Abstract
Context: Parthenolide (a sesquiterpene lactone), a bioactive compound of Tanacetum parthenium (L.) Schultz Bip. (Asteraceae) herb, has been reported for antioxidant and anticancer activities.
Objective: The present study evaluated the effect of parthenolide on growth and apoptosis-regulatory genes of human cervical cancer (SiHa) and breast cancer (MCF-7) cell lines.
Materials and methods: The cytotoxic activity of parthenolide (3.5–21 µM) was examined by MTT and LDH assays at 24 and 48 h time intervals. Apoptotic activity was evaluated by expression analysis of multiple apoptosis-regulatory genes (i.e., p53, Bcl-2, Bax, caspase-3, -6, and -9) by reverse transcriptase-PCR and DNA fragmentation assay.
Results: Parthenolide inhibited the growth of SiHa and MCF-7 cell lines in a concentration-dependent manner at 24 and 48 h time intervals (p < 0.001). The IC50 value of parthenolide against SiHa and MCF-7 cells were 8.42 ± 0.76 and 9.54 ± 0.82 μM, respectively. Parthenolide-treated cells showed up-regulation of p53, Bax, caspase-3, -6, and -3 genes and down-regulation of Bcl-2 gene (p ≤ 0.008). At IC50, the p53 gene was up-regulated by 9.67- and 3.15-fold in SiHa and MCF-7 cells, respectively. The Bax to Bcl-2 ratio was 3.4 and 2.3 for SiHa and MCF-7 cells, respectively. Also, the fragmented genomic DNA in parthenolide-treated cells showed the signs of apoptosis.
Conclusion: Our study endorsed the biological activity of parthenolide and demonstrated the parthenolide-induced growth inhibition and apoptosis in SiHa and MCF-7 cells by modulating the expression of apoptosis-regulatory genes.
Introduction
Parthenolide (a sesquiterpene lactone) is a bioactive compound of Tanacetum parthenium (L.) Schultz Bip. (Asteraceae), commonly known as feverfew herb (Cretnik et al., Citation2005; Hamedi et al., Citation2013). Various biological activities, like antioxidant, anticancer, antimicrobial, and anti-inflammatory properties of T. parthenium, are attributed to the presence of parthenolide (Jain & Kulkarni, Citation1999; Kwok et al., Citation2001; Lesiak et al., Citation2010; Mohsenzadeh et al., Citation2011; Wu et al., Citation2006). Also, parthenolide has been reported for its anti-inflammatory, antileishmanial, antifungal, antioxidant, and anticancer properties (Hehner et al., Citation1999; Herrera et al., Citation2005; Mathema et al., Citation2012; Tiuman et al., Citation2005). The pharmacological properties of parthenolide are mostly due to α-methylene-γ-lactone ring and epoxide, which interact with nucleophilic sites of various biological molecules (Koprowska & Czyz, Citation2010).
Parthenolide promotes apoptosis in neoplastic cells by inducing oxidative stress and by inhibiting the cancer promoting transcription factor, nuclear factor kappa B (NFκB) (Bork et al., Citation1997). Parthenolide reduces the cellular level of glutathione and other thiol groups present in the cancer cells, which result in the accumulation of reactive oxygen species (ROS) and activation of caspases and ultimately apoptosis in hepatocellular carcinoma cells (Wen et al., Citation2002). Also, parthenolide inhibited NFκB DNA binding in prostate cancer cells (Sweeney et al., Citation2004). Additionally, parthenolide decreases the NFκB DNA binding by inhibiting the IκB kinase and by preventing p65 protein binding to DNA (Hehner et al., Citation1998; Sobota et al., Citation2000). It has been reported that the inhibition of NFκB leads to reduced expression of anti-apoptotic proteins in addition to tumor necrosis factor receptor-associated factor (TRAF)-1 and TRAF-2 (Sweeney et al., Citation2004). The downstream consequences of these effects lead to activation of p53 and caspases, which results in cell-cycle arrest and ultimately cell death (Gopal et al., Citation2009; Mendonca et al., Citation2007).
The process of apoptosis is regulated by differential expression of various apoptotic genes and their proteins. Bcl-2 is an anti-apoptotic gene that protects the cell death by inhibiting the apoptosis pathway (Pearson et al., Citation2000). In contrast, Bax gene is a pro-apoptotic regulator of apoptosis (Sedlak et al., Citation1995), whereas p53 regulates the apoptosis pathway by interacting with genes of the Bcl-2 family (Marin et al., Citation1994). It has been reported that p53 up-regulates Bax gene expression via direct transcriptional activation of the Bax gene promoter and concomitant down-regulation of Bcl-2 gene expression (Pearson et al., Citation2000). Caspase-9 initiates the cascade of apoptosis after release of mitochondrial cytochrom-C, whereas caspase-3 and -6 are downstream caspases which play a pivotal role in the terminal phase of apoptosis (Parrish et al., Citation2013; Slee et al., Citation2001). Hence, the present study was undertaken to evaluate the effect of parthenolide on growth and p53, Bax, Bcl-2, caspase-3, -6, and -9 gene regulation in cervical cancer (SiHa) and breast cancer (MCF-7) cells. For the first time we have examined the effect of parthenolide against SiHa and MCF-7 cells.
Materials and methods
Reagent and chemicals
Parthenolide (purity ≥ 98% HPLC) was purchased from Sigma-Aldrich (St. Louis, MO). Various tissue culture media components were purchased from HiMedia (Mumbai, India). All chemicals and solvents were of analytical grade and purchased from Merck (Mumbai, India).
Cell culture
Human cervical cancer (SiHa) and breast cancer (MCF-7) cell lines were obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Cells were grown as a monolayer in Dulbecco's Modified Eagle's Medium (DMEM) [supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 100 mg/L streptomycin)] at 37 °C in a humidified atmosphere of 5% CO2.
MTT assay
Cytotoxicity was evaluated by the MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide) assay as described by Mehdi et al. (Citation2011). The SiHa and MCF-7 cells (∼2 × 104 per well) were seeded overnight in a flat bottom 96-well plate (HiMedia, India) and incubated at 37 °C in a humidified air atmosphere enriched with 5% (v/v) CO2. Both SiHa and MCF-7 cells were treated with various concentrations of parthenolide ranging between 3.5 and 21 µM for 24 and 48 h time points. Afterwards, the culture medium was replaced with fresh medium and 20 µL of MTT (5 mg/mL in PBS) was added to it and kept at 37 °C for 4 h. Formazan crystals formed in live cells by mitochondrial reduction of MTT were solubilized in DMSO (200 µL/well) and the absorbance was measured at 570 nm on iMark Microplate Reader (Bio-Rad, Hercules, CA). All cytotoxicity assays were performed in triplicate and the percentage of cell survival was calculated using the following formula:
The mean percentage [± standard error of mean (SEM)] cell survival was plotted against the corresponding parthenolide concentration and the “best fit” was employed to derive the IC50 value.
Lactate dehydrogenase enzyme leakage assay
For lactate dehydrogenase enzyme (LDH) leakage assay, SiHa and MCF-7 cells (∼2 × 104 per well) were seeded overnight in a flat bottom 96-well plate (HiMedia, India) and incubated at 37 °C in a humidified air atmosphere enriched with 5% (v/v) CO2. Various concentrations of parthenolide ranging from 3.5 to 21 µM were used to treat the cells for 24 and 48 h. All parthenolide concentrations were tested on both the cells in triplicate. The treated cells were centrifuged at 3000 rpm for 5 min at 4 °C. The cell free medium was used for the quantification of LDH enzyme following the commercially available Cytoscan™-LDH assay kit (G-Biosciences, Maryland Heights, MO) protocol. The absorbance of the reaction mixture was measured at 490 nm on the iMark Microplate Reader (Bio-Rad, Hercules, CA). The assay was performed in triplicate and the percent cytotoxicity was calculated as
Reverse transcriptase-polymerase chain reaction
The mRNA expression of apoptosis regulatory genes were examined after treating both the cell lines with different concentrations (4.5–11.5 µM) of parthenolide for 48 h. Treated and untreated SiHa and MCF-7 cells were harvested and washed with phosphate buffer saline (PBS) at 4 °C. Total RNA was extracted using a TRIZOL reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instruction. RNA preparations were analyzed by agarose gel 1.8 (w/v) electrophoresis and found to be free of DNA contamination. Total RNA (1 μg) was used for cDNA synthesis using a RevertAidTM fisrt stranded cDNA synthesis Kit (Fermentas Life Science, Rockford, IL) with random hexamers. cDNA was used for the detection of mRNA expression of p53, Bcl-2, Bax, caspase-3, -6, and -9 genes using specific oligonucleotide primers (). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. The volume of PCR mixture was 25 µL containing 2 μL of cDNA, 1U taq DNA polymerase, 1.5 mM MgCl2, 0.2 mM dNTP, and 20 pmole of each gene-specific oligonucleotide primer. The PCR reaction conditions were denaturation at 94 °C for 30 s, annealing at 52.7 °C, 56.3 °C, 65.4 °C, 54.6 °C, 49.3 °C, 52.6 °C, and 55.2 °C for GAPDH, p35, Bcl-2, Bax, caspase-3, -6, and -9, respectively, for 30 s, and extension at 72 °C for 30 s (Eppendorf, Norwalk, CT). The amplified products were checked on 2% agarose gel and documented on the Gel-doc system (Bio-Rad, Hercules, CA).
Table 1. Oligonucleotide primer sequences.
DNA fragmentation assay
In order to perform DNA fragmentation assay, parthenolide (at IC50 concentration for 48 h) treated SiHa and MCF-7 cells were harvested and washed with PBS at 4 °C. The cell pellets were used for genomic DNA fragmentation assay according to the commercially available DNA Ladder Assay Kit (G-Biosciences, Maryland Heights, MO). The fragmented DNA was analyzed on 1.8% (w/v) agarose gel and documented using the Gel Doc system (Bio-Rad, Hercules, CA).
Statistical analysis
The mean value ± standard error of mean (SEM) was calculated from the samples (triplicate) for each experimental group. The statistical significance was determined with analysis of variance (ANOVA) test and statistical significance level was maintained at p ≤ 0.05.
Results
Cytotoxicity assays
MTT assays were performed for the screening of cytotoxicity at various concentrations of parthenolide. The assay results showed a dose-dependent decrease in viability of SiHa and MCF-7 cells at 24 and 48 h time points (p < 0.001) (). The IC50 values (evaluated after 48 h) of parthenolide against SiHa and MCF-7 cells were 8.42 ± 0.76 and 9.54 ± 0.82 µM, respectively (p ≤ 0.05). Whereas 24 h parthenolide-treated SiHa and MCF-7 cells exhibited 30.8 and 28.03% cytotoxicity at the IC50 concentrations, respectively.
Figure 1. Cytotoxicity of parthenolide against MCF-7 and SiHa cells evaluated by MTT and LDH assays.
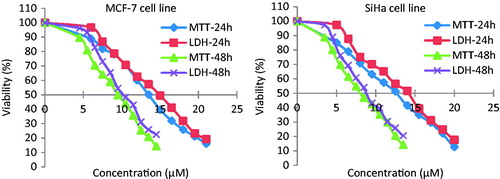
Likewise, the LDH enzyme leakage assay gave a positive response in both the cell lines after treating with different concentrations of parthenolide. The cytotoxic activity profile of parthenolide against SiHa and MCF-7 cells evaluated by MTT and LDH assays were significantly correlated at 24 and 48 h time points (r ≥ 0.983). However, the LDH assay demonstrated less toxicity against SiHa (0.8–9.1%) and MCF-7 (4.5–12.5%) cells at parthenolide concentrations ranging between 3.5 and 21 µM. Interestingly, both bioactivity assays showed parthenolide toxicity against SiHa and MCF-7 cell lines, despite their different working principles. As in the MTT assay, only metabolically active cells reduce MTT salt to purple formazan by mitochondrial succinate dehydrogenase enzyme, whereas, in LDH assay, the LDH enzyme is released into the culture medium after disruption of cell membrane integrity.
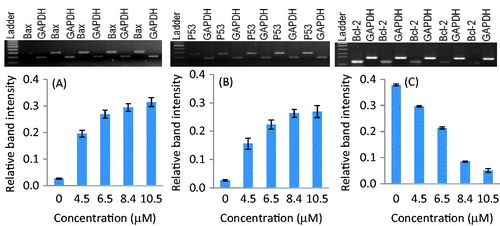
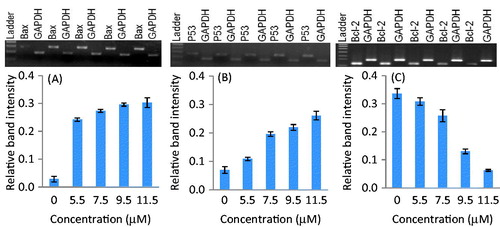
Expression analysis of Bcl-2, Bax, and p53 genes
Reverse transcriptase-PCR was performed to study the expression analysis of Bcl-2, Bax, and p53 genes in SiHa and MCF-7 cells. The changes in mRNA expression levels were standardized by GAPDH expression. Densitometry analysis revealed the relative mRNA band intensity on the gel-doc system (Bio-Rad, Hercules, CA). The treatments of parthenolide up-regulated the p53 gene in a concentration-dependent manner in both the cell lines ( and ). At the IC50 of parthenolide, the p53 gene mRNA relative band intensity increased by 9.67- and 3.15-fold in SiHa and MCF-7 cells, respectively. Similarly, parthenolide-treated SiHa and MCF-7 cells showed down-regulation of Bcl-2, up-regulation of Bax, and increased Bax to Bcl-2 ratio in a dose-dependent manner (p ≤ 0.006) ( and ). A densitometry study demonstrated that Bcl-2 gene was up-regulated in SiHa- and MCF-7-untreated cells, whereas down-regulated in parthenolide-treated SiHa and MCF-7 cells. At the IC50, the Bax to Bcl-2 ratio for SiHa cells was estimated to be 3.4 whereas, for MCF-7 cells it was 2.3.
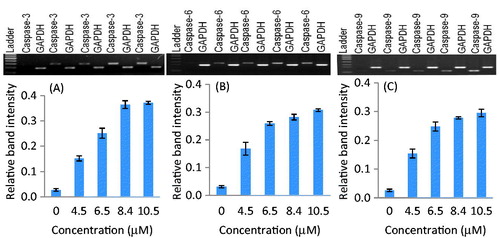
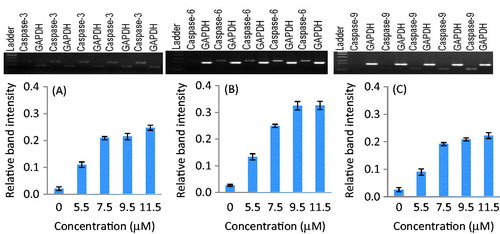
Expression analysis of caspase genes
The apoptosis activity in parthenolide-treated SiHa and MCF-7 cells was determined by the expression analysis of caspase genes. The results showed that parthenolide-treated SiHa and MCF-7 cells exhibited significant up-regulation of caspase-3, -6, and -9 genes in a concentration-dependent manner ( and ). Densitometry analysis revealed that, at the IC50 of parthenolide, caspase-3, -6, and -9 genes were up-regulated in SiHa cells by 13.4-, 9.2-, and 10.8-fold, respectively. Likewise, in MCF-7 cells, the caspase-3, -6, and -9 genes were up-regulated by 12.0-, 10.2- and 8.1-fold, respectively (p < 0.001–0.008).
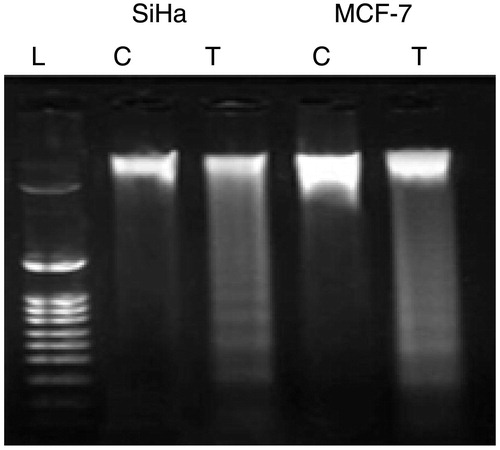
DNA fragmentation analysis
In order to analyze the hallmark of apoptosis, a genomic DNA fragmentation assay was performed for parthenolide-treated SiHa and MCF-7 cells. DNA fragmentation assay revealed ladder-like appearance in the gel (). The “laddering phenomenon” is a characteristic feature of apoptosis process in which the genomic DNA is cleaved into fragments by the endogenous endonucleases.
Discussion
Parthenolide is a natural compound and has been previously reported for anticancer activities; however, the regulatory mechanism of apoptotic pathway is still unclear. A recent report suggested that parthenolide-induced cell death of ovarian carcinoma cells by activating caspase-8 and Bid-dependent apoptotic pathway (Kwak et al., Citation2014). Apoptosis is regulated by the families of pro- and anti-apoptotic factors (Wong, Citation2011). In the present study, the IC50 of parthenolide against SiHa and MCF-7 cells ranged between 8.4 and 9.5 µM which was consistent with the previous report in which parthenolide inhibited the growth of pancreatic cancer (BxPC-3) at 5.5–15 µM concentration (Liu et al., Citation2010). The pro-apoptotic genes (e.g., p53 and Bax) and anti-apoptotic genes (e.g., Bcl-2) are generally involved in cellular proliferation and apoptosis (Basu & Haldar, Citation1998). Semi-quantitative RT-PCR revealed down-regulation of Bcl-2 gene and up-regulation of p53 and Bax genes in SiHa and MCF-7 cells treated with parthenolide ( and ). Also, increased ratio of Bax to Bcl-2 gene regulation was noticed for both the parthenolide-treated cell lines. An earlier report showed that the increased ratio of Bax to Bcl-2 gene translational product induces the cell death process via apoptosis (Wong, Citation2011). Pro-apoptotic gene, Bax, is the most characteristic death-promoting member of the Bcl-2 family (Murphy et al., Citation2000). The Bax gene encodes a protein that is primarily localized to the cytosol where apoptotic stimulation is translocated to the mitochondria (Fulda & Debatin, Citation2006). In mitochondria, it activates the release of cytochrome-c and forms a complex with other co-factors that trigger the activation of caspase-9 and initiate downstream caspase cascade leading to cell death (Budihardjo et al., Citation1999; Parrish et al., Citation2013). The Bcl-2 gene product acts as an anti-apoptotic agent by binding and antagonising with executioner molecules, such as Bax and Bak (Youle & Strasser, Citation2008). In response to apoptotic stimuli, like growth factor deprivation, ultraviolet radiation, chemotherapeutic drugs, and loss of cell survival factors, the pro-apoptotic protein Bim binds to and antagonises Bcl-2. As a result, Bax and Bak activate and permeate the outer membrane of the mitochondria. Consequently, cytochrome-c is released and leads to the activation of caspase enzymes (Ganesan et al., Citation2010). Also, p53 expression leads to an increase in the p53 protein concentration which ultimately enhances the expression of Bax gene which is probably associated with further activation of pro-caspase genes (Ganesan et al., Citation2010; Youle & Strasser, Citation2008). Along similar lines, Basu and Haldar (Citation1998) reported that the p53 gene is a negative regulator of Bcl-2 gene and acts as a transcriptional activator of the Bax gene.
Caspase enzymes belong to family of cysteine proteases, which are mainly involved in the apoptotic cascade and leads to proteolysis of specific substrates associated with programmed cell death (Slee et al., Citation2001). Consistent with this possibility, parthenolide-treated both SiHa and MCF-7 cells showed increased up-regulation of caspase-9 gene and consequently activate caspase-3 gene up-regulation (). In addition, the up-regulation of caspase-6 gene in parthenolide-treated SiHa and MCF-7 cells are also capable of processing and activating the pro-caspase-3 (Allsopp et al., Citation2000). The caspase-3 is an important executioner caspase, which is activated by any of the initiator caspases. Active caspase-3 has a variety of functions including activation of a latent cytosolic endonuclease, caspase-activated deoxyribonuclease that cleaves DNA into oligonucleosomal fragments (Fahy et al., Citation1999; Janicke et al., Citation1998). These findings can be correlated with a recent report in which parthenolide induced cleavage of apoptotic proteins, such as, caspase-8, -9, -3 and PARP1 in lung cancer cells, have been given and suggested that apoptosis was trigged after parthenolide exposure (Zhao et al., Citation2014). However, the present study reports the consequences of apoptosis as a DNA fragmentation in the parthenolide-treated SiHa and MCF-7 cells for the first time (). These findings provided information about the therapeutic function of parthenolide in human cervical and breast cancer cells.
Conclusion
We conclude that parthenolide inhibited the growth of MCF-7 and SiHa cancerous cells. The cytotoxic activity of parthenolide is possibly due to the induction of apoptosis via p53 and caspase dependent pathway. Hence, we suggest that parthenolide is a promising compound and can be successfully exploited in cancer chemoprevention or chemotherapy. However, further studies are warranted to decipher the precise molecular mechanism of this bioactive compound to evaluate its anticancer properties.
Acknowledgements
The authors sincerely acknowledge the Department of Pharmacology, Institute of Pharmacy, NIMS University, for providing the laboratory facility for this research study.
Declaration of interest
The authors have no declaration of interest.
References
- Allsopp TE, McLuckie J, Kerr LE, et al. (2000). Caspase-6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ 7:984–93
- Basu A, Haldar S. (1998). The relationship between Bcl-2, Bax and p53: Consequences for cell cycle progression and cell death. Mol Hum Reprod 4:1099–109
- Bork PM, Schmitz ML, Kuhnt M, et al. (1997). Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett 402:85–90
- Budihardjo I, Oliver H, Lutter M, et al. (1999). Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–90
- Cretnik L, Skerget M, Knez Z. (2005). Separation of parthenolide from feverfew: Performance of convectional and high pressure extraction techniques. Sep Purif Technol 41:13–20
- Fahy RJ, Doseff AI, Wewers MD. (1999). Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol 163:1755–62
- Fulda S, Debatin KM. (2006). Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798–811
- Ganesan V, Perera MN, Colombini D, et al. (2010). Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis 15:553–62
- Gopal YN, Chanchorn E, van Dyke MW. (2009). Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther 8:552–62
- Hamedi A, Zarshenas MM, Sohrabpour M, Zargaran A. (2013). Herbal medicinal oils in traditional Persian medicine. Pharm Biol 51:1208–18
- Hehner SP, Heinrich M, Bork PM, et al. (1998). Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem 273:1288–97
- Hehner SP, Hofmann TG, Droge W, Schmitz ML (1999). The anti-inflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J Immunol 163:5617–23
- Herrera F, Martin V, Rodriguez-Blanco J, et al. (2005). Intracellular redox state regulation by parthenolide. Biochem Biophys Res Commun 332:321–5
- Jain NK, Kulkarni SK. (1999). Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol 68:251–9
- Janicke RU, Sprengart ML, Wati MR, Porter AG. (1998). Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–60
- Koprowska K, Czyz M. (2010). Molecular mechanisms of parthenolide's action: Old drug with a new face. Postepy Hig Med Dosw 16:100–14
- Kwak SW, Park ES, Lee CS. (2014). Parthenolide induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits FAK-mediated cell invasion. Mol Cell Biochem 385:133–44
- Kwok BH, Koh B, Ndubuisi MI, et al. (2001). The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IκB kinase. Chem Biol 8:759–66
- Lesiak K, Koprowska K, Zalesna I, et al. (2010). Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anticancer activity against human melanoma cells in vitro. Melanoma Res 20:21–34
- Liu JW, Cai MX, Xin Y, et al. (2010). Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J Exp Clin Cancer Res 29:1–7
- Marin MC, Hsu B, Meyn RE, et al. (1994). Evidence that p53 and Bcl-2 are regulators of a common cell death pathway important for in vivo lymphomagenesis. Oncogene 9:3107–12
- Mathema VB, Koh YS, Thakuri BC, Sillanpaa M. (2012). Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation 35:560–5
- Mehdi SJ, Ahmad A, Irshad M, et al. (2011). Cytotoxic effect of carvacrol on human cervical cancer cells. Biol Med 3:307–12
- Mendonca MS, Chin-Sinex H, Gomez-Millan J, et al. (2007). Parthenolide sensitizes cells to X-ray-induced cell killing through inhibition of NF-kappaB and split-dose repair. Radiat Res 168:689–97
- Mohsenzadeh F, Chehregani A, Amiri H. (2011). Chemical composition, antibacterial activity and cytotoxicity of essential oils of Tanacetum parthenium in different developmental stages. Pharm Biol 49:920–6
- Murphy KM, Ranganathan V, Farnsworth ML, et al. (2000). Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ 7:102–11
- Parrish AB, Freel CD, Kornbluth S. (2013). Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5:1–24
- Pearson AS, Spitz FR, Swisher SG, et al. (2000). Up-regulation of the proapoptotic mediators Bax and Bak after adenovirus-mediated p53 gene transfer in lung cancer cells. Clin Cancer Res 6:887–90
- Sedlak TW, Oltvai ZN, Yang E, et al. (1995). Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA 92:7834–8
- Slee EA, Adrain C, Martin SJ. (2001). Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–6
- Sobota R, Szwed M, Kasza A, et al. (2000). Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem Biophys Res Commun 267:329–33
- Sweeney C, Li L, Shanmugam R, et al. (2004). Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res 10:5501–7
- Tiuman TS, Ueda-Nakamura T, Cortez DAG, et al. (2005). Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother 49:176–82
- Wen J, You KR, Lee SY, et al. (2002). Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 277:38954–64
- Wong RS. (2011). Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res 30:1–14
- Wu C, Chen F, Wang X, et al. (2006). Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem 96:220–7
- Youle RJ, Strasser A. (2008). The Bcl-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
- Zhao X, Liu X, Su L. (2014). Parthenolide induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung cancer cells. J Exp Clin Cancer Res 33:1–11