Abstract
Context: Euphorbia is an important Euphorbiaceae genus that is traditionally being used for various infections, inflammation, and cancer.
Objective: The present study investigated the possible in vitro immunomodulatory effect of three species of Euphorbia genus including Euphorbia microciadia Boiss, Euphorbia osyridea Boiss, and Euphorbia heteradenia Jaub. & Sp. on lymphocyte activation and cytokine secretion.
Materials and methods: Human lymphocytes were cultured in the presence of various concentrations (0.1–200 µg/ml) of the butanol/hexane extracts of the plants in the presence or absence of phytohemmagglutinin (PHA). The activation of lymphocytes after 48 h was determined by a proliferation assay. The release of T cell cytokines was studied to determine the dominant T cell subsets involved in the immune response.
Results: All three plant extracts increased the proliferation of PHA-treated lymphocytes (maximum; 132% of control). Extract treatment of lymphocytes in the absence of PHA resulted in an increased proliferation of the cells indicating their lymphocyte mitogenic activity (maximum at 10 µg/ml E. microciadia extract; 494.5 ± 42.2% of control, p < 0.01). The extracts of E. microciadia and E. osyridea could increase IL-4 and IL-10 secretion but not IFN-γ production showing their capacity to deviate immune response toward a Th2 pattern. Euphorbia heteradenia did not change the release of IL-4 and IFN-γ cytokines but increased IL-10 production. The three extracts stimulated lymphocytes to produce IL-17 which showed their possible effects on Th17 cells activation.
Conclusion: The studied extracts had the ability to modulate T cell responses suggesting their possible beneficial effects on immune host defense.
Introduction
Immunomodulation using medicinal plants can provide an alternative to conventional chemotherapy for a variety of diseases, especially when the host defense mechanism has to be activated under conditions of impaired immune response, or when a selective immunosuppression is desired in situations such as autoimmune disorders. An immunomodulator may be defined as a substance, biological or synthetic, which can stimulate, suppress, or modulate any of the components of the immune system including both innate and adaptive arms of the immune responses (Kumar et al., Citation2012). In various studies, the beneficial immunomodulatory effects of medicinal plants in infections and immune-related diseases have been reported (Amirghofran et al., Citation2011a,Citationb; Gao et al., Citation2004). Medicinal plants such as Thymus vulgaris L. and Zataria multiflora Boiss. (Lamiaceae) that are being used widely for infections and inflammatory diseases in folk medicine have been shown to decrease the proliferation of mitogen-stimulated lymphocytes (Amirghofran et al., Citation2011a,Citationb). Curcumin, a major curcumanoid found in the spice turmeric, has shown anti-inflammatory activities and inhibited cell proliferation, cell-mediated cytotoxicity, and cytokine production by inhibiting NF-κB target genes involved in the induction of the immune responses (Gao et al., Citation2004). Several medicinal plants, including Calendula officinalis L. (Asteraceae), Cichorium intybus L. (Asteraceae), and Stachys obtusicrena Boiss (Lamiaceae) growing in Iran, have shown an inhibitory effect on the proliferation of lymphocytes in the presence of phytohemagglutinin (PHA) (Amirghofran, Citation2010; Amirghofran et al., Citation2007). Glycyrol, an effective immunosuppressant from Glycyrrhiza uralensis Fisch. (Fabaceae), significantly inhibited the proliferation of murine spleen T lymphocytes induced by concanavalin A and mixed lymphocyte reaction (Li et al., Citation2010). Coriolus versicolor L. (Polyproraceae), a kind of Chinese medicinal herb known to have anti-tumor activities, has been suggested to be a lymphocyte mitogen by differentially enhancing the production of T helper (h)1-related cytokines (Ho et al., Citation2004). Morinda citrifolia L. (Rubiaceae) have revealed immunostimulant activity (Nayak & Mengi, Citation2010), E. hirta anti-arthritic (Ahmad et al., Citation2013), and Astragali membranaceus Bunge. (Fabaceae), anti-inflammatory activity through modulation of Th1/Th2 cytokine balance (Kang et al., Citation2004).
Plants of the Euphorbia genus (Euphorbiaceae) include over 1000 species spread broadly in both temperate and tropical regions. Seventeen out of 70 reported species endemic in Iran have traditional medical uses against inflammation, swelling, skin infections, gonorrhea, migraines, intestinal parasites, and warts (Mir Heydari, Citation2010). In our previous study, the immunostimulatory effect of the methanolic extract of E. cheradenia, a native medicinal plant from this genus on lymphocyte proliferation, was shown (Amirghofran et al., Citation2008). In another study, the immunostimulatory effects of the butanolic and hexane extracts of several other native Euphorbia species were demonstrated (Amirghofran et al., Citation2011a,Citationb). In the present study, we sought to determine the immunomodulatory effects of three of these Euphorbia species including E. microciadia Boiss, E. osyridea Boiss, and E. heteradenia Jaub. & Sp. on lymphocyte activation in the presence and absence of mitogen and determine the cytokine secretion pattern of the treated lymphocytes. The data obtained would help us to identify the underlying mechanism responsible for the beneficial of these plants for various infectious and/or immune-related diseases.
Materials and methods
Preparation of the extracts
The plants were collected in May, at the time of flowering from Fars Province, Iran and authenticated by Dr. Khosravi from the Department of Biology, Shiraz University, Shiraz, Iran. Voucher specimens were deposited in the Shiraz University Herbarium. Extraction was performed as previously described (Amirghofran et al., Citation2011a,Citationb). Briefly, the aerial parts of the plants were subjected to maceration in methanol at room temperature (RT) for 48 h. The methanol extracts were filtered and concentrated under reduced pressure. The resultant extracts with of yields (w/w) 5.4%, 11.1%, and 11% for E. microciadia, E. heteradenia, and E. osyridea, respectively, were subsequently suspended in 500 ml of water and re-extracted three-times with n-butanol (for E. microciadia and E. osyridea) and hexane (for E. heteradenia). The preparation of n-butanol and hexane extracts from the plants was based on the results of our previous study in which these extracts had shown the highest immunomodulatory activity (Amirghofran et al., Citation2011a,Citationb). The extracts were concentrated under reduced pressure using a rotary evaporator and then freeze-dried. They were negative for endotoxin contamination. For the experiments, samples were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO) and then they were diluted in the RPMI 1640 culture medium (Sigma) to obtain a 20 µg/ml solution. The study was approved by the Ahwaz University of Medical Sciences Ethics Committee.
Lymphocyte proliferation assay
Peripheral blood lymphocytes (PBLs) were isolated from 20 to 30 year-old, healthy male individuals (with their consent) by gradient centrifugation with Ficoll–Hypaque (Baharafshan, Tehran, Iran). The viability of cells was more than 95% determined by the Trypan blue dye exclusion test. The effect of the extracts on the PBLs (1 × 105/well) in the presence or absence of phytohemagglutinin (PHA, Fluka, Germany, 1/1750) as the mitogen was determined using 5-bromo-2′-deoxy-uridine (BrdU) incorporation assay kit (Roche, Germany) as described previously (Gharagozloo et al., Citation2013). Cells were treated with various concentrations of the extracts and cultured in flat-bottom 96-well plates for 48 h. The positive control was extract-untreated cells containing only PHA and the negative control was those cells treated with neither the extract nor PHA. In all wells without the extract, DMSO as the solvent at the highest concentration used in the tests (e.g. 0.05%) was added. After labeling with BrdU for the final 18 h of the incubation period, DNA was denatured and the cells were incubated with anti-BrdU monoclonal antibody for detecting incorporated BrdU. The optical density (OD) related to the BrdU level was measured with a microplate reader at 450 nm. The experiments were plated in triplicate wells and were performed at least three times. The same experiments with the similar conditions were performed in parallel to collect the supernatant of the cells for measurement of cytokines.
The measurement of cytokines
The level of cytokines including interferon (IFN)-γ, interleukin (IL)-4, IL10, and IL-17 was measured in the supernatant of extract-treated cultures with the highest proliferatory effect on the PBLs. Cytokine levels were determined by using the related enzyme-linked immunosorbent assay (ELISA) kit obtained from eBioscience (Frankfurt, Germany). The sensitivity of kits was 4, 2, 2, and 4 pg/ml for the above cytokines. Briefly, the plates were coated with the related capture antibody at 4 °C for an overnight. Then the wells were washed with buffer three times, and they were covered by blocking protein for 1 h at RT. After washing, standards and samples were added and the plates left at 4 °C for a further overnight. The plates were again washed and detected antibody was then added for 1 h at RT. The supernatant was aspirated and after washing, avidin-HRP solution was added and the plates were left at RT for 30 min. At the final step, the substrate and then stopping solution was added and the OD at 450 nm wave length was read by an ELISA reader (Biotek, Winooski, VT). The level of each cytokine was determined by using a standard curve.
Results
The effect of Euphorbia plant extracts on the PBLs proliferation in the presence or absence of PHA
In order to test the effect of the extracts on lymphocyte proliferation, cells were stimulated with PHA at suboptimal concentration and simultaneously various concentrations of the extracts were added. represents the influence of each plant extract on the proliferation of the lymphocytes.
Figure 1. The effect of E. microsciadia (A), E. heteradenia (B), and E. osyridea (C) extracts on the proliferation of the lymphocytes in the presence (1) and absence (2) of phytohemagglutinin (PHA). Peripheral blood lymphocytes were treated with various concentrations of the extracts for 48 h and then the cell proliferation was measured by Brdu incorporation assay. The positive control (c+) was lymphocytes treated only with PHA and without the extracts and the negative control (c−) was those without PHA and the extracts. The asterisks on the graphs show significant difference with the positive control (for cultures in the presence of PHA; A1, B1, C1) and with the negative control (for cultures in the absence of PHA; A2, B2, C2). *p < 0.05, **p < 0.01. The experiments were performed in triplicates and repeated at least three times.
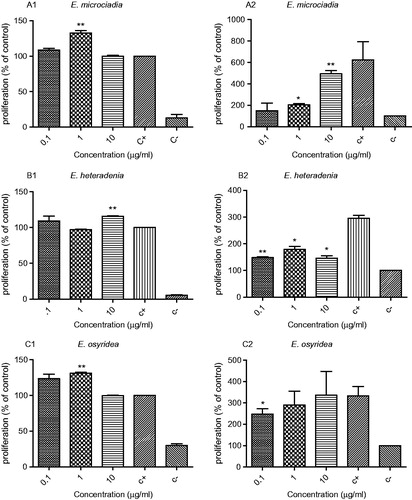
E. microciadia – as shown in , the extract of this plant at a concentration of 1 µg/ml significantly increased lymphocyte proliferation in comparison with the positive control (132.7 ± 4.5% of positive control) (p < 0.01). Considering the stimulatory effect of the extract on the proliferation and activation of lymphocytes, its possible mitogenic effects were also investigated. For this, cells were only treated with the extract and PHA was not added to the culture. As shown in , the extract concentrations of 1 and 10 µg/ml have significantly increased lymphocyte proliferation in comparison with the negative control (203.9 ± 19.04% and 494.5 ± 42.17% of the negative control, p < 0.01).
E. heteradenia – this extract at concentrations of 10 µg/ml slightly increased the proliferation of lymphocytes (115 ± 1.26% of the positive control, p < 0.01) (). The extract at 0.1–10 µg/ml showed a mitogenic effect and caused an increase in the proliferation of lymphocytes in the absence of PHA (maximum at 1 µg/ml; 178.8 ± 16.3% of the negative control). This effect at all three concentrations was significant (p < 0.05) compared with the negative control ().
E. osyridea – the results related to the effect of this extract on the activation and proliferation of lymphocytes in the presence and absence of PHA mitogen are shown in . The extract at concentrations of 0.1 and 1 µg/ml had a stimulatory effect on lymphocyte proliferation in the presence of PHA (123.4 ± 8.8 and 131.09 ± 2.1% of the positive control), but in comparison with the positive control, this effect was significant only at 1 µg/ml (p < 0.01). Concentrations of 0.1–10 µg/ml of the extract also increased the proliferation of lymphocytes in the absence of PHA (), although it was only significant at 0.1 µg/ml compared with the negative control.
The effect of Euphorbia plant extracts on the cytokine secretion
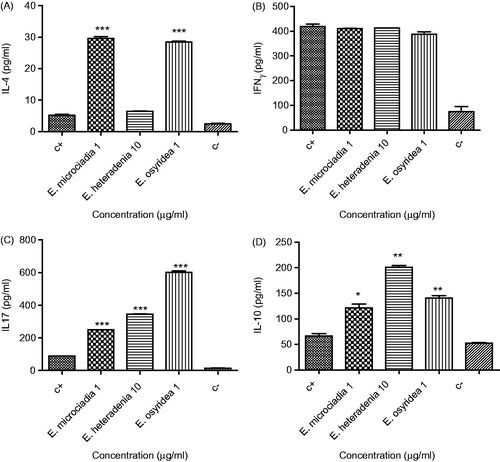
The levels of four cytokines including IFN-γ, IL-4, IL-10, and IL-17 were measured in the supernatant of the extract-treated lymphocytes in the presence or absence of PHA. As shown in , a concentration of 1 µg/ml E. microciadia (29.6 ± 0.6 pg/ml) and E. osyridea (28.5 ± 0.3 pg/ml) extract significantly increased the release of IL-4 in the presence of PHA compared with the positive control (5.17 ± 0.2 pg/ml), whereas E. heteradenia extract had no significant effect.
Figure 2. The effect of plant extracts on the production of IL-4 (A), IFN-γ (B), IL-17 (C), and IL-10 (D) by the lymphocytes in the presence of PHA. Peripheral blood lymphocytes were treated with 1 or 10 µg/ml of the extracts for 48 h and then the supernatants were collected for cytokine measurement by ELISA assay. The positive control (c+) was lymphocytes stimulated with PHA and without extract and the negative control (c−) was without PHA and extract. The asterisks on the graphs show significant difference with the positive control. *p < 0.05, **p < 0.01, ***p < 0.001. The experiments were performed in triplicate and repeated at least three times.
The effect of the above plants on the induction of IL-4 release by lymphocytes in the absence of PHA was also investigated and it appeared that none of the plants had significant effects on IL-4 levels compared with the negative control ().
Figure 3. The effect of plant extracts on the production of IL-4 (A), IFN-γ (B), and IL-17 (C) by lymphocytes in the absence of PHA. Peripheral blood lymphocytes were treated with 1 or 10 µg/ml of the extracts for 48 h and then the supernatants were collected for cytokine measurement by ELISA assay. The positive control (c+) was lymphocytes stimulated with PHA and without extract and the negative control (c−) was without PHA and extract. The asterisks on the graphs show significant difference with the negative control. *p < 0.05, **p < 0.01, ***p < 0.001. The experiments were performed in triplicate and repeated at least three times.
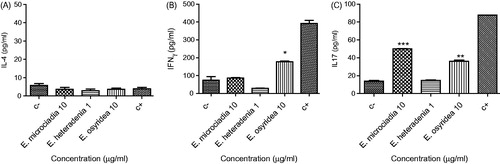
Regarding the effect of these plants on the production of IFN-γ by the lymphocytes, none of the extracts caused a significant change in IFN-γ production in the presence of PHA (). In the absence of PHA, E. microciadia and E. heteradenia had no effect on the production of IFN-γ by the lymphocytes, whereas E. osyridea increased the secretion of this cytokine (178.2 ± 2.6 pg/ml) compared with the negative control (74.2 ± 20.9 pg/ml) ().
As shown in , all three extracts significantly increased the secretion of IL-17 by mitogen-stimulated lymphocytes. Among the extracts, the effect of E. osyridea (602.7 ± 8.6 pg/ml) was stronger than two other plants. indicates that E. microciadia and E. osyridea significantly increased IL-17 release in the absence of PHA; however, E. heteradenia (14.8 ± 0.4 pg/ml) did not have any effect on the production of this cytokine.
All three extracts significantly increased the secretion of IL-10 by mitogen-stimulated lymphocytes. Among the extracts, the effect of E. heteradenia (201 ± 3.3 pg/ml) was stronger than two other plants ().
Discussion
In the present study, the immunomodulatory properties of the extracts of three Euphorbia species that are native to Iran were investigated. The genus Euphorbia is known as “Farfion (Shirsag)” in Persian. The plants E. microciadia, E. heteradenia (Farfioun-e Esfahani) and E. osyridea (Farfioun-e Khashbi) are distributed broadly in the center and south of Iran and less in the north (Mir Heydari, Citation2010; Tyler et al., Citation1998). There are a few reports regarding the chemical compositions and the biological effects of these plants. Several flavonol glycosides from E. microciadia with immunomodulatory activities have been reported. These flavonoids mostly have inhibited lymphocyte proliferation at a high concentration of 50 µg/ml (Ghanadian et al., Citation2012). The crude extract of E. osyridae plant has shown antifungal activity on various phytopathogenic fungi (Abdolmaleki et al., Citation2011).
Stimulation of peripheral blood mononuclear cells under the influence of PHA is a reliable method to study the immunomodulatory effects of the plant extracts. PHA is a mitogen with the ability to stimulate T cells proliferation. In this method, T cells proliferate and the quantity of cell proliferation shows the level of cell activation which is accompanied by the production of various cytokines. As results of this study showed extracts of E. microciadia and E. osyridea at concentrations of 1 µg/ml increased the proliferation of PHA-treated lymphocytes, indicating the immunostimulatory effects of these extracts. Considering this effect, the plants were added to the culture of lymphocytes in the absence of PHA to find their possible mitogenic effects. Euphorbia microciadia extract at concentrations of 1 and 10 µg/ml was able to induce lymphocyte proliferation. Similarly, E. osyridea at 0.1 µg/ml significantly stimulated lymphocyte proliferation in the absence of PHA. These data indicated the mitogenic effect of these plants for human lymphocytes. With respect to E. heteradenia, a slight increased proliferation of the mitogen-activated lymphocytes and also increased cell proliferation in the absence of PHA was observed. The rate of cell proliferation indicated that compared with other plants, E. heteradenia had less proliferative inducing activity. In cytokine assays, E. microciadia and E. osyridea extracts significantly augmented the release of IL-4 by the mitogen-activated lymphocytes whereas no effects on the release of IFN-γ were observed, suggesting the ability of these extracts to deviate immune response toward a Th2 pattern. Increased levels of IL-10 in the culture of mitogen-activated lymphocytes treated with these extracts supported this capability of the plants. In a normal immune response, naive T cells, when encountering an antigen or a mitogen are activated and can differentiate into two types of effector cells called Th1 and Th2. Th1 cells secrete mainly IFN-γ proinflammatory cytokine, while Th2 cells produce mainly IL-4 and IL-10 (Franciotta et al., Citation2003), the key cytokines of anti-inflammatory pathway. IL-4 can act as an anti-inflammatory cytokine in autoimmune diseases by inhibiting Th1 cell formation through driving Th0 cell differentiation towards the Th2 cell type (Li et al., Citation2011). Accordingly, increasing Th2 response is considered to be beneficial in Th1-mediated diseases such as multiple sclerosis. Moreover, in various infections, Th2 response plays an important role in host protection against pathogenic agents, e.g., extracellular bacteria and also some viruses and parasites (Taylor et al., Citation2012).
In recent years, Th17 a new subset of T cells, has been identified (Chen & Oshea, Citation2012). These cells, by producing IL-17 cytokine, have shown significant roles in several types of diseases, ranging from inflammation and autoimmunity to infectious diseases and cancer (Kurebayashi et al., Citation2013; Wilke et al., Citation2011). Although both pathological and protective roles have been explained with respect to the Th17 subset in infectious diseases (Lee et al., Citation2012; Marwaha et al., Citation2012), the role of these cells in host defense against extracellular bacteria and fungi has been documented (Awasthi & Kuchroo, Citation2009). In infections caused by bacteria and fungi, the pathogen induced Th17 response has been considered as an important mediator of protective mucosal host defense (Awasthi & Kuchroo, Citation2009; Hernández-Santos & Gaffen, Citation2012; McGeachy & McSorley, Citation2012). Th17 cells make mucosal barrier function better during infection by release of anti-microbial factors and additional chemokine signaling for neutrophil reinforcements (Hernández-Santos & Gaffen, Citation2012; Rendon & Choudhry, Citation2012). As results of this study showed that the extracts increased IL-17 release from activated lymphocytes, which in conjunction with their Th2 stimulatory effect, may suggest possible usefulness of these extracts in infections in which Th17 and Th2 have a protective role. Furthermore, these data may justify the traditional usefulness of these plants for infectious diseases and may show the underlying mechanism.
Among the extracts, E. heteradenia had no effect on the production of IFN-γ and IL-4, but increased the secretion of IL-10, which is suggestive of its possible immune regulatory effects. IL-10 is essential for the effective function of the regulatory T cell population (Yao et al., Citation2013). Regulatory T cells control immune responses and, therefore, can prevent autoimmune disease (Dennis et al., Citation2013). Indeed further studies to prove such possible effect for E. heteradenia plant are needed.
We also measured the level of cytokines in the absence of PHA to compare the mitogenic effect of the extracts with PHA and find their effects in the absence of previous stimulation. Euphorbia osyridea showed a relatively similar effect to PHA; it did not change IL-4 release but slightly increased IFN-γ and IL-17 production. Euphorbia microciadia increased IL-17 but in contrast to PHA did not have any effects on IFN-γ secretion suggesting its different modes of action compared with PHA. With respect to E. heteradenia, no significant change in the cytokine production was observed suggesting that the proliferation of cells had not reached to a level that influences cytokine secretion.
Taken together, from the data obtained it can be concluded that both E. microciadia and E. osyridea are able to stimulate lymphocyte proliferation and increase IL-4, IL-10, and IL-17 cytokines secretion from T cells without any effect on IFN-γ production, suggesting that these plants have a capability to induce T cells differentiation towards Th2 and Th17 subsets. With respect to E. heterodenia, an increased IL-10 secretion support the conduct of further studies on its possible immunoregulatory effects. Identification of the responsible compounds for the observed effects may help us to clarify the potential usefulness of these plants in certain infections and immune-mediated diseases.
Declaration of interest
The authors report no declaration of interest. We would like to express our specific thanks to the Deputy of Research Affairs of Ahvaz Jundishapur University of Medical Sciences for financial support and also from Shiraz University of Medical Sciences.
Acknowledgements
This study is from MSc thesis of Neda Khosravi (grant no. U-91254).
References
- Abdolmaleki M, Bahraminejad S, Abbasi S. (2011). Antifungal activity of some plant crude extracts on four phytopathogenic fungi. J Med Plants 10:148–55
- Ahmad SF, Bani S, Sultan P, et al. (2013). TNF-α inhibitory effect of Euphorbia hirta in rats. Pharm Biol 51:411–17
- Amirghofran Z. (2010). Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J Immunol 7:65–73
- Amirghofran Z, Azadmehr A, Bahmani M, Javidnia K. (2008). Stimulatory effects of Euphorbia cheiradenia on cell mediated immunity and humoral antibody synthesis. Iran J Immunol 5:115–23
- Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. (2007). Immunomodulatory and apoptotic effects of Stachys obtusicrena on proliferative lymphocytes. Med Sci Monit 13:BR145–50
- Amirghofran Z, Hashemzadeh R, Javidnia K, et al. (2011a). In vitro immunomodulatory effects of extracts from three plants of the Labiatae family and isolation of the active compound(s). J Immunotoxicol 8:265–73
- Amirghofran Z, Malek-Hosseini S, Gholmoghaddam H, Kalalinia F. (2011b). Inhibition of tumor cells growth and stimulation of lymphocytes by Euphorbia species. Immunopharmacol Immunotoxicol 33:34–42
- Awasthi A, Kuchroo VK. (2009). Th17 cells: From precursors to players in inflammation and infection. Int Immunol 21:489–98
- Chen Z, Oshea JJ. (2012). T cells in helminth infection: The regulators and the regulated. Trends Immunol 33:181–9
- Dennis KL, Blatner NR, Gounari F, Khazaie K. (2013). Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol 25:637–45
- Franciotta D, Zardini E, Bergamaschi R, et al. (2003). Interferon γ and interleukin 4 producing T cells in peripheral blood of multiple sclerosis patients undergoing immunomodulatory treatment. J Neurol Neurosurg Psychiatry 74:123–6
- Gao X, Kuo J, Jiang H, et al. (2004). Immunomodulatory activity of curcumin: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol 1:51–61
- Ghanadian SM, Ayatollahi AM, Afsharypour S, et al. (2012). Flavonol glycosides from Euphorbia microsciadia Bioss. with their immunomodulatory activities. Iranian J Pharmaceut Res 11:925–30
- Gharagozloo M, Karimi M, Amirghofran Z. (2013). Immunomodulatory effects of silymarin in patients with β-thalassemia major. Int Immunopharmacol 16:243–7
- Hernández-Santos N, Gaffen SL. (2012). Th17 cells in immunity to Candida albicans. Cell Host Microbe 17:425–35
- Ho CY, Lau C, Kim CF, et al. (2004). Differential effect of Coriolus versicolor (Yunzhi) extract on cytokine production by murine lymphocytes in vitro. Int Immunopharmacol 4:1549–57
- Kang H, Ahn KS, Cho C, Bae HS. (2004). Immunomodulatory effect of Astragali Radix extract on murine TH1/TH2 cell lineage development. Biol Pharm Bull 27:1946–50
- Kumar A, Manjunath C, Thaminzhmani T, et al. (2012). A review on immunomodulatory activity plants. Indian J Novel Drug Deliv 4:93–103
- Kurebayashi Y, Nagai S, Ikejiri A, Koyasu S. (2013). Recent advances in understanding the molecular mechanisms of the development and function of Th17 cells. Genes Cells 18:247–65
- Lee Y, Awasthi A, Yosef N, et al. (2012). Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13:991–9
- Li J, Tu Y, Tong L, et al. (2010). Immunosuppressive activity on the murine immune responses of glycyrol from Glycyrrhiza uralensis via inhibition of calcineurin activity. Pharm Biol 48:1177–84
- Li Z, Zhang Y, Sun B. (2011). Current understanding of Th2 cell differentiation and function. Protein Cell 2:604–11
- Marwaha AK, Leung NJ, McMurchy AN, Megan K. (2012). TH17 cells in autoimmunity and immunodeficiency: Protective or pathogenic? Levings Front Immunol 3:129. doi: 10.3389/fimmu.2012.00129
- McGeachy MJ, McSorley SJ. (2012). Microbial-induced Th17: Superhero or supervillain? J Immunol 189:3285–91
- Mir Heydari H. (2010). Moaref-e Giahi. Daftar-e Nashr-e Farhang-e Eslami. Tehran. (In Persian)
- Nayak S, Mengi S. (2010). Immunostimulant activity of noni (Morinda citrifolia) on T and B lymphocytes. Pharm Biol 48:724–31
- Rendon JL, Choudhry MA. (2012). Th17 cells: Critical mediators of host responses to burn injury and sepsis. J Leukoc Biol 92:529–38
- Taylor MD, van der Werf N, Maizels RM. (2012). T cells in helminth infection: The regulators and the regulated. Trends Immunol 33:181–9
- Tyler E, Brady R, Roberts E. (1998). Pharmacognost. 9th ed. Philadelphia: Lea and Febiger
- Wilke CM, Bishop K, Fox D, Zou W. (2011). Deciphering the role of Th17 cells in human disease. Trends Immunol 32:603–11
- Yao Y, Simard AR, Shi FD, Hao J. (2013). IL-10-producing lymphocytes in inflammatory disease. Int Rev Immunol 32:324–36

