintained at 37 °C in a 5% CO2 atmosphere. All cells were grown in RPMI medium 1640 (Gibco, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT), 2 mM l-glutamine (Invitrogen, San Diego, CA), 1% antibiotics (streptomycin–penicillin, (Sigma, St. Louis, MO), and 10 mM HEPES buffer (pH 7.4) (Hangzhou Jinuo, Zhejiang, China).
Cytotoxicity assay
A microassay for cytotoxicity was performed using a MTT [3-(4,5-dimethyl-2-thiazol)-2,5-diphenyl-2H tetrazolium bromide] (Sigma, Steinheim, Germany) assay (Iizuka et al., Citation1999). Briefly, 5.0 × 103 cells/100 μL were seeded in 96-well microplates and preincubated for 6 h to allow cell attachment. The media were aspirated, and then 100 μL of fresh media containing designed various concentrations (50, 10, 5, and 1 μg/mL) of either the total alkaloids or each of the isolated compounds were added to the cultures. After 48 h incubation in the presence or absence–negative control of the compounds, the cell viability was evaluated by adding 10 μL MTT solution (1 mg MTT/mL in PBS). After 4 h incubation at 37 °C, 100 μL DMSO (Sigma, Steinheim, Germany) were added. Microplates were then shaken for 15 min before reading absorbance at 450 nm with ELx800 scanning multiwell spectrophotometer (Shanghai Bio-Tech Co., Ltd, China). The absorbance revealed directly correlates to the number of viable cells and the results were expressed as a percentage of cells viability to negative control (100%). Cytotoxicity was expressed as the concentration of the compounds inhibiting cell growth by 50% (IC50). Each condition was tested in triplicate. Hydroxycamptothecine (HCPT) was used as a positive control and DMSO only as the negative control.
Results
Chemical composition of the alkaloids
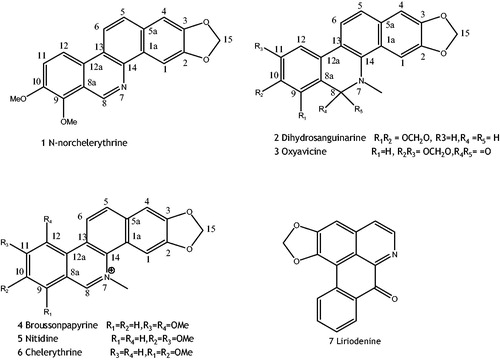
By detailed analysis of 1D- and 2D-NMR, MS spectral data and chemical methods, two compounds were determined, namely N-norchelerythrine (compound 1) and dihydrosanguinarine (compound 2), respectively. All seven isoquinoline alkaloids’ structures are showed in . The following are the physical properties of compounds 1 and 2:
Compound 1 (N-norchelerythrine): yellow grainy crystal in appearance (CHCl3/MeOH 15:1), positive result of Dragendorff test, mp 218–220 °C, ESI-MS [M]+ m/z 333, [M + H]+ 334.1. UV (MeOH) λmax: 219, 242, 255, 275, 325, and 385 nm. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.72 (1H, s, C1–H), 7.25 (1H, s, C4–H), 7.82 (1H, d, J = 9 Hz, C5–H), 8.32 (1H, d, J = 9 Hz, C6–H), 9.74 (1H, s, C8–H), 7.57 (1H, d, J = 9 Hz, C11–H), 8.31 (1H, d, J = 9 Hz, C12–H), 4.05 (3H, s, C9–O–CH3), 4.12 (3H, s, C10–O–CH3), 6.32 (2H, s, –OCH2O–) (C15). 13C-NMR (DMSO-d6) δ: 100.8 (C-1), 126.4 (C-1a), 148.1 (C-2), 147.5 (C-3), 104.3 (C-4), 123.8 (C-5), 130.9 (C-5a), 119.1 (C-6), 48.8 (C-8), 126.3 (C-8a), 146.3 (C-9), 152.3 (C-10), 111.2 (C-11), 118.7 (C-12), 124.3 (C-13), 132.4 (C-14), 124.0 (C-12a), 102.6 (–O–CH2–O–), 61.1 (9-O–CH3), 57.2 (10-O–CH3), its structure was identified as N-norchelerythrine (Balawant et al., Citation1991).
Compound 2 (dihydrosanguinarine): colorless crystal in appearance (CHCl3/MeOH 1:1), positive result of Dragendorff test, mp 190–192 °C. ESI-MS [M]+ m/z 333. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 7.66 (1H, d, J = 8.4 Hz, H-11), 7.65 (1H, s, H-4), 7.47 (1H, d, J = 8.4 Hz, H-12), 7.28 (1H, d, J = 8.0 Hz, H-10), 7.09 (1H, s, H-1), 6.83 (1H, d, J = 8.0 Hz, H-9), 6.03 (4H, d, J = 5.2 Hz, –O–CH2–O–), 4.19 (2H, s, H-6), 2.61 (3H, s, H-N-CH3); 13C-NMR (100 MHz, CDCl3) δ: 104.3 (C-1), 148.1 (C-2), 147.5 (C-3), 100.7 (C-4), 126.5 (C-4a), 142.5 (C-4b), 48.4 (C-6), 113.6 (C-6a), 144.6 (C-7), 147.1 (C-8), 107.2 (C-9), 116.2 (C-10), 127.2 (C-10a), 124.4 (C-10b), 120.3 (C-11), 124.0 (C-12), 130.8 (C-12a), 101.0 (2,3–OCH2O–), 101.3 (7, 8–OCH2O–), 41.5 (N-CH3). So we identified compound 2 as dihydrosanguinarine (Oechslin et al., Citation1991).
Cytotoxic activity
The anticancer properties of the total alkaloids and the seven isoquinoline alkaloids were assessed against A375, Hela, and BEL-7402 cancer cell lines, and human skin epidermal cells. All the cells were subjected to grow in the media containing the designed concentrations of the total alkaloids and each of the seven isoquinoline alkaloids, respectively, for 48 h. The results indicated that total alkaloids and the seven individual isoquinoline alkaloids inhibited four different cell lines BEL-7402, A375, Hela, and human skin epidermal cells to different degrees. The cell survival curves showed a decreasing trend with the increase of the concentrations of the tested compounds (). The concentration of each of the tested compounds causing 50% inhibition (IC50) of the survival rate of the each cell line was determined (). The total alkaloids and the seven individual alkaloids had higher activities on BEL-7402 and Hela cell lines with IC50 values 6.61–47.41 and 5.97–40.17 μg/mL (<50 μg/mL), respectively. In addition to dihydrosanguinarine and N-norchelerythrine, the other tested alkaloids had significant inhibitory effect on A375 with IC50 value in the range of 5.38–26.33 μg/mL. Especially, liriodenine and chelerythrine, their cell growth rate curves were lower than the positive control of HCPT on three kinds of cancer cells. Nitidine, broussonpapyrine, and chelerythrine had also lower curves on non-cancer cells, so for clinical utility, it is necessary to consider their pros and cons.
Figure 2. Cytotoxic activity of B. papyrifera fruits total alkaloids and the seven individual isoquinoline alkaloids against three different tumor cell lines (A) BEL-7402, (B) A375, (C) Hela, and (D) human skin epidermal cells. The values of the cell survival represent the means of triplicate independent cultures with standard deviations.
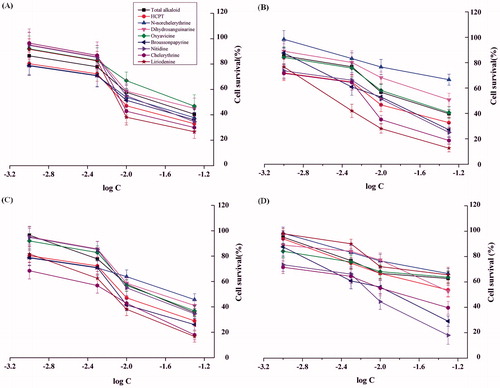
Table 1. IC50 values (μg/mL) of total alkaloid and seven isoquinoline alkaloids from B. papyrifera fruits against different cell lines after 48 h incubation.
All the tested compounds and HCPT had inhibitory effects on tumor cells BEL-7402 and Hela with low IC50 values (<50 μg/mL). Except N-norchelerythrine and dihydrosanguinarine, the other compounds and HCPT exhibited high inhibitory activity on A375. Moreover, liriodenine and chelerythrine with lower IC50 values exhibited a slightly higher effect on three tumor cell lines than HCPT (positive control). The data also showed that three individual alkaloids, nitidine, broussonpapyrine, and chelerythrine had strong toxic on non-cancer cells with IC50 value 18.01, 19.91, and 22.31 μg/mL, respectively.
Discussion
Cancer is a widespread disease responsible for millions of deaths each year worldwide. Chemotherapy is an essential strategy for the treatment of cancer; however, its efficiency restricted by both intrinsic and acquired cell resistance to the drugs remains to be improved. New classes of chemotherapeutic agents have been continuously developed, among which naturally occurring compounds have increasingly attracted to be used as lead compounds for drug development.
In the present study, the total alkaloids and seven individual compounds from B. papyrifera fruits were investigated for their anticancer activity against three human cancer cell lines and normal human skin epidermal cells as control. Most of those compounds showed strong toxic activity on the three cancer cell lines. The total alkaloid exhibited low cytotoxicity (IC50 > 50 μg/mL) on normal human skin epidermal cells while high cytotoxicity (IC50 < 50 μg/mL) on all the three tested cancer cell lines. This property could used to explain the anti-cancer effect of B. papyrifera fruits in traditional Chinese medicine (Xu et al., Citation1990).
The differences of the cytotoxicities of the seven isoquinoline alkaloids were directly correlated with their structures. From these results, we could deduce structure-activity relationships. First, in all the seven isoquinoline alkaloids, liriodenine is the only oxoaporphine alkaloid and exhibited the strongest toxic effect. It was observed that the significant antitumor activity should be ascribed to the hyperconjugational planar structure of oxoaporphine as parent nucleus (Woo et al., Citation1997, Citation1999). Second, the quaternary nitrogen at the ring junction (position 7, ) is the most important structure for cytotoxicity among compounds 1–6, they are all benzophenanthridines structures. It has been reported (Makhey et al., Citation1996) that most of the benzophenanthridines were topoisomerase inhibitors, and their activities were relied on the mother nucleus structure and substituents. The rigid structure of parent nucleus of aromatic ring is the key to anticancer activity and methylene-dioxy substitutes in positions 2 and 3 have strong activity against topoisomerase to kill cancer cells. We also found that the cytotoxic properties of the six isoquinoline alkaloids seemed to be correlated with their substituents of methoxylation (Prado et al., Citation2004), especially at positions 9, 10 or 10, 11. Nitidine, chelerythrine, broussonpapyrine, and oxyavicine have these structures with high toxicity on three cancer cells.
Of those seven individual compounds, liriodenine and chelerythrine were indicated to be worth further evaluation of anti-tumor activity. It has been reported that liriodenine could quickly and efficiently pass through the cell membrane, induce cross-linking of topoisomerase II and SV-40 DNA, so it could interfere the process of DNA replication of tumor cells that depended on topoisomerase II (Woo et al., Citation1997). Chelerythrine can inhibit the growth of all four kinds of the tested cells, and the mechanism may be related to its blockage of cell cycle and apoptosis induction (Liu et al., Citation2009). However, in our study, chelerythrine, nitidine, and broussonpapyrine also inhibited non-cancer cells, which will not be suitable for clinical utility directly. Our future work will focus on the improvement of their effects, high toxic on cancer cells while low toxic on normal cells, by modification of the relevant groups from their molecular structures.
In contrast, the total alkaloid is rich in B. papyrifera fruits with the content of 7.98–23.53 mg/g from different habitats (Pang et al., Citation2010), and it is easy to be separated and purified, has high antitumor effect and low toxicity to normal cells. So the further study will focus on the development of antitumor drugs, and we believe there will be good prospects.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by the Quanzhou Science & Technology Fund (2007Z12).
Acknowledgement
The authors thank Dr. Xu Xian-xiang from School of Biomedical Sciences, Huaqiao University of China for identification of the plant and fruits.
References
- Balawant SJ, Kristi M, Moore S, William P. (1991). Alkaloids of Zanthoxylumbu-drunga Wall. NMR assignments of dihydrochelerythrine, (±)-evodiamine and zanthobungeanine. Phytochem Annu 2:202–51
- Iizuka N, Hirose K, Noma T, et al. (1999). The nm23-H1 gene as a predictor of sensitivity to chemotherapeutic agents in oesophageal squamous cell carcinoma. Br J Cancer 81:469–75
- Lee D, Bhat KPL, Fong HHS. (2001). Aromatase inhibitors from Broussonetia papyrifera. J Nat Prod 64:1286–93
- Liu F, Huang ZF, Wei XH. (2009). Inhibition effects of chelerythrine on Hela cells. Prog Mod Biomed 9:514–16
- Makhey D, Gatto B, Yu C. (1996). Coralyne and related compounds as mammalian topoisomerase I and topoisomerase II poisons. Bioorg Med Chem 4:781–91
- Oechslin SM, Konig KGM. (1991). An NMR study of four benzophenanthridine alkaloids. J Nat Prod 54:519–24
- Pang SQ, Wang GQ, Qin LP, Huang BK. (2006). Antioxidation activities of Fructus Broussonetia haematochrome in vitro. J Chin Med Mater 29:262–5
- Pang SQ, Wang GQ, Huang BK, Qin LP. (2007a). Cytotoxic activities of total alkaloids isolated from Fructus Broussonetia in vitro. J Chin Med Mater 30:826–8
- Pang SQ, Wang GQ, Huang BK, Qin LP. (2007b). Isoquinoline alkaloids from fruits of Broussonetia papyrifera. Chem Nat Compd 43:100–2
- Pang SQ, Wang GQ, Huang BK, Qin LP. (2009). Studies on chemical constituents of Fructus Broussonetiae. J Chin Med Mater 32:1229–31
- Pang SQ, Wang GQ, Huang BK, Qin LP. (2010). Comparison of total alkaloids content of Fructus Broussonetiae from different habitats. J Pharm Pract 28:223–4
- Pharmacopoeia Commission of People’s Republic of China. (2010). Pharmacopoeia of People's Republic of China. Beijing, China: Chin Med Sci & Tech Press, 315
- Prado S, Michel S, Tillequin F. (2004). Synthesis and cytotoxic activity of benzo [c] [1,7] and [1,8] phenanthrolines analogues of nitidine and fagaronine. Bioorg Med Chem 12:3943–53
- Woo SH, Reynolds MC, Sun NJ. (1997). Inhibition of topoisomerase II by liriodenine. Biochem Pharm 54:467–73
- Woo SH, Sun NJ, Cassady JM. (1999). Topoisomerase II inhibition by aporphine alkaloids. Biochem Pharm 57:1141–5
- Xu JP, Huang JJ, Yang F. (1990). Therapy to lung cancer of FU-FEI-JIAN. Chin J Tradi Chin Med Pharm 5:37–9