Abstract
Context: Alstonia boonei De Wild (Apocyanaceae) is used in ethnomedicine for the management of malaria, ulcer, rhematic pain, toothache, and inflammatory disorders.
Objective: To investigate the anti-inflammatory potential of β-amyrin and α-amyrin acetate isolated from the stem bark of Alstonia boonei using animal models.
Materials and methods: Chromatographic purification of the crude methanol extract led to the isolation and structure elucidation of β-amyrin and α-amyrin acetate. Their anti-inflammatory activities were evaluated in rodents using egg albumen-induced paw edema and xylene-induced ear edema models. The gastric ulcerogenic, in vivo leucocyte migration, and RBC membrane stabilization tests were also investigated.
Results: α-Amyrin acetate at 100 mg/kg showed significant (p < 0.05) inhibition of egg albumen-induced paw edema with % inhibition of 40 at the 5th hour. Oral administration up to 100 mg/kg did not produce significant (p > 0.01) irritation of the gastric mucosa while significant (p < 0.01) ulceration was recorded for indomethacin at 40 mg/kg compared with the negative control. At 100 μg/mL, both β-amyrin and α-amyrin acetate inhibited heat-induced hemolysis to as much 47.2 and 61.5%, respectively, while diclofenac sodium (100 μg/mL) evoked only 40.5% inhibition. Both compounds at 100 µg/ear produced significant (p < 0.01) inhibition of ear edema in mice by 39.4 and 55.5%, respectively. Also at 100 mg/kg (p.o.) α-amyrin acetate evoked 60.3% reduction in total leucocyte count and significant (p < 0.05) suppression (47.9%) of neutrophil infiltration.
Discussion and conclusion: This study generally provided evidence of profound anti-inflammatory activity of β-amyrin and α-amyrin acetate isolated from the Alstonia boonei stem bark.
Introduction
Alstonia boonei De Wild (Apocyanaceae) is a large deciduous tree, which grows up to 45 m tall. It is widely distributed in Angola, Central African Republic, Ghana, Democratic Republic of Congo, Cote d’Ivoire, and Nigeria (Adotey et al., Citation2012). The plant thrives very well in damp riverbanks. All the plant parts are useful medicinally but the thick bark is the most commonly used for therapeutic purposes (Iyiola et al., Citation2011). It contains important minerals like calcium, phosphorus, iron, sodium, potassium, and magnesium (Akinmoladun et al., Citation2007). An infusion of the stem bark in cold water is drunk as a cure for venereal disease, worms, snakebites, and rheumatic pains and to relax muscles (Orwa et al., Citation2009). The root and stem bark decoctions are used as a remedy for asthma while the stem bark and fruit decoctions are taken daily to treat impotence (Akinmoladun et al., Citation2007). In Nigeria, the bark and the root are used in the treatment of malaria, ulcer, and sores. The bark is also used as an antidote in persons hit by poisoned arrow (Idu et al., Citation2010; Olajide et al., Citation2000). In India, the bark is used for treatment of malaria and chronic diarrhea, while In Cote d’Ivoire, it is used in treating sores and fractures. The bark, leaves, and root are all used to relieve rheumatic pain (Asuzu & Anaga, Citation1991; Iwu, 1993). The bark is used in treating tooth ache and given after childbirth to promote expulsion of placenta (Orwa et al., Citation2009).
The essential oil compositions of the leaf, stem bark, and root have been reported (Moronkola & Kunle, Citation2012). (Z)-9-Octadecenoic acid was found to be the most abundant volatile oil in the leaf and stem bark while methyl (7 E)-7-octadecenoate was most abundant in the root. Alkaloids, triterpenes, and other chemical compounds have been isolated from the plant and have been demonstrated to possess some pharmacological activities (Akinmoladun et al., Citation2007). Echitamine (main alkaloid) and other alkaloids, and the triterpenes β-amyrin, lupenol, and ursolic acid have all been isolated from leaves and stem bark (Adotey et al., Citation2012). Some of the alkaloids isolated from the plant have been shown to possess diuretic, spasmolytic, and hypotensive properties (Ojewole, Citation1984) while the triterpenes demonstrated anti-inflammatory activity (Kweifio-Okai, Citation1991). There is also a report on the formulation of the extract of the stem bark as tablet dosage form (Majekodunmi et al., Citation2008).
In a preliminary fractionation process, we noticed a precipitation of a large quantity of crude triterpenoid fraction from the methanol stem bark extract. This stimulated our interest to carry out further chemical investigation of the precipitate. In this paper, we report the isolation, structure elucidation, and anti-inflammatory effect of two pentacyclic triterpenes, β-amyrin, and α-amyrin acetate, obtained from the stem bark of Alstonia boonei.
Materials and methods
General experimental procedures
NMR spectra (1H, 13C, DEPT, HMQC, and HMBC) were recorded with Bruker ARX 500 NMR spectrometer. MS (EI) was obtained with a Finnigan MAT 8430 mass spectrometer. Analytical HPLC was carried out with a Dionex P580 HPLC system coupled to a photodiode array detector (UVD340S). Routine detection was at 235, 254, 280, and 354 nm. The separation column (125 × 4 mm, length × internal diameter) was prefilled with Eurospher-10 C18 (Knauer, Germany), and a linear gradient of nanopure water (adjusted to pH 2 by addition of formic acid) and methanol was used as an eluent. Silica gel 60 F254 (layer thickness 0.2 mm, E. Merck, Darmstadt, Germany) was used for analytical TLC. Compounds were detected under UV absorbance at 254 nm (fluorescence absorption) and 366 nm (fluorescence), followed by spraying the TLC plates with anisaldehyde-H2SO4 reagent and subsequent heating at 110 °C. Silica gel 60 (mesh 70–230, Merck, Darmstadt, Germany) was used for column chromatography while Sephadex LH-20 (25–100 μm mesh size, Merck) was used for gel chromatography.
Plant material
Fresh stem bark of Alstonia boonei was harvested in December, 2010 with the help of a taxonomist, Mr. Alfred Ozioko of Bioresources Development and Conservation Program, Nsukka, Enugu State, Nigeria, who also authenticated the plant material. A voucher specimen has been deposited at the herbarium of Bioresources Development and Conservation Program. The plant material was air dried for 2 weeks and finally pulverized.
Isolation and purification of the compounds
About 500 g of powdered stem bark of Alstonia boonei was extracted with 2.5 L of methanol by cold maceration at room temperature for 1 week with the solvent changed every 24 h. The combined extracts were evaporated to one-fourth of the volume and kept at room temperature after which a crude precipitate occurred. The crude precipitate was washed in methanol and about 1.5 g was separated on silica gel column eluting with gradient of hexane and ethyl acetate. Fractions were monitored with TLC and similar fractions combined from which compounds 1 (40 mg) and compound 2 (1 g) were obtained. Compound 2 was further purified with Sephadex LH-20 eluting with dichloromethane:methanol (1:1). The purity of the compounds was confirmed by TLC and analytical HPLC. Compounds 1 and 2 were subjected to NMR and mass spectral measurements.
Pharmacological tests
Animals
Adult albino rats (150–200 g) and mice (25–30 g) of both sexes obtained from the animal house of the Department of Pharmacology and Toxicology, Faculty of Pharmaceutical sciences, Nnamdi Azikiwe University, Awka, Nigeria, were used. Animals were housed in institutional facilities under standard conditions (25 ± 2°C and a 12 h light/dark cycle) and maintained on standard pellets and drinking water ad libitum. The use and care of laboratory animals in the study were in accordance with ethical guidelines as contained in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (EEC Directive 86/609/EEC) of 1986.
Egg albumen-induced rat paw edema tests
The test was carried out as previously described by Osadebe and Okoye (Citation2003). The animals (5 per group) were fasted overnight and deprived of water only during the experiment. They were given oral administration of α-amyrin acetate solubilized in 10% Tween 80 at doses of 50 and 100 mg/kg. Control animals received 0.4 mL of 10% Tween 80 or 100 mg/kg aspirin (p.o.); all substances were administered 30 min before the sub-plantar injection of the phlogistic agent (0.1 mL of fresh undiluted egg albumen). Paw volumes were measured by the water displacement method at 0, 1, 2, 3, 4, 5, and 6 h after induction of edema. The anti-inflammatory effect was calculated at each time of observation as percentage inhibition of edema in the animals treated with the test substances in comparison with the vehicle-treated animals. The percentage inhibition of edema was calculated as described by Backhouse et al. (Citation1996) using the following formula:
V0 is the volume of edema of the control (vehicle treated) group and Vt is the volume of edema of the treated group.
Ulcerogenic effects in rats
The method of Cashin et al. (Citation1979) was used. Adult Albino rats of both sexes were fasted for 24 h. After the fasting period, 50 and 100 mg/kg of α-amyrin acetate were administered orally to treatment groups (n = 5, per group). Control animals received either indomethacin 40 mg/kg or equivalent volume of vehicle (10 mL/kg normal saline). Three hours after drug administration, animals were sacrificed, the stomach was removed and cut along the larger curvature and opened to expose the mucosal surface. The mucosa was washed with normal saline and observed with magnifying lens (×10). The ulcer index was determined according to the method described by Main and Whittle (Citation1975).
Membrane stabilization test
The method described by Shinde et al. (Citation1999) was used with little modification. Fresh whole human blood (5 mL) was collected and transferred to an EDTA centrifuge tube. The tube was centrifuged at 2000 rpm for 5 min, and washed three times with equal volume of normal saline. The volume of the blood was measured and reconstituted as a 40% v/v suspension with an isotonic buffer solution (pH 7.4). The composition of the buffer solution (g/l) was NaCl (4.4 g), NaH2PO4 (1.6 g), and Na2HPO4 (7.6 g).
Heat-induced hemolysis
The isotonic buffer solution (5 mL) each containing 50 or 100 µg/mL of the test compound was put in four sets (per concentration) of centrifuge tubes. Control tubes contain vehicle or 5 mL of 100 µg/mL diclofenac sodium. Erythrocyte suspension (0.005 mL) was added to each tube and gently mixed. A pair of the tubes was incubated at 54 °C for 20 min in a regulated water bath. The other pair was maintained at 0–4 °C in a freezer for 20 min. At the end of the incubation, the reaction mixture was centrifuged at 1000 rpm for 3 min and the absorbance of the supernatant measured spectrophotometrically at 540 nm. The percentage inhibition of hemolysis was calculated as follows:
OD1 is the absorbance of test sample unheated, OD2 is the absorbance of test sample heated, and OD3 is the absorbance of control sample heated.
Hypotonicity-induced hemolysis
The hypotonic solution (distilled water, 5 mL) containing 50 or 100 µg/mL of the test compound was put in two pairs (per concentration) of centrifuge tube. Control tubes contain 5 mL of the vehicle or 100 µg/mL diclofenac sodium. Erythrocyte suspension (0.005 mL) was added to each tube and after gentle mixing, the mixture was incubated for 1 h at room temperature (30 °C). At the end of the incubation, the reaction mixture was centrifuged at 1000 rpm for 3 min and the absorbance of the supernatant measured at 540 nm using a spectrophotometer. The percentage inhibition of hemolysis was calculated thus
OD1 is the absorbance of test sample in isotonic solution, OD2 is the absorbance of test sample in hypotonic solution, OD3 is the absorbance of control sample in hypotonic solution.
Xylene-induced mouse ear topical edema tests
The effect of the test compound on acute topical inflammation was evaluated by a modification of a previously reported method (Okoye et al., Citation2010). Adult Swiss albino mice were divided into four groups of five animals each. The treatment groups received 50 and 100 µg/ear applied on the anterior surface of the right ear. Topical inflammation was instantly induced on the posterior surface of the same ear by application of xylene (0.05 mL). Control animals received either the vehicle (normal saline) or indomethacin (100 µg/ear). Two hours after induction of inflammation, mice were killed by overdose of chloroform anesthesia and both ears removed. Circular sections (7 mm diameter) of both the right (treated) and left (untreated) ears were punched out using a cork borer, and weighed. Edema was quantified as the weight difference between the two earplugs. The anti-inflammatory activity was evaluated as percent edema reduction/inhibition in the treated animals relative to control animals (Okoye et al., Citation2010) using the following relation:
where Rt is the mean weight of right ear plug of treated animals, Lt is the mean weight of left ear plug of treated animals, Rc is the mean weight of right ear plug of control animals, Lc is the mean weight of left earplug of control animals.
In vivo leucocytes migration test
The effect of the test compound on cell migration in vivo was evaluated in albino rats using the method described by Ribeiro et al. (Citation1991). One hour after oral administration of 50 and 100 mg/kg of the test compound, the animals received intraperitoneal injection of 1 mL of 3%, w/v agar suspension in normal saline. Four hours later, the animals were killed and the peritoneal cavities washed with 5 mL of phosphate buffer saline containing 0.5 mL of 10% EDTA. The peritoneal fluid was recovered and the total and differential leucocytes counts (TLC and DLC) were performed on the perfusates.
Statistical analysis
The results were analyzed using SPSS version 16 (SPSS Inc., Chicago, IL) and presented as mean ± SEM. Significance between the control and the treated group were determined using Student's t-test and a one way ANOVA. Significant differences between mean were considered at p < 0.05 and p < 0.01.
Results and discussion
Alstonia boonei stem bark was extracted with methanol and the extract concentrated to one-fourth the volume and allowed to stand at room temperature after which crude triterpenoid mixture precipitated. The precipitate was washed with methanol and subjected to silica gel column chromatography and final purification with Sephadex LH-20 to obtain compounds 1 and 2 ().
Compound 1 (40 mg, representing 0.008% of starting material) was obtained as white powder. The mass spectrum showed a molecular ion peak at m/z 426, which corresponded to a molecular formula C30H50O. The formula showed six double bond equivalents, five of which were adjusted in a pentacyclic carbon framework and the remainder in a CC double bond. The mass spectrum also showed fragments at 219 and 203 generated due to a retro-Diels Alder fragmentation indicative of the presence of double bond at position 12 in ring C (Budzikiewicz, Citation1964; Juang et al., Citation1989). The NMR spectrum showed the presence of eight methyl singlets, one olefenic proton at δH 5.16 t (J = 3.5 Hz), and an oxygenated proton at δH 3.20 dd (J = 4.4, 11.5), all suggestive of olealane type triterpenoid. All the proton and carbon signals were assigned based on the 1H1HCOSY, DEPT analysis and HMQC and HMBC as shown in . Compound 1 was elucidated as 3β-hydroxylolean-12-ene (β-amyrin) () and the spectral data compared well with those previously reported (Dias et al., Citation2011; Oliveira et al., Citation2010). Compound 2 (800 mg, representing 0.16% of starting material) was obtained as a white powder. The mass spectrum showed a molecular ion peak at m/z 468, which corresponded to a molecular formula of C32H52O2. The formula showed seven double bond equivalents, five of which were adjusted in a pentacyclic carbon framework and the remainder in CC and CO double bonds. Similar to 1, the mass spectrum also showed fragments at 219 and 203 generated due to a retro-Diels Alder fragmentation indicative of the presence of double bond at position 12 in ring C (Budzikiewicz, Citation1964; Juang et al., Citation1989). The NMR spectrum of 2, however, showed the presence seven methyl singlets and two methyl doublets. One of the methyl singlets, which appeared downfield at δH 2.02 s, is indicative of the presence of acetate moiety, and this was confirmed by the presence of carbonyl carbon at δC 171.5. The presence of two methyl doublets is indicative of ursane-type triterpenoid. The compound also showed one olefenic proton at δH 5.10 t (3.6) assigned to H-12 and an oxygenated proton at δH 4.48 m assigned to H-3. The downfield shift of the oxygenated proton suggested the attachment of the acetate moiety at position 3. The attachment was also confirmed by correlation of H-3 with carbonyl carbon at δC 171.5 in HMBC. Similarly, all the proton and carbon signals were assigned based on the 1H1HCOSY, DEPT analysis, and HMQC and HMBC data as shown in . Compound 2 was thus identified as 3β-acetoxyurs-12-ene (α-amyrin acetate) () and the spectral data compared favorably well with those previously reported (Ali, Citation2013; Dias et al., Citation2011; Manguro et al., Citation2009; Sob et al., Citation2010). Both compounds were used for the xylene-induced mouse ear edema test and membrane stabilization effect, while only compound 2 (α-amyrin acetate) was used for the egg albumen-induced rat paw edema test, ulcerogenic effect and effect on in vivo leukocyte migration.
Table 1. NMR spectroscopic data of 1 (β-amyrin) and 2 (α-amyrin acetate).
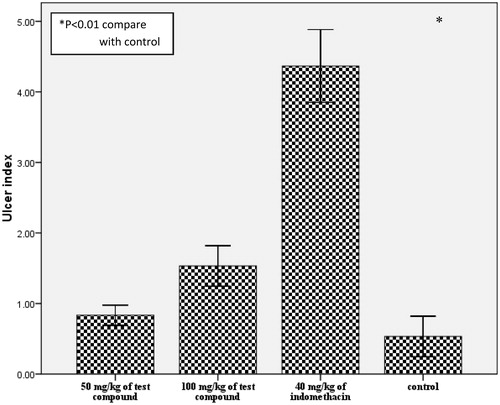
Compound 2 at 100 mg/kg showed significant (p < 0.05) reduction in paw edema from the 5th hour while aspirin recorded significant (p < 0.05) activity from the 2nd hour untill the 6th hour () in the egg albumen-induced rat paw edema test. Prostaglandin-induced vascular permeability has been associated with late phase inflammatory mediator response in acute inflammation (Corea et al., Citation2005). The significant inhibition of the later phase of acute inflammation by this compound suggests the involvement of inhibition of prostaglandins possibly via inhibition of cycloxygenase (COX) enzyme activity. The isoforms of cyclooxygenase enzyme (COX1 and COX2) contribute to both the toxicity and the activity of most anti-inflammatory agents (Mitchell et al., Citation1994). Non-selective inhibition of cycloxygenase enzyme is a major setback of most traditional NSAID and anti-inflammatory drugs (Valiollah et al., Citation2009). COX-2 is implicated in inflammation while COX-1 helps in the maintenance of gastric mucosal integrity (Mitchell et al., Citation1994). We carried out a simple ulcerogenic activity test to ascertain the involvement of cyclooxygenase in the anti-inflammatory activity of the compound. The compound at 50 and 100 mg/kg did not evoke significant (p > 0.01) irritation compared with the control (), while indomethacin at 40 mg/kg evoked significant (p < 0.01) irritation of the gastric mucosa. This result suggests the involvement of a different mechanism of anti-inflammatory activity, although a reduction of COX-2 expression was observed in a previous study (Victor et al., Citation2009). Moreover, the observed reduced GIT side effect could offer some advantage in the management of chronic inflammation.
Figure 2. Effect of compound 2 on the development of acute inflammation after sub-plantar injection of egg albumen in rats. Animals received 50 and 100 mg/kg (p.o.) of test compounds. Treatment animals (n = 5) were compared with control animals (n = 5) which had received vehicle only.
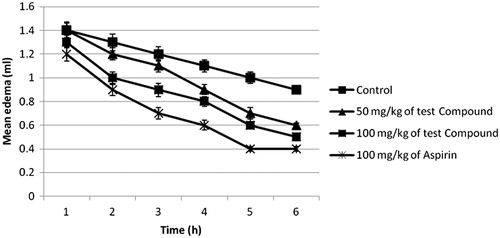
Figure 3. Effect of compound 2 on gastrointestinal irritation in rats. Animals received 40, 50, or 100 mg/kg (p.o.) of test compounds. Treatment animals were compared with control animals which had received vehicle only.
The methanol extract of the stem bark of A. boonei has been previously reported to cause significant (p < 0.05) inhibition of carrageenan-induced paw edema and cotton pellet granuloma in rats (Kweifio-Okai, Citation1991). Also, α- and β-amyrin acetate, β-amyrin, and lupeol acetate isolated from the petroleum ether extract of A. boonei de Wild root bark were demonstrated to exhibit activity against complete Freund’s adjuvant-induced arthritis (Kweifio-Okai & Carrol, Citation1993). Other studies have also supported the relatively strong and rapid onset of the systemic anti-inflammatory property for these plant triterpenes (Otuki et al., Citation2005a,Citationb; Recio et al., Citation1995)
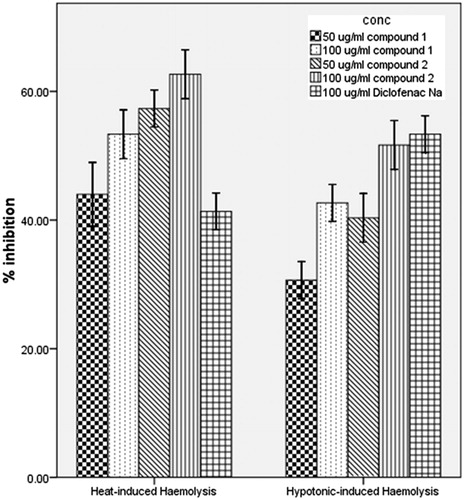
To further delineate the probable mechanism of anti-inflammatory activity of the compounds, we investigated their effects on the integrity of the membrane of human red blood cell (RBC) in vitro. Reports have shown that the structure of human RBC is similar to that of lysosomal membrane components (Weissmann, Citation1967). The release of inflammatory mediators involves the degranulation of these sub-cellular organelles and as such the stabilization of their membranes will diminish inflammatory process (Okoye et al., Citation2010; Weissmann, Citation1967). The compounds inhibited both the heat and the hypotonicity-induced hemolysis of human RBC (). At all the experimental doses, compounds 1 and 2 showed more inhibition of heat-induced hemolysis than diclofenac sodium. However, diclofenac sodium showed more protection against hypotonic induced hemolysis. The hemolytic effect of heat and hypotonic solution are related to membrane denaturation by high temperature and excessive accumulation of fluid within the cell, respectively, resulting in the rupture of the cell membrane (Umukoro & Ashorobi, Citation2006). The ability of these compounds to offer protection against hemolysis of human RBC membrane induced by both the heat and the hypotonic medium is an evidence of their membrane stabilizing property. Compounds with membrane stabilizing properties are known to interfere with the release of cytoplasmic contents like phospholipase A2 (Umukoro & Ashorobi, Citation2006) that trigger the formation of inflammatory mediators.
Figure 4. Effect of the test compounds on heat-induced and hypotonicity-induced hemolysis of human erythrocytes in vitro. Erythrocyte membrane stabilization was determined at drug concentrations of 50 and 100 μg/mL in isotonic phosphate buffer, pH 7. Treatment animals were compared with control animals which had received vehicle only.
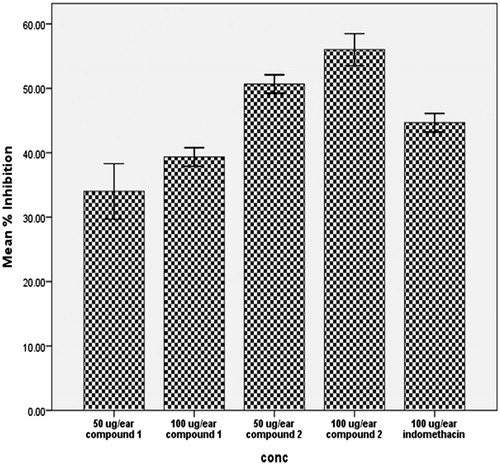
We also evaluated the potential of the isolated compounds as topical anti-inflammatory agents using xylene-induced ear edema in mice. Xylene-induced ear inflammation is a recognized method for assay of acute topical inflammation. Compounds 1 and 2 significantly (p < 0.01) inhibited xylene-induced ear edema in mice at 50 and 100 µg/ear (). At 100 µg/ear, compound 2 showed 59.4% inhibition while indomethacin showed 44.7% inhibition. This finding corroborated the pronounced topical anti-inflammatory properties of α-amyrin against 12-O-tetradecanoylphorbol-acetate-induced ear edema previously reported (Otuki et al., Citation2005b). Myeloperoxidase (MPO) enzymatic activity has been established to be elevated in xylene-induced inflammation (Ravelo-Calzado et al., Citation2011) with corresponding increase in prostaglandin-E2, a powerful vasodilator that together with other inflammatory mediators contribute to redness and increased blood flow in areas of acute inflammation (Foyet et al., Citation2011). There is direct relationship between MPO activity and leucocyte migration in inflamed tissues (Jyothibasu et al., Citation2011). Inhibition of xylene-induced topical inflammation could be as a result of reduction in MPO activity leading to decreased vasodilation and leucocyte migration. We, therefore, investigated the effect of the compounds on total and differential leucocyte migration in vivo.
Figure 5. Effect of the test compounds on xylene-induced acute topical edema of the mouse ear. The test compounds were applied topically at 50 and 100 μg/ear. Treatment animals were compared with control animals which had received 5 μL/ear of vehicle.
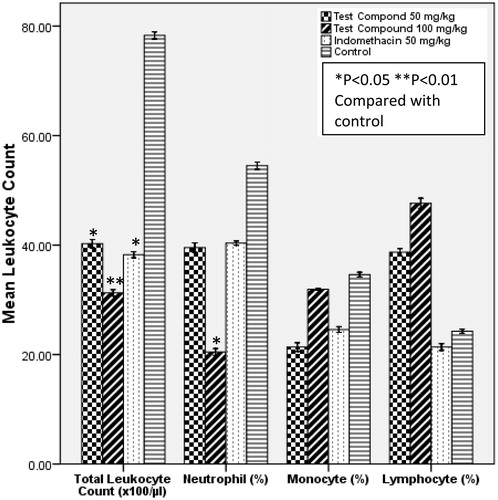
At 100 mg/kg, compound 2 produced significant (p < 0.01) inhibition of leucocyte migration and neutrophil infiltration compared with the control (). The recruitment of leucocyte in response to inflammation is an important step in phagocytosis. Leukocyte extravasation is usually mediated by prostaglandins, bradykinin and histamine which increase capillary permeability (Rang et al., Citation2003). Neutrophils are the first leucocyte to respond in inflammatory conditions (Anosike et al., Citation2012). Their interaction with other inflammatory mediators leads to the production of cytokins, degrading enzymes, and reactive species that may further amplify the inflammatory response (Tanaka et al., Citation2006). The decreased responsiveness of leucocytes and in particular neutrophils to the chemotactic stimulus induced by agar following oral administration of compound 2 may have in part been accounted for by inhibition of these mediators (prostaglandins, bradykinin, and histamine).
Conclusion
The present study generally provided evidence of strong anti-inflammatory activity of the isolated triterpenoids. One of these compounds, α-amyrin acetate, was isolated in very large amount and may account for the efficacy of Alstonia boonei stem bark extracts in ethnomedicinal management of toothache, rhematic pain, and other inflammatory related disorders. Previous studies (Medeiros et al., Citation2007; Victor et al., Citation2009) proposed inhibition of nuclear factor kappa-B and cAMP response element-binding protein activation as the main mechanism through which these triterpenes exert their anti-inflammatory action, along with a reduction of COX-2 expression. Our findings, however, could support a combination of membrane stabilization and inhibition of leukocyte migration as the probable mechanisms of anti-inflammatory activity of the isolated triterpenes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are grateful to the Institut für Pharmazeutische Biologie, Universität Düsseldorf, Germany, for providing the facilities for structure elucidation of the isolated compounds.
References
- Adotey JPK, Adukpo GE, Boahen YO, Armah FA. (2012). A review of the ethnobotany and pharmacological importance of Alstonia boonei De Wild (Apocynaceae). Int Scholar Res Net 1:1–9
- Akinmoladun AC, Ibukun EO, Afor E, et al. (2007). Chemical constituents and antioxidant activity of Alstonia boonei. Afri J Biotech 6:1197–201
- Ali N. (2013). Brine shrimp cytotoxicity of crude methanol extract and antispasmodic activity of α-amyrin acetate from Tylophora hirsuta Wall. BMC Comp Alt Med 13:135
- Anosike CA, Obidoa O, Ezeanyika LUS. (2012). Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum acthiopicum). DARU J Pharm Sci 20:1–7
- Asuzu IU, Anaga AO. (1991). Pharmacological screening of the aqueous extract of Alstonia boonei stem bark. Fitoterapia 63:411–17
- Backhouse N, Delporte C, Negrete R, et al. (1996). Anti-inflammatory and antipyretic activities of Cuscuta chilensis, Cestrum parqui, and Psolarea glandulosa. Int J Pharmacog 34:53–7
- Budzikiewicz H, Djerassi C, Williams DH. (1964). Structure Elucidation of Natural Products by Mass Spectrometry, Vol II: Steroids, Terpenoids, Sugars, and Miscellaneous Classes. San Francisco: Holden Day Publisher, 50–63
- Cashin CH, Dawson W, Kitchen EA. (1979). The pharmacology of benoxaprofen (2-4-chlorophenyl-methyl-5-benzoxazole acetic acid) LRC.L3694, a new compound with anti-inflammatory activity apparently unrelated to inhibition of prostaglandin synthetase. J Pharm Pharmacol 29:330–6
- Corea G, Fattorusso E, lanzotti V, et al. (2005). Discovery and biological evaluation of the novel naturally occurring diterpene pepluanone as anti-inflammatory agent. J Med Chem 48:7055–62
- Dias MM, Hamerski L, Pinto A. (2011). Separacao semipreparative de α and β-amyrina por cromatografia líquida de alta eficiencia. Qumica Nova 34:704–6
- Foyet HS, Abdou BA, Ponka R, et al. (2011). Effect of Hibiscus asper leaves extracts on carrageenan induced oedema and complete Freund’s adjuvant induced arthritis in rats. J Cell Anim Biol 5:69–75
- Idu MD, Erhabor OJ, Efijuemue MH. (2010). Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop J Pharm Res 9:110–18
- Iwu MM. (1993). Handbook of African Medicinal Plants. Boca Raton, (FL): CRC Press
- Iyiola OA, Tijani AY, Lateef KM. (2011). Antimalaria activity of ethanol stem bark extract of Alstonia boonei in mice. Asia J Biol Sci 4:235–43
- Juang JK, Huang HW, Chen CM, Liu HJ. (1989). A new compound, with angulatin A, promotes type II DNA topoisomerase-mediated DNA damage. Biochem Biophys Res Commun 159:1128–34
- Jyothibasu T, Ramana KV, Yuvaray G, et al. (2011). Biological evaluation of anti-inflammatory effect of Physalis minima. Asian J Biochem Pharm Res 3:581–9
- Kweifio-okai G. (1991). Anti-inflammatory activity of a Ghanaian antiarthritic herbal preparation. J Ethnopharmacol 33:263–7
- Kweifio-Okai G, Carrol AR. (1993). Antiarthritic effect of lupeol acetate. Phytother Res 7:213–15
- Main IHM, Whittle NB. (1975). Investigation of vasodilator and anti-secretary role of prostaglandin in the rat mucosa by use of NSAIDs. Br J Pharmacol 53:217–24
- Majekodunmi SO, Adegoke OA, Odeku OA. (2008). Formulation of the extract of the stem bark of Alstonia boonei as tablet dosage form. Trop J Pharm Res 7:987–94
- Manguro LOA, Opiyo SA, Herdtweck E, Lemmen P. (2009). Triterpenes of Commiphora holtziana oleo-gum resin. Can J Chem 87:1173–9
- Medeiros R, Otuki MF, Avellar MC, Calixto JB. (2007). Mechanisms underlying the inhibitory actions of the pentacyclic triterpene alpha-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Eur J Pharmacol 559:227–35
- Mitchell JA, Akarasereenont P, Thiemermann C, et al. (1994). Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natt Acad Sci USA 90:11693–7
- Moronkola DO, Kunle OF. (2012). Essential oil compositions of leaf, stem bark and root of Alsonia boonei de Wild (Apocyanaceae). Int J Biol Pharm Res 3:51–60
- Ojewole JAO. (1984). Studies on the pharmacology of echitamine, an alkaloid from the stem bark of Alstonia boonei (Apocynaceae). Int J Crude Drug Res 22:121–43
- Okoye FBC, Osadebe PO, Proksch P, et al. (2010). Anti-inflammatory and membrane-stabilizing stigmastane steroids from Alchornea floribunda leaves. Planta Med 76:172–7
- Olajide OA, Awe SO, Makinde JM, et al. (2000). Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol 71:179–86
- Oliveira AP, Silva LR, Andrade PB, et al. (2010). Further insight into the látex metabolite profile of Ficus carica. J Agri Food Chem 58:10855–63
- Orwa C, Mutua A, Kindt R, et al. (2009). Agroforest free Database: A tree reference and section guide version 4.0. Available from: http//www.worldagroforestry.org/af/treedb/ [last accessed 25 Nov 2013]
- Osadebe PO, Okoye FBC. (2003). Anti-inflammatory effects of crude methanol extracts and fractions of Alchornea cordifolia leaves. J Ethnopharmacol 89:19–24
- Otuki MF, Ferreira J, Lima FV, et al. (2005a). Antinociceptive properties of mixture of alpha-amyrin and beta-amyrin triterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J Pharmacol Exp Ther 313:310–18
- Otuki MF, Vieira-Lima F, Malheiros A, et al. (2005b). Topical antiinflammatory effects of the ether extract from Protium kleinii and alpha-amyrin pentacyclic triterpene. Eur J Pharmacol 507:253–9
- Rang HP, Dale MM, Ritter JM, Moore PK. (2003). Pharmacology, 5th ed. London: Churchill Livingstone
- Ravelo-Calzado YV, Molina-Cuevas S, Jimenez-Despaine Y, et al. (2011). Effects of D-002 on xylene-induced oedema in ear mice. Revista CENIC Sci 42:13–16
- Recio MC, Giner RM, Manez S, Rios JL. (1995). Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med 61:182–5
- Ribeiro RA, Flores CA, Cunha FQ, Ferreira SH. (1991). IL-8 causes in vivo neutrophil migration by a cell dependent mechanism. Immunology 73:472–7
- Shinde UA, Phadke AS, Nair AM, et al. (1999). Membrane stabilizing activity a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70:251–7
- Sob SVT, Wabbo K, Tchinda HK, et al. (2010). Anthraquinones, sterols, triterpeniods and xanthones from Cassia obtusifolia. Biochem Syst Ecol 38:342–5
- Tanaka D, Kagari T, Doi H, Shimozato T. (2006). Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide induced arthritis. Immunology 119:195–202
- Umukoro S, Ashorobi RB. (2006). Evaluation of anti-inflammatory and membrane stabilizing property of aqueous leaf extract of Momordica charantia in rats. Afr J Biomed Res 9:119–24
- Valiollah H, Seyed ES, Mojtaba H. (2009). Anti-inflammatory and analgesic properties of Heradeum persicum essential oil and hydroalcoholic extract in animal models. J Ethnopharmacol 124:475–80
- Victor CE, Figueiredo CP, Hara DB, et al. (2009). Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, α- and β-amyrin, in a mouse model of colitis. Br J Pharmacol 157:1034–44
- Weissmann G. (1967). The role of lysosomes in inflammation and disease. Annu Rev Med 18:97–112