Abstract
Context: Ferulago carduchorum Boiss. & Hausskn. (Apiaceae) is known as Chavil in Persian which grows in west of Iran. Local people add Chavil to dairy and oil ghee as a natural preservative to extend the expiration date.
Objective: The goal of this survey is the safety evaluation of the total extract of F. carduchorum in rats by determining both oral acute and subchronic toxicities; furthermore, the anticoagulant activity of isolated coumarins was evaluated.
Materials and methods: The aerial parts of F. carduchorum were extracted by the percolation method. The anticoagulant activity of isolated coumarins was evaluated and the total extract was used to investigate acute and subchronic toxicity in rats. In the subchronic toxicity model, doses of 250, 500, and 1000 mg/kg of the extract were administered to treated groups for 30 consecutive days by gavage.
Results: According to the results of acute toxicity, the LD50 of Chavil extract was more than 2000 mg/kg. The subchronic study showed no significant difference (p > 0.05) between the groups treated with extract and control groups in hematological (erythrocyte, total and differential leukocyte, hematocrit, hemoglobin, platelet count) and biochemical parameter (glucose, albumin, cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) evaluations. The isolated coumarins (suberosin and suberenol) prolonged the prothrombin time (PT) at doses of 3 and 6 mg/kg compared with control (p < 0.05). The longest PT was for suberosin at 6 mg/kg (17.4 s).
Conclusion: In conclusion, oral administration of the Chavil extract did not cause either acute or subchronic toxicities although the coumarins showed anticoagulant effect in rats.
Introduction
Ferulago (called Chavil in Persian) is a genus belonging to the Apiaceae family and consists of 40 species, eight of which exist in Iran (Mozaffarian, Citation2007; Rechinger et al., Citation1987). These plants have been used in traditional medicine for their sedative, tonic, digestive, and aphrodisiac properties, and for treatment of ulcer, snake bite, headache, intestinal worms, and hemorrhoids (Demetzos et al., Citation2000). Ferulago carduchorum Boiss. & Hausskn. is a perennial shrub with yellow color flowers and the height of about 60–150 cm which grows in west of Iran at an altitude of 1900–3200 m above sea level as an endemic plant in Iran (Mozaffarian, Citation2007). Local people add Chavil to meat, dairy, and oil ghee as a natural preservative to extend the expiration date and give them a pleasant taste.
Several studies have documented different coumarins isolated from some Ferulago species (Apiaceae), Ferulago bernardii Tomk. & M. Pimen. (Khalighi-Sigaroodi et al., Citation2006) Ferulago meoides (L.) Boiss. (Ognyanov & Bocheva, Citation1969), Ferulago turcomanica Schisch. (Andrianova et al., Citation1975; Serkerov et al., Citation1976), Ferulago sylvatica Rchb. (Sklyar et al., Citation1982), Ferulago granatensis Boiss. (De Pascual et al., Citation1979), Ferulago aucheri Boiss. (Doganca et al., Citation1991), Ferulago asparagifolia Boiss. (Doganca et al., Citation1992), Ferulago nodosa (L.) Boiss. (Ruberto et al., Citation1994), Ferulago capillaris (Link ex Spreng.) Cout., and Ferulago brachyloba Boiss. & Reut. (Jimenez et al., Citation2000). Antibacterial and antioxidant activities of some coumarins isolated from Ferulago campestris (Besser.) Grec. have previously been investigated (Basile et al., Citation2009). Moreover, in previous studies, the acetylcholinesterase inhibitory (Dall'Acqua et al., Citation2010) and cytotoxic activities of F. campestris coumarins were evaluated (Rosselli et al., Citation2009). Coumarins with medical or toxic anticoagulant activity are natural products which have been isolated several times from Ferulago species (Arora & Mathur, Citation1963; Khalighi-Sigaroodi et al., Citation2006). There are some reports related to traditional uses of Chavil in Persian folk medicine, but we could not find any scientific evidence on the safety of the plant. Therefore, the goal of this survey was to evaluate the safety of total extract of F. carduchorum in rats by determining both oral acute and subchronic toxicities; furthermore, the anticoagulant activity of isolated coumarins was evaluated.
Materials and methods
Plant material
The aerial parts of F. carduchorum were collected from Ilam province, Iran, on June 2011. The voucher specimen is deposited in the herbarium of Institute of Medicinal Plants (ACECR).
Instruments and materials
The 1H and 13C-NMR spectra were recorded on a Brucker Avance TM 500 DRX (500 MHz for 1H and 125 MHz for 13C) spectrometer with tetramethylsilane as internal standard and chemical shifts are given in δ (ppm) and coupling constants (J) in Hz. The silica gel 60F254 precoated plates for thin-layer chromatography (TLC) were purchased from Merck Company (Darmstadt, Germany). The spots were detected under UV 254 and 365 nm and by spraying a common reagent (anisaldehyde-H2SO4) followed by heating. Silica gel from Merck Company (Darmstadt, Germany) was used for column chromatography (meshes 70–230 and 230–400).
Extraction and isolation
The plant material (1500 g) was dried for 7 d at room temperature (17–22 °C) and powdered. The air-dried and ground aerial parts of F. carduchorum were extracted using a percolation method with MeOH/H2O (80/20) and hexane separately, at room temperature, and the extracts were evaporated with a rotary evaporator and freeze-dried. The extracts were stored in a refrigerator for the phytochemical study, acute and sub-chronic toxicity in rats. The hexane extract (15.24 g) was selected for phytochemical analysis. Then, it was subjected to silica gel (mesh 230–400) column chromatography (CC) with hexane: AcOEt (19:1–0:20) and MeOH as an eluent, to give several fractions (1–21). Fraction 11 was pure compound 1 (500 mg). Fraction 20 was fractionated on silica gel CC with n-hexane/AcOEt (7:3–0:1) to obtain five fractions 20a–20e. Fraction 20c was the pure compound 2 (50 mg).
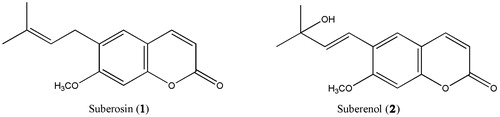
Compound 1: (suberosin). White crystal. 1H-NMR (500 Hz, CDCl3): δ ppm: 7.63 (d, 1H, J = 9.6 Hz, H-4), 7.19 (s, 1H, H-5), 6.78 (s, 1H, H-8), 6.24 (d, 1H, J = 9.6 Hz, H-3), 5.29 (t, 1H, J = 7.2 Hz, H-2′), 3.91 (s, 3H, OCH3), 3.31(d, 2H, J = 7.2 Hz, H-1′), 1.71, 1.78 (s, each 3H, H-4′, H-5′). 13C-NMR (CDCl3): 161.5 (C-2), 160.6 (C-7), 154.5 (C-9), 143.6 (C-4), 133.6 (C-3′), 127.5 (C-6), 127.4 (C-5), 121.3 (C-2′), 112.7 (C-3), 111.9 (C-10), 98.5 (C-8), 55.8 (OCH3), 27.8 (C-1′), 25.8 (C-4′), 17.74 (C-5′).
Compound 2: (suberenol). White crystal. 1H-NMR (DMSO-d6): δ (ppm): 7.98 (d,1H, J = 9.5 Hz, H-4), 7.79 (bs, 1H, H-5), 7.031 (bs, 1H, H-8), 6.78 (d, 1H, J = 16.5 Hz, H-1′), 6.39 (d, 1H, J = 16.5 Hz, H-2′), 6.29 (d,1H, J = 9.5 Hz, H-3), 4.77(s, 1H, OH) 3.90 (s, 3H, OCH3), 1.27(s, 6H, H-4′, H-5′). 13C NMR (DMSO-d6): δ (ppm)160.3 (C-2), 159.34 (C-7), 154.4 (C-9), 144.4 (C-4), 140.5 (C-2′), 125.3 (C-1′), 123.2 (C-6), 118 (C-5), 112.8 (C-3), 112.10 (C-10), 99.2 (C-8), 69.5 (C-3′), 56.3 (OCH3), 30.1 (C-4′), 30.1(C-5′).
Animals
Adult male rats (Wistar) weighing 270–280 g were used in this experiment. Animals were maintained in 22 ± 2 °C temperature, 40 ± 10% relative humidity on a 12 h light/dark cycles with free access to standard diet and tap water throughout the experimental period.
Acute toxicity study
To study acute toxicity in rats (Wills & Velikkakathu, Citation2012) five animals was treated a single oral dose of 2000 mg/kg body weight and tap water was given to the other group of rats as a control. All rats were followed at 1, 2, 3, 4, 5, 6, 7, and 8 h post-dosing and thereafter once daily over 14 d for clinical signs of toxicity, mortality, and any abnormalities in food and water consumption. Animal weight changes before and during the administration of the extract were measured.
Subchronic toxicity study
The rats were randomly divided into four groups of six animals. Doses of 250, 500, and 1000 mg/kg of the total extract were administered to groups for 30 consecutive days by gavage. One group as a control group was considered and only received tap water by gavage in the same volume. During this period, changes in weight and motor behavior in rats were investigated. At the end of the study, animals were fasted overnight and anesthetized with diethyl ether for blood collection from common carotid artery. Blood (1.5 mL) was collected into tubes containing anticoagulant for hematology tests [erythrocyte (RBC), total and differential leukocyte (WBC), hematocrit (Hct), hemoglobin (Hb), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, neutrophils, lymphocyte, monocyte, eosinophil, basophil] and 3 mL of blood were used for separation serum for determining biochemical parameters [glucose, uric acid, creatinine, albumin, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, total protein, and lactate dehydrogenase (LDH); calcium, phosphorus, sodium, potassium, and magnesium]. After the blood collection, liver and kidney were removed, weighed, and fixed in 10% buffered formalin solution then, paraffin sections of the tissues were cut at 5 µm thickness and stained with hematoxylin and eosin for histopathological evaluation.
Anticoagulant activity
The isolated coumarins (suberosin and suberenol) dissolved in ethanol/H2O (50:50) and were orally administered to male Wistar rats at the doses 3 and 6 mg/kg body weight once a day for 3 consecutive days. Also, total extract at doses 250 and 500 mg/kg body weight and warfarin (positive control) at an oral dose 1 mg/kg were administered daily for 3 d. One group as a control group was considered and only received tap water by gavage. On the fourth day, the rats were anesthetized with diethyl ether for blood collection from common carotid artery. The blood was anticoagulated with trisodium citrate (3/8% w/v; 1/10) and centrifuged to prepare platelet poor plasma (PPP). Then, 100 µL of PPP were incubated in 37 °C for 3 min and the prothrombin time (PT) was evaluated by adding 200 µL of prothrombin reagent (Ortho Diagnostic Systems, Rochester, NY) (Rosselli et al., Citation2006).
Analysis of data
Data were expressed as mean ± SD. An one-way ANOVA and LSD post hoc multicomparison tests were used for the analyses. Statistically significance level was set at p < 0.05.
Results
Acute study
All rats treated with the extract were alive throughout the study period (14 d). During the observation, the animals did not show any signs of toxicity. As a result, acute toxicity (LD50) of the total extract of F. carduchorum is more than 2000 mg/kg body weight.
Sub-chronic toxicity
Body and organ weights in rats
All rats survived the 30 d period. There were no significant differences in body weight gain or food and water consumption when compared with the control group (p > 0.05) ( and ). There were also no significant differences in relative organ weights (ROW) between treated and control groups (p > 0.05) ().
Figure 1. Changes in rat body weight with the duration of subchronic treatment. Each point represents mean ± SD.
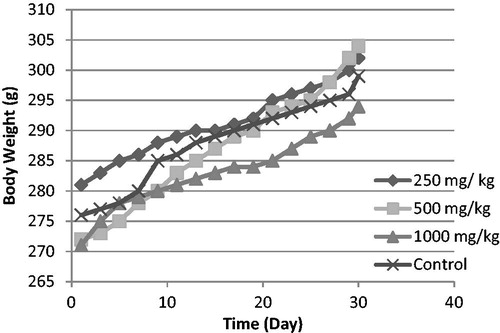
Figure 2. Changes in food consumption with the duration of subchronic treatment. Each point represents mean ± SD.
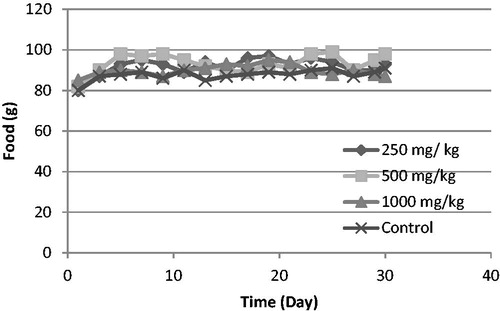
Table 1. Relative organ weight (ROW) at end of treatment with Ferulago carduchorum (g% body weight).
Hematological parameters
The results showed no significant differences between the groups treated with various concentrations of extract and control groups in hematological parameters. Evaluations included erythrocyte (RBC), total and differential leukocyte (WBC), hematocrit (Hct), hemoglobin (Hb), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, neutrophils, lymphocyte, monocyte, eosinophil, and basophil (p > 0.05) ().
Table 2. Hematologic parameters in Wistar rats after 30 d treatment with Ferulago carduchorum.
Serum biochemical parameters
During 30 d, there were no significant differences between the groups treated with different concentrations of extract and control groups. Biochemical parameters of evaluation included glucose, uric acid, creatinine, albumin, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, total protein, lactate dehydrogenase (LDH); calcium, phosphorus, sodium, potassium, and magnesium (p > 0.05) ().
Table 3. Biochemical parameters of Wistar rats after 30 d treatment with Ferulago carduchorum.
Histopathological changes
At the end of the acute and sub-chronic toxicity experiments, rats were sacrificed and liver and kidney tissues were removed for histopathological evaluation. Microscopic examination of tissues revealed no significant damage in the internal organs ( and ).
Figure 3. Histopathological picture of liver of control and treated groups of animals. (A) The section of liver from control animals showed normal liver architecture composed of normal sinusoidal pattern and normal hepatocytes; (B), (C), and (D) The section of liver from rats treated with Ferulago carduchorum 250, 500, and 1000 mg/kg body weight exhibited normal architecture of hepatocytes indicating no toxicity of the extract (40× magnification).
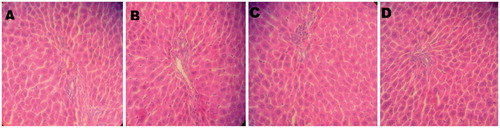
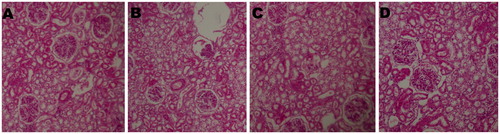
Figure 4. Histopathological picture of kidney of control and treated groups of animals. (A) The section of kidney from control animals revealed normal sizes of glomeruli with normal tubules; (B), (C) and (D) The section of kidney from rats treated with Ferulago carduchorum 250, 500, and 1000 mg/kg body weight showed the normal sizes of glomeruli with normal tubules indicating safety of the extract (20× magnification).
Phytochemical analysis
Isolated compounds () from the n-hexane extract of F. carduchorum were identified as suberosin (1) and suberenol (2). Although they are reported later in other herbal medicine, it is the first time we could find them in Ferulago species (Cazal et al., Citation2009; Reisch et al., Citation1980).
Anticoagulant activity
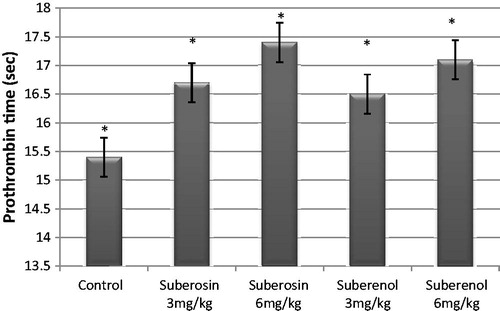
The prothrombin time (PT) of total extract of Chavil at doses of 250 and 500 mg/kg were 15.5 and 15.6 s, respectively, indicating that the extract did not show a significant effect compared with the control group (p > 0.05). The results of anticoagulant activity of coumarins are shown in which showed the longest PT for suberosin at 6 mg/kg (17.4 s). Suberosin prolonged the PT at doses of 3 and 6 mg/kg (16.7 and 17.4 s, respectively) compared with the control group (p < 0.05). Also, suberenol prolonged the PT at doses of 3 and 6 mg/kg (16.5 and 17.1 s, respectively) compared with the control group (p < 0.05).
Discussion
The use of herbal medicines has been growing worldwide and consumers believe that traditional herbal remedies are safe, whereas they could cause some adverse effects so their safety and efficacy evaluation is required (Abotsi et al., Citation2011). During our efforts to investigate the oral acute toxicity of F. carduchorum extract, there were no mortality and adverse effects observed, indicating that the median lethal dose (LD50) is higher than 2000 mg/kg in rats, which suggests that F. carduchorum is a safe additive in traditional uses.
According to subchronic toxicity results, doses of 250, 500, and 1000 mg/kg body weight did not produce significant changes in behavior, food and water consumption, body weight gain, breathing, and gastrointestinal effects in rats. Blood parameters were also evaluated for hematological toxicity after 30 d. Results indicated that the extract has no effect on blood cell count, hemoglobin, and other related parameters. In serum biochemical parameter evaluation, the normal values of blood urea and creatinine (renal biochemical parameters) indicated that the extract did not produce any deleterious effect on renal function. In contrast, there were no treatment-related effects on the heart biochemical parameters (LDL, HDL) evaluated during the administration of extract. In the present study, there was also no significant change in the levels of hepatic enzymes AST, ALT, and ALP in treated groups as compared with the control group. The histopathological evaluation of the important organs (liver and kidney) of the treated animals did not show any changes when compared with the control group. The liver of control and exposed rats revealed hepatocytes, portal triads which were of standard dimensions and vasculature appeared within normal limit. The liver of rats treated with extract did not reveal any pathological alterations (necrosis, inflammation, or fibrosis) and no macrophages were found. No specific changes were detected in the liver parenchyma in the exposed rats. Sinusoidal and perisinusoidal cells did not show any changes.
The kidney of control and treated rats was observed normal structure of the cortex and medulla. Also, normal sizes of glomeruli with normal tubules were seen. No changes were observed in any parameters evaluated, including alveolar collapse, septal thickness, interstitial infiltrate, intra-alveolar neutrophil counts, and alveolar edema.
In summary, the extract did not show any conspicuous toxicity on vital organs that indicates its use as a safe traditional plant. Furthermore, histopathological studies provide support for the safety data of other physiological, biochemical, and hematological parameters of oral administration of Chavil. Notwithstanding, Ferulago genus consist of different coumarins (Khalighi-Sigaroodi et al., Citation2006; Jimenez et al., Citation2000) with anticoagulant effects but in this study during treatment rats with F. carduchorum, it showed no observed abnormal bleeding in animals.
The isolated coumarins (suberosin and suberenol) prolonged the prothrombin time (PT) at doses of 3 and 6 mg/kg compared with the control group significantly (p < 0.05), but warfarin showed more potent effect than samples. In a previous study, the anticoagulant activity of some coumarins was reported; tested coumarins at doses of 3 and 10 mg/kg prolonged the PT compared with the control group (Rosselli et al., Citation2006). The results of recent and previous studies indicated that different coumarins had anticoagulant activity. Warfarin, as a synthetic derivative of coumarin, has intensive anticoagulant activity and is used for the prophylaxis of thrombosis and embolism in some diseases. Warfarin’s anticoagulant effect is due to its interference with vitamin K metabolism (Greaves, Citation2005).
Conclusion
The results indicate that the extract of Ferulago carduchorum did not produce significant damage in internal organs such as liver and kidney, also, no significant effect was observed on any of hematological and biochemical parameters. Oral administration of the total extract from the aerial parts of Chavil did not cause either acute or subchronic toxicity in rats. The isolated coumarins from this plant showed anticoagulant activity which could be more effective as a new industrial formulation with anticoagulant agent or a toxic compound in excess uses.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This research has been supported by Tehran University of Medical Sciences (Grant no. 91-03-33-19324).
Acknowledgements
We would like to thank Dr. Ali Shafiee for assisting with histopathological examinations.
References
- Abotsi KMW, Ainooson KG, Boakye Gyasi E. (2011). Acute and sub-acute toxicity studies of the ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae) in rodents. West Afric J Pharmacy 22:27–35
- Andrianova VB, Sklyar YuE, Pimenov MG. (1975). Coumarins of Ferulago turcomanica roots. Khim Prir Soedin 11:514. Chemical Abstracts 1976;84:27990u
- Arora RB, Mathur CN. (1963). Derivatives, relationship between structure and anticoagulant activity of coumarin. Brit J Pharmacol 20:29–35
- Basile A, Sorbo S, Spadaro V, et al. (2009). Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 14:939–52
- Cazal Cde M, Domingues VC, Batalhão JR, et al. (2009). Isolation of xanthyletin, an inhibitor of ants’ symbiotic fungus, by high-speed counter-current chromatography. J Chromatogr A 1216:4307–12
- Dall'Acqua S, Maggi F, Minesso P, et al. (2010). Identification of nonalkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae). Fitoterapia 81:1208–12
- Demetzos C, Perdetzoglou D, Gazouli M, et al. (2000). Chemical analysis and antimicrobial studies on three species of Ferulago from Greece. Planta Med 66:560–3
- De Pascual TJ, Jimenez B, Corrales B, Grande M. (1979). Coumarins from Ferulago granatensis group: Isovaleryl marmesin. An Quim 75:175–6
- Doganca S, Tuzlaci E, Ulubelen A. (1992). Constituents of Ferulago asperigifolia. Fitoterapia 63:552
- Doganca S, Ulubelen A, Tuzlaci E. (1991). 1-Acetylhydroquinone 4-galactoside from Ferulago aucheri. Phytochemistry 30:2803–5
- Greaves M. (2005). Pharmacogenetics in the management of coumarin anticoagulant therapy: The way forward or an expensive diversion? PLoS Med 2:e342
- Jimenez B, Grande MC, Anaya J, et al. (2000). Coumarins from Ferulago capillaris and F. brachyloba. Phytochemistry 53:1025–31
- Khalighi-Sigaroodi F, Hadjiakhoondi A, Shafiee A, et al. (2006). Phytochemical analysis of Ferulogo bernardii Tomk and M. Pimen. Daru 14:214–21
- Mozaffarian V. (2007). A Dictionary of Iranian Plant Names. Tehran: Farhange Moaser
- Ognyanov I, Bocheva D. (1969). Natural coumarins. II: Coumarins in Ferulago meoides. Planta Med 17:65–70
- Rechinger KH, Hedge IC, Lamond JM. (1987). Flora Iranica. Graz: Akademische Druck-u. Verlagsanstalt
- Reisch J, Mester I, Sofowora EA. (1980). Rare coumarins from Citrus nobilis. J Med Plant Res 40:56–9
- Rosselli S, Maggio AM, Faraone N, et al. (2009). The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelino. Nat Prod Commun 4:1701–6
- Rosselli S, Maggio A, Bellone G, et al. (2006). Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magidaris tomentosa. Planta Med 72:116–20
- Ruberto G, Cannizzo S, Amico V, et al. (1994). Chemical constituents of Ferulago nodosa. J Nat Prod 57:1731–3
- Serkerov SV, Kagramanov AA, Abbasov RM. (1976). Coumarins of Ferulago turcomanica. Khim Prir Soedin 1:94. Chemical abstracts 1976;85:59559x
- Sklyar YE, Andrianova VB, Pimenov MG. (1982). Coumarins of the roots of Ferulago sylvatica. Chem Nat Comp 18:488–9
- Wills JP, Velikkakathu V. Asha. (2012). Acute and subacute toxicity studies of Lygodium flexuosum extracts in rats. APJTM S200–S202:200–2