Abstract
Context: DTD is a Chinese herb prescription used for centuries to treat atherosclerosis or dizziness. Previous studies show that DTD could inhibit ICAM-1 expression induced by TNF-α. However, its mechanism has never been clearly described.
Objective: To examine the hypothesis that DTD might inhibit TNF-α-induced ICAM-1 expression through regulating the expression of p53 and p21.
Materials and methods: The rats were orally treated with DTD for 3 d (2.3 g/kg per day), and then the serum was collected. HUVECs were cultured and stimulated by TNF-α with or without DTD serum (5, 10, and 20%). The expression of ICAM-1 mRNA was examined by RT-PCR and the expression of p53 and p21 was examined by western blot analysis.
Results: The ICAM-1 mRNA levels induced by TNF-α were significantly reduced from 23 to 47%, and the expression of p53 and p21 mRNA levels were significantly reduced from 13 to 43% and 14 to 42%, as the concentration of DTD serum increased. In western blot, TNF-α-induced the expression of p53 and was inhibited from 15 to 53%, by DTD serum in a concentration-dependent manner. TNF-α-induced expression of p21 was inhibited from 2 to 37%, by DTD serum in a concentration-dependent manner.
Discussion and conclusion: DTD has a function of “dissolving phlegm”, thus it is chosen for the treatment of atherosclerosis. This study demonstrated that DTD could significantly inhibit the expression of ICAM-1, p53 and p21, which are important factors of atherosclerosis. Therefore, the present study indicates the pharmacological basis for treatment of atherosclerosis with DTD.
Introduction
Atherosclerosis is known to be a chronic inflammatory disease characterized by a series of highly specific cellular and molecular responses (Ross, Citation1993; Ross & Glomset, Citation1973). Adhesion of circulating polymorphonuclear leukocytes to the vascular endothelium is a critical step in the inflammatory response. This interaction is mediated by inducible cell adhesion molecules, such as ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, which are expressed on the surface of endothelial cells, through a series of complex events (Güray et al., Citation2004; Huo & Ley, Citation2001).
As an inducible cell surface glycoprotein, ICAM-1 belongs to the immunoglobulin superfamily, and is widely expressed at a low level in leukocytes and endothelial cells and can be upregulated by proinflammatory cytokines (Staunton, Citation1988). ICAM-1 plays a central role in a number of inflammatory and immune responses, because it serves as a counter-receptor for leukocyte β2-integrins CD11a/CD18 (lymphocyte function-associated antigen-1) and CD11b/CD18 (Mac-1) and mediates leukocytes and their migration across the vessel wall barrier (Smith et al., Citation1989).
ICAM-1 expression is regulated primarily at the level of transcription (Bevilacqua, Citation1993), and induced by various stimuli, including TNF-α, interferon (INF)-γ, IL-1β (Dustin et al., Citation1986), and thrombin (Kaplanski et al., Citation1998). A growing body of evidence implicates that the tumor suppressor protein, as p53 and p21, which are essential molecules in cell proliferation and apoptosis, work as mediators of leukocyte adhesion in response to classical inflammatory stimuli, induced by TNF-α (Akca et al., Citation2002; Kim et al., Citation2009), which may promote adhesion molecule surface expression and result in atherosclerosis (Gutiérrez et al., Citation2007).
DTD is a safe and effective traditional Chinese medicinal formula used in the treatment of dizziness for centuries. Traditionally, DTD has the functions of eliminating dampness and phlegm, promoting qi circulation and relieving depression. Oral administration of DTD has benefic effects in the treatment of atherosclerosis (Duan & Huang, Citation2011), stroke (Zhang, Citation2008), hyperlipidemia (Guo, Citation2000), and fatty liver (Chen, Citation2001). In our previous study, we used serum from animals which received DTD orally instead of DTD itself, since most of the herbs contain complex components, and their pharmacokinetics remain unknown. It is difficult to investigate their pharmacological actions and mechanisms in vitro by directly adding crude extracts into cultured cells. It is, therefore, more reasonable to use the cell-free portion of a blood specimen, which contains the absorbed components and their metabolites, in pharmacological experiments in vitro (Bochu et al., Citation2005). We found that the DTD could inhibit ICAM-1 expression induced by TNF-α, in the cerebrovascular endothelial cells of the mouse (Chen et al., Citation2008; Huang et al., Citation2012). Bearing in mind that p53 and p21 are involved in the modulation of ICAM-1 expression, we wanted to examine the hypothesis that DTD might inhibit TNF-α-induced ICAM-1 expression, and could regulate the expression of p53 and p21, in cultured HUVEC.
Materials and methods
Plant materials and preparation of DTD
The component herbs of DTD were used as follows (with voucher numbers): Rhizoma Pinelliae [Pinellia ternata (Thunb.) Makino ex Breitenbach.] (091102), Rhizoma Arisaematis [Arisaema erubescens (Wall.) Schott] (100501), Poria [Poria cocos (Schw) Wolf] (100555), Fructus Aurantii Immaturus [Citrus sinensis (Linn.) Osbeck] (100234), Exocarpium Citri Grandis [Citrus maxima (Burm) Osbeck.] (218209101), Radix Glycyrrhizae (Glycyrrhiza uralensis Fisch. ex DC) (100206), and Rhizoma zingiberis recens (Zingiber officinale Rosc.) (108610101) were mixed based on a dry weight ratio of 9:3:3:3:3:2:3. All the herb plants were extracted by standard methods according to the Chinese Pharmacopoeia (China, 2000). All herbs were purchased from the Pharmacy Department of Xuanwu Hospital, in 2011. All herbs were identified by Chinese herbalist Lixin Han in the Pharmacy Department of Xuanwu Hospital. The authenticated voucher specimens are available in the Pharmacy Department of Xuanwu Hospital.
The ingredients were decocted in 10-fold volumes of water at 100 °C for 30 min twice, and then the two decocted solutions were mixed together. The solution was freeze-dried under vacuum, and made into a powder. The powder was dissolved in distilled water to a final concentration of 1 g/ml (equivalent to dry weight of raw materials).
DTD serum preparation
Male Sprague–Dawley rats (Laboratory Animal Center of Xuanwu Hospital, Beijing, China) initially weighting 200–250 g were used in this study. The animals were housed individually at 21 °C with a 12 h light/dark cycle. Rats had free access to a standard diet and drinking water.
Sprague–Dawley rats were randomly divided into two groups: control and DTD groups (10 animals per group). The control animals were fed with saline 5 ml/d/rat for 3 d, by oral administration. The DTD-treated animals were treated with DTD (2.3 g/kg body weight per day) for 3 d, by oral administration. Blood was collected from the abdominal aorta at 2 h after the final administration, then centrifuged at 2500 rpm for 20 min, collected serum, and filtered with 0.2 μm cellulose acetate membrane. The serum was treated in 56 °C water bath for 30 min, and then was stored at −20 °C until use.
All animals received humane care in compliance with the National Institute of Health Criteria for the Care of Laboratory Animals, and the study was approved by the Ethics Committee of Xuanwu Hospital.
Reagents and antibodies
Tissue culture medium 199 and fetal bovine serum (FBS) were obtained from GIBCO (Grand Island, NY). Endothelial cells growth factor (ECGF) was acquired from Roche Inc. (Basel, Switzerland). TNF-α was obtained from Pepro Tech Inc. (Rocky Hill, NJ). p53 inhibitor-alpha (PFT-α) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). TRIzol and electrophoresis reagents were from Invitrogen (Carlsbad, CA). TaKaRa RNA PCR Kits (AMV) Version 3.0 was from Takara Bio (Tokyo, Japan). All reagents for cell culture were of tissue culture grade, and for RNA extraction, reagents were of molecular biology grade. All other materials were purchased from Sigma Co. (St. Louis, MO) except where indicated, and were of analytical grade.
Cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured based on previously described methods (Jaffe et al., Citation1973). HUVEC were cultured in M199 media supplemented with 20% FBS, 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l glutamine, 20 μg/ml ECGF, and 95 μg/ml heparin. Cells were grown in 75 cm2 flasks and maintained at 37 °C in a 95%/5% humidified air/CO2 atmosphere. The cells were split 1:4 every 3 d; experiments were performed on cells at passage 4 to passage 8 when at 80–90% confluence.
The cells were divided into seven groups: (1) control group: cells were cultured with 20% FBS, (2) control serum group: cells were cultured with 10% DTD serum, (3) TNF-α group: cells were co-treated with 200 U/ml TNF-α for 15 min and then cultured with 20% FBS, (4) 5% DTD serum group: cells were pretreated with 5% DTD serum for 6 h and then co-treated with TNF-α for 15 min, (5) 10% DTD serum group: cells were pretreated with 10% DTD serum for 6 h and then co-treated with TNF-α for 15 min, (6) 20% DTD serum group: cells were pretreated with 20% DTD serum for 6 h and then co-treated with TNF-α for 15 min, (7) PFT-α group: cells were pretreated with PFT-α (25 μM, 30 min) (Misra et al., Citation2010), which is a specific inhibitor of p53, and then co-treated with TNF-α for 15 min. We used a normal adult oral dose of DTD as 10% serum dilution in the medium, and then used geometric series as 5% and 20% serum dilutions (data not shown).
RNA extraction and reverse transcription
Total RNA was extracted from HUVEC using Trizol according to the manufacturer's protocol. HUVEC were starved for 12 h in serum-free medium. The concentration of RNA was determined by absorbance at 260 nm and RNA was reverse transcribed to cDNA. cDNA was synthesized from 1 μl of RNA. cDNA synthesis was conducted in a reaction mixture containing MgCl2 2 μl, 10 × RT buffer 1 μl, RNase free H2O 3.75 μl, dNTP 1 μl, RNase inhibitors 0.25 μl, AMV 0.5 μl and 0.5 μl primer; the total reaction volume was 10 μl. Reverse transcription was performed at 42 °C, 30 min; 99 °C, 5 min; 5 °C, 5 min. cDNA was analyzed immediately or stored at −20 °C for reverse transcription polymerase chain reaction (RT-PCR). The details of all oligonucleotide primer sequences, predicted product lengths and gene bank accession by amplification in RT-PCR are listed in . The RT-PCR consisted of upstream PCR primer 0.5 μl, downstream PCR primer 0.5 μl, 5 × PCR buffer 10 μl, Taq enzyme 0.25 μl, sterilized distilled water 28.75 μl in a 40 μl reaction mixture under the following conditions: pre-denaturation at 94 °C for 2 min, denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, extension at 72 °C for 45 s for a total of 30 cycles, after which a final extension step was performed at 72 °C for 7 min. RT-PCR assay was carried out with ABI GeneAmp® PCR System 9600 (Applied Biosystems, San Francisco, CA). After RT-PCR, 5 μl from each product was run out on a 1.5% agarose gel, stained with ethidium bromide and viewed over UV light. The production of RT-PCR was compared with Image Master VDS (Pharmacia Biotech, Uppsala, Sweden) according the area and brightness of every band.
Table 1. Sequences of primers used for RT-PCR.
Western blot analysis
HUVEC were starved for 12 h in serum-free medium. The cells were washed with ice-cold phosphate-buffered saline (PBS; pH 7.4) and lysed in the sample buffer (20 mmo1/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 50 mmol/l NaF, 2 mmol/l EDTA, 1 mmo1/l Na3VO4, 1% NP-40, 1 μg/ml leupeptin, 1 μg/ml Pepstatin, 1 mmol/l PMSF), 100 μl per well of a 6-well plate, then sonicated for 15 s to shear DNA and reduce sample viscosity. Insoluble material was pelleted in a centrifuge at 16 000 × g for 10 min. 20 μg of protein (measured by the method of Bradford) was separated on 15% SDS-polyacrylamide gels and electrophoresis for 1 h at 110 V. Proteins separated by SDS–PAGE were then transferred to polyvinylidene difluoride membranes for 2 h at 100 V. The membranes were blocked and then incubated at 4 °C overnight with a primary antibody. The primary antibodies used in this study were as follows: mouse polyclonal anti-p53, anti-p21 (1:1000). The next day, membranes were washed three times with TBST. Then incubated for 1 h with a polyclonal goat-anti-mouse IgG antibody (1:2000) conjugated with horseradish peroxidase at room temperature and then washed with TBST three times for 5 min. And the detection was performed by using enhanced chemiluminescence system (Pierce, Rockford, IL). Quantitation of the intensity of the bands were scanned with an image scanner and analyzed with NIH image 1.61.
Statistical analysis
Data are expressed as means ± S.D. of the indicated the number of independent experiments. Statistical analyses were performed by a one-way ANOVA, and the Tukey–Kramer post test was used to locate any significant differences identified in the ANOVA. A value of p < 0.05 was considered significant for all tests.
Results
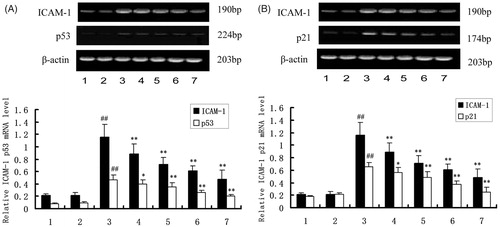
To investigate the role of DTD serum, p53 and p21 on the expression of ICAM-1, the expression of ICAM-1 was determined after stimulation of HUVEC with TNF-α. The cells were pretreated with DTD serum (5, 10, and 20%) for 6 h and then co-treated with TNF-α (200 U/ml) for 12 h. The ICAM-1 mRNA levels induced by TNF-α were significantly reduced from 23 to 47%, and the expression of p53 and p21 mRNA levels were significantly reduced from 13 to 43% and 14 to 42%, as the concentration of DTD serum increased (). Also, the ICAM-1 mRNA level decreased significantly after the addition of PFT-α (25 μM), a p53 inhibitor.
Figure 1. The mRNA expression of ICAM-1, p53 and p21. The cells were pretreated with DTD serum (5, 10, and 20%, 6 h), PFT-α (25 μM, 30 min), and then co-treated with TNF-α (200 U/mL, 15 min). (1) Control group, (2) Control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, and (7) PFT-α group. (A) The expression of ICAM-1 and p53. (B) The expression of ICAM-1 and p21. The band intensities were assessed by scanning densitometry. Data were presented as means ± S.D. of three independent experiments. A one-way analysis of variance was used to compare the multiple group means followed by Newman–Keuls test (##p < 0.01, versus control group; *p < 0.05, **p < 0.01, versus TNF-α group).
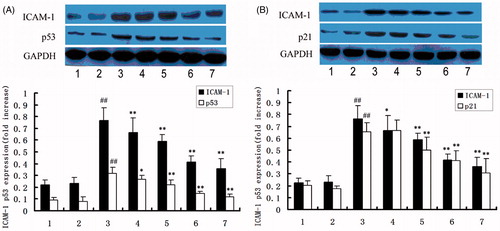
HUVEC was also exposed to 200 U/ml TNF-α, p53, and p21 were expressed within 30 min (data are not shown) to clarify whether DTD serum affects TNF-α-induced the expression of p53 and p21. We examined the effect of various concentrations of DTD serum on TNF-α-induced expression of p53 and p21 in HUVEC (). The cells were pretreated with DTD serum for 6 h before the addition of TNF-α (200 U/ml) for 15 min for the expression of p53 and p21. TNF-α-induced the expression of p53 was inhibited from 15 to 53%, by DTD serum in a concentration-dependent manner. TNF-α-induced the expression of p21 was inhibited from 2 to 37%, by DTD serum in a concentration-dependent manner.
Figure 2 The protein expression of ICAM-1, p53 and p21. Cells were pretreated with DTD serum for 6 h and then co-treated with 200 U/mL TNF-α for 15 min. Cells were extracted and protein levels were determined by Western blots. The cells were pretreated with DTD serum (5, 10, and 20%, 6 h), PFT-α (25 μM, 30 min) and then co-treated with TNF-α. (1) Control group, (2) control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, (7) PFT-α group. (A) The expression of ICAM-1 and p53. (B) The expression of ICAM-1 and p21. The band intensities were assessed by scanning densitometry. Data are presented as means ± S.D. of three independent experiments. A one-way analysis of variance was used to compare the multiple group means followed by Newman–Keuls test (##p < 0.01, versus control group; *p < 0.05, **p < 0.01, versus TNF-α group).
Discussion
Monocyte adhesion to the vascular endothelium and migration into the subendothelial areas of the artery are key initial steps in the development of atherosclerosis. It has also been reported that ICAM-1 was expressed in human atherosclerotic plaques (Haverslag et al., Citation2008), suggesting that ICAM-1 plays a role in atherosclerosis. In senescent human cells, ICAM-1 could be induced by p53 in an NF-κB-independent manner (Gorgoulis et al., Citation2003), and ICAM-1 is also over expressed at the mRNA and protein levels in parallel with the p21, which is a classical p53 target (Gorgoulis et al., Citation2005).
Age-associated changes of the blood vessels include a decrease in compliance and an increase of inflammation (Marín et al., Citation1995). It has also been reported that angiogenesis becomes impaired with advancing age (Rivard et al., Citation1999, Citation2000) and that aging decreases the antithrombogenic properties of the endothelium (Schneiderman et al., Citation1992; Wilkerson & Sane, Citation2002). These changes of vascular structure and function have been suggested to have a role in the increased risk of atherosclerotic cardiovascular disease in the elderly (Lakatta, Citation2003). The p53 plays a pivotal role in the regulation of apoptosis in human cells growth, and may participate in the regulation of vascular apoptosis during the development of atherosclerosis (Aoki et al., Citation1999; Geng, Citation2001), by inducing a cyclin-dependent protein kinase inhibitor (p21) (Hunter & Pines, Citation1994; Levine, Citation1997). p53 immunoreactivity is detectable in vascular cells from sites of chronic inflammation in human atheroma (Ihling, Citation1997; Minamino et al., Citation2003). p53 is also known to interact with the tumor progression factor MDM2 (Barak et al., Citation1993; Chen et al., Citation1994), which promotes p53 degradation and can inhibit p53 transactivation and transrepression (Kubbutat et al., Citation1997; Momand et al., Citation1992). p21 is a transcriptional target of the tumor suppressor gene p53 (el-Deiry et al., Citation1993). Expression of p21 is inducible by wild type p53 but not mutant p53. Several studies have shown that expression of p53 in the cell can induce cell growth arrest through transcriptional activation of p21 (He et al., Citation2005; Hsu & Lee, Citation2011; Igata et al., Citation2005). p21 Immunoreactivity is also detected in human atheroma, but not in normal vessels, and it co-localizes with p53. These observations suggest a pathological role of p53 and p21 in atherogenesis. Thus, overexpression of p53 and p21 by vascular cells may have a deleterious effect in human atherosclerosis.
Since injury of vascular endothelial cells has been implicated in the pathogenesis of atherosclerosis, and endothelial cell-adhesion molecules play a key role in this process, we examined the role of DTD serum on the expression of these adhesion molecules in the present study. Our results demonstrate that DTD serum-inhibited TNF-α-induced ICAM-1 expression. It was also found that DTD serum-inhibited TNF-α-induced ICAM-1 expression through regulating the expression of p53 and p21 in HUVEC.
Significant progress has been made in the treatment of atherosclerosis with Chinese medicine, the mechanism of compound recipes, single Chinese herbs or effective ingredients of Chinese herbs on atherosclerosis were observed. According to the theory of Traditional Chinese Medicine (TCM), the pathogenesis of atherosclerosis is mainly because “blood” and “phlegm” stayed in vessels (Lei et al., Citation2009; Song et al., Citation2008). On account of this, the TCM method of “dissolving phlegm” was chosen for the treatment of atherosclerosis patients. Previous study had shown that this method could alleviate these patients’ symptoms, reverse or delay the course of atherosclerosis, with mechanisms that are possibly related to the function of protecting vascular endothelium (Huang et al., Citation2007). Meanwhile, some other studies showed that some Chinese medicine decoctions, which have the function of “dissolving phlegm”, could influence atherosclerosis by modulating the metabolism of proteoglycans (Wang et al., Citation2008).
DTD, a typical TCM prescription, can promote blood circulation, remove blood stasis, and scavenge the channels or blood vessels, by “dissolving phlegm” in the body, because in Chinese medicine theory, phlegm is thought as the main reason of blood stasis. In clinical practice, it is applied to treat stroke (Wang et al., Citation1986), headache (Sun, Citation2009), high blood lipids (Zhang, Citation1998) and some diseases (Liu & Wang, Citation2009), which are caused by “phlegm stasis” in TCM. The DTD was mainly made by Rhizoma Pinelliae, Rhizoma Arisaematis, Exocarpium Citri Grandis, Fructus Aurantii Immaturus. β-Sitosterol and hesperidin, which function as antioxidation, anticoagulant and antihyperlipidemics (Gupta et al., Citation2010; Jain & Parmar, Citation2011), are two main components of these four medicines (Wei et al., Citation2010; Zhao et al., Citation2009). Also, β-sitosterol and hesperidin could inhibit the superoxide anion generated by the xanthine oxidase/hypoxanthine system. Hesperidin and β-sitosterol had obvious synergies in eliminating free radicals, and their free radical scavenging capacity increases with the increase of drug concentration, in a certain range of concentration. In addition, Fructus Aurantii Immaturus has various constituents, as N-acetyoctopamine, synephrine, N-methyltyramine and GABA, which have active functions of vascular arteries strip (Peng et al., Citation2001). Additionally, Rhizoma Arisaematis could increase glutathione peroxidase activity in the whole blood and catalase activity in red cells of rat (Zhang et al., Citation1996).
So, different from previous studies which mainly examined DTD’s function of decreasing lipids (Guo, Citation2000), our results demonstrated that DTD could inhibit the expression of ICAM-1, and significantly inhibit the expression of p53 and p21.
Conclusions
Taken together, our results suggest that the expression of p53 and p21, which are involved in ICAM-1 expression, induced by TNF-α, are specifically sensitive to DTD in HUVEC. To our knowledge, we first examined the inhibitory effect of DTD serum on ICAM-1 is connected, at least in part, with the inhibition of p53 and p21. Although the mechanisms of the beneficial effect of DTD serum against the expression of p53 and p21 remain to be elucidated, the findings of the present study may shed light on the pharmacological basis for the clinical application of Chinese medicine DTD, for the treatment of atherosclerosis.
Declaration of interest
The authors report no declarations of interest. This study was supported by grants from the National Natural Science Foundation of China, No. 30801481 (PI: Wenqiang Chen), the National Natural Science Foundation of China, No. 81001586 (PI: Fen Wang), and the Beijing Nova Program, No. 2008A090 (PI: Wenqiang Chen).
References
- Akca H, Yenisoy S, Yanikoglu A, Ozes ON. (2002). Tumor necrosis factor-alpha-induced accumulation of tumor suppressor protein p53 and cyclin-dependent protein kinase inhibitory protein p21 is inhibited by insulin in ME-180S cells. Clin Chem Lab Med 40:764–8
- Aoki M, Morishita R, Matsushita H, et al. (1999). Inhibition of the p53 tumor suppressor gene results in growth of human aortic vascular smooth muscle cells. Potential role of p53 in regulation of vascular smooth muscle cell growth. Hypertension 34:192–200
- Barak Y, Juven T, Haffner R, Oren M. (1993). mdm2 expression is induced by wild type p53 activity. EMBO J 12:461–8
- Bevilacqua MP. (1993). Endothelial-leukocyte adhesion molecules. Annu Rev Immunol 11:767–804
- Bochu W, Liancai Z, Qi C. (2005). Primary study on the application of serum pharmacology in Chinese traditional medicine. Colloids Surf B Biointerfaces 43:194–7
- Chen CY, Oliner JD, Zhan Q, et al. (1994). Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA 91:2684–8
- Chen H. (2001). Clinical effect of daotan decoction on fatty liver. Chin J Integr Med 21:457–8
- Chen W, Li Z, Huang X, et al. (2008). Influence of daotan decoction on the expression of ICAM-1 in cerebrovascular endothelial cells of rat. Chin J Inf Trad Chin Med 15:28–9
- Duan X, Huang S. (2011). Clinical observation on 68 cases of angina pectoris treated by daotan decoction. Hun J Trad Chin Med 27:12–4
- Dustin ML, Rothlein R, Bhan AK, et al. (1986). Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 137:245–54
- el-Deiry WS, Tokino T, Velculescu VE, et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–25
- Geng YJ. (2001). Biologic effect and molecular regulation of vascular apoptosis in atherosclerosis. Curr Atheroscler Rep 3:234–42
- Gorgoulis VG, Pratsinis H, Zacharatos P, et al. (2005). p53-dependent ICAM-1 overexpression in senescent human cells identified in atherosclerotic lesions. Lab Invest 85:502–11
- Gorgoulis VG, Zacharatos P, Kotsinas A, et al. (2003). p53 activates ICAM-1 (CD54) expression in an NF-kappaB-independent manner. EMBO J 22:1567–78
- Guo X. (2000). The research of daotan decoction on hyperlipidemia. Chin J Tradit Chin Med Pharm 15:71–2
- Gupta P, Balwani S, Kumar S, et al. (2010). beta-Sitosterol among other secondary metabolites of Piper galeatum shows inhibition of TNFalpha-induced cell adhesion molecule expression on human endothelial cells. Biochimie 92:1213–21
- Güray U, Erbay AR, Güray Y, et al. (2004). Levels of soluble adhesion molecules in various clinical presentations of coronary atherosclerosis. Int J Cardiol l96:235–40
- Gutiérrez G, Mendoza C, Zapata E, et al. (2007). Dehydroepiandrosterone inhibits the TNF-alpha-induced inflammatory response in human umbilical vein endothelial cells. Atherosclerosis 190:90–9
- Haverslag R, Pasterkamp G, Hoefer IE. (2008). Targeting adhesion molecules in cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets 8:252–60
- He G, Siddik ZH, Huang Z, et al. (2005). Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 24:2929–43
- Hsu SP, Lee WS. (2011). Progesterone receptor activation of extranuclear signaling pathways in regulating p53 expression in vascular endothelial cells. Mol Endocrinol 25:421–32
- Huang F, Yao GX, Huang XL, Liu YN. (2007). Clinical observation on acupuncture for treatment of hypertension of phlegm-stasis blocking collateral type. Zhong guo Zhen Jiu 27:403–6
- Huang X, Wang F, Chen W, et al. (2012). Dao-Tan decoction inhibits tumor necrosis factor-α-induced intercellular adhesion molecule-1 expression by blocking JNK and p38 signaling pathways in human umbilical vein endothelial cells. Pharm Biol 50:1111–17
- Hunter T, Pines J. (1994). Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79:573–82
- Huo Y, Ley K. (2001). Adhesion molecules and atherogenesis. Acta Physiol Scand 173:35–43
- Igata M, Motoshima H, Tsuruzoe K, et al. (2005). Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97:837–44
- Ihling C, Menzel G, Wellens E, et al. (1997). Topographical association between the cyclin-dependent kinases inhibitor p21, p53 accumulation, and cellular proliferation in human atherosclerotic tissue. Arterioscler Thromb Vasc Biol 17:2218–24
- Jaffe EA, Nachman RL, Becker CG, Minick CR. (1973). Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–56
- Jain M, Parmar HS. (2011). Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res 60:483–91
- Kaplanski G, Marin V, Fabrigoule M, et al. (1998). Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106). Blood 92:1259–67
- Kim HJ, Yoo EK, Kim JY, et al. (2009). Protective role of clusterin/apolipoprotein J against neointimal hyperplasia via antiproliferative effect on vascular smooth muscle cells and cytoprotective effect on endothelial cells. Arterioscler Thromb Vasc Biol 29:1558–64
- Kubbutat MH, Jones SN, Vousden KH. (1997). Regulation of p53 stability by Mdm2. Nature 387:299–303
- Lakatta EG. (2003). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 107:490–7
- Lei Y, Wang ZH, Zhao H, Liu JG. (2009). Study of the relationship between carotid intima-media thickness and traditional Chinese medicine syndrome of dyslipidemia. Chin J Integr Med 15:112–16
- Levine AJ. (1997). p53, the cellular gatekeeper for growth and division. Cell 88:323–31
- Liu Jia, Wang F. (2009). From the sputum on the treatment of coronary heart disease and angina. J Pract Tradit Chin Intern Med 12:36–7
- Marín J. (1995). Age-related changes in vascular responses: A review. Mech Ageing Dev 79:71–114
- Minamino T, Yoshida T, Tateno K, et al. (2003). Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation 108:2264–9
- Misra UK, Pizzo SV. (2010). PFT-alpha inhibits antibody-induced activation of p53 and pro-apoptotic signaling in 1-LN prostate cancer cells. Biochem Biophys Res Commun 391:272–6
- Momand J, Zambetti GP, Olson DC, et al. (1992). The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69:1237–45
- Peng G, Niu H, Xu L. (2001). Studies on the active constituents offructus aurantii immaturus. J Nanj TCM Un 17:91–2
- Rivard A, Berthou-Soulie L, Principe N, et al. (2000). Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem 275:29643–7
- Rivard A, Fabre JE, Silver M, et al. (1999). Age-dependent impairment of angiogenesis. Circulation 99:111–20
- Ross R, Glomset JA. (1973). Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 180:1332–9
- Ross R. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362:801–9
- Schneiderman J, Sawdey MS, Keeton MR, et al. (1992). Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci USA 89:6998–7002
- Smith CW, Marlin SD, Rothlein R, et al. (1989). Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest 83:2008–17
- Song JN, Liu JL, Fang XZ, et al. (2008). Relationship between plasma protein expression profiles and states of Zang-Fu organs in patients with phlegm or blood stagnation syndromes due to hyperlipidemia and atherosclerosis. Zhong Xi Yi Jie He Xue Bao 6:1233–7
- Staunton DE, Marlin SD, Stratowa C, et al. (1988). Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 52:925–33
- Sun Z. (2009). 43 Headache patients of phlegm and blood stasis syndrome were treated by dissolving phlegm and blood method. J Em Trad Chin Med 18:1156–7
- Wang J, Huang S, Xu Z. (2008). Effect of gualou xiebai banxia decocation on metablism of proteoglycans in aorta in rabbits of atherosclerosis model. Chin J Art 16:290–2
- Wang Y, Li X, Deng Z, et al. (1986). 158 stroke patients were treated by dissolving phlegm and bowel relaxing method. Chin J Trad Chin Med Pharm 2:86–8
- Wei H, Xie F, Rao Y, et al. (2010). Determination of flavone ingredients in Citrus aurantium, bitter orange immature, walnut peel and tangerine peel by internal standard multi-control method. Chin J Exp Trad Med Form 16:75–7
- Wilkerson WR, Sane DC. (2002). Aging and thrombosis. Semin Thromb Hemost 28:555–68
- Zhang H. (2008). Daotan decoction is used in treating with 30 hemorrhagic stroke patients. Guid J Trad Chin Med Pharm 14:26–7
- Zhang Q, Zheng Y, Zhang R, Jiang H. (1996). Experimental studies on the anti-oxidation action of the tuber of Pedate Pinellia (Pinellia Pedatisecta) and the root of common monkshood (Acoitum carmichaeli). Chin Trad Herb Drugs 27:544–6
- Zhang X. (1998). 32 High blood lipid patients were treated by dissolving phlegm method. Beij J Trad Chin Med 5:33–4
- Zhao Q, Guo H, Cui G. (2009). The similarity research of several Araceae medicinal plants. Heb Med J 31:2975–7

