Abstract
Context: Consumption of Sarpa salpa Linn. (Sparidae) in certain periods of the year is inadvisable because it can cause central nervous system disorders resulting in sea food poisoning.
Aims: The present study assesses the cytotoxic effects of compounds, not-yet identified, present in the organ extracts of S. salpa, collected in autumn, the period corresponding to the peak in human health problems.
Materials and methods: The toxicity was assessed by mouse bioassay of aqueous extract of the fish organs. Wistar rats received daily extracts of different organs of S. salpa by gastric gavage for 7 d (0.3 mL of extract/100 g body weight BW). The dose of tissue extracts of viscera, liver, brain, and flesh of S. salpa administered to rats was as follows: 172, 313, 2050, and 2660 mg/kg BW, respectively. No deaths occurred during the period of treatment.
Results: The lethal dose (LD50) determined for the crude ciguatoxin (neurotoxins) extracts of viscera, liver, brain, and flesh of S. salpa was as follows: 1.2, 2.2, 14.4, and 18.6 g/kg mouse, respectively. Changes in locomotor activity during the first 2 h and failure in breathing and no evident signs of gastrointestinal problems were recorded. We observed (1) induction of oxidative stress, indicated by an increase in lipid peroxidation (TBARS) in groups that received extracts of liver (+425%) or viscera (+433%), and a significant decrease in antioxidant enzyme activities (SOD, CAT, and GPx) in cerebral cortex tissue by 13%, 25%, and 25% (LT: animals receiving liver extracts) and by 16%, 26%, and 27% (VT: animals receiving viscera extracts), respectively. In contrast, the administration of extracts of flesh and brain induced an increase in antioxidant enzyme activities (SOD, CAT, and GPx) in cerebral cortex tissue by 26%, 23%, and 44% (FT: flesh extract) and 28%, 24%, and 46% (BT: brain extract), respectively; (2) a significant decrease for acetylcholinesterase (AChE) activity in cerebral cortex was recorded in FT, BT, LT, and VT by 27, 34, 58, and 78%, respectively. Moreover, a significant decrease of AChE activity in plasma was recorded in FT, BT, LT, and VT by 16, 21, 38, and 48%, respectively; (3) the histological findings confirmed the biochemical results.
Conclusions: Liver and especially the visceral part of S. salpa presented toxicity, which clearly indicates the danger of using this fish as food.
Introduction
Sarpa salpa Linn. (Sparidae) also known as salema porgy, a species of bream, is an herbivorous sea fish that preferentially feeds on the sea grass Posidonia oceanica (L.) Delile (Posidoniaceae) (Peirano et al., Citation2001; Prado et al., Citation2008); it is used for human consumption in the Mediterranean region (e.g., Tunisia, region Gulf of Gabes). Due to its low cost, this fish is predominantly on the menu of the lower income classes. The consumption of the S. salpa is, however, inadvisable in certain periods of the year because it causes a hallucinatory syndrome and central nervous system disorders. Most poisonings involving S. salpa consumption have been reported in spring, summer, and autumn (Bellassoued et al., Citation2012; Chevaldonne, Citation1990; Raikhlin-Eisenkraft et al., Citation1989). An important observation in this context is the presence of ciguateric species that live as epiphytes on P. oceanica leaves (Ben Brahim et al., Citation2010) and that are co-ingested by the S. salpa as a part of their diet (Velimirov, Citation1984).
With respect to this, the Gulf of Gabes is a threatened biotope mainly due to the pressure of anthropogenic expansion and dumping of large quantities of phosphogypsum and other chemical products that severely impacted benthic habitats (Hamza-Chaffai et al., Citation1999). It has been shown that epiphytes of sea grass are sensitive to environmental changes (Giovannetti et al., Citation2010). For example, various studies reported increases in epiphyte biomass parallel with nutrient enrichment (Armitage et al., Citation2006), eutrophication (Frankovich et al., Citation2009), and water quality (Meric et al., Citation2005). This has led to a substantial proliferation of microalgae and particularly of toxic dinoflagellates in the Gulf of Gabes (Turki et al., Citation2006). Proliferation of such undesirable microalgae has resulted in increased problems in both coastal and estuarine environments (Leong & Taguchi, Citation2005; Smayda, Citation1997).
For instance, ciguatera food poisoning increases due to the presence of a toxin in fish produced by the benthic alga Gambierdiscus toxicus Adachi & Fukuyo (Goniodomaceae) and other coral microalgae, most of them belonging to these genera: Prorocentrum, Ostreopsis, and Amphidinum. Another less common form of poisoning is ichthyoallyeinotoxism that is characterized by the development of central nervous system disturbances, especially hallucinations and nightmares (Chateau-Degat, Citation2003; Halstead, Citation1988). Several toxins increase to dangerous levels for humans during their transmission through herbivorous and carnivorous fish (Vaillant et al., Citation2001).
The brain exhibits distinct variations in cellular as well as regional distribution of antioxidant defenses (Verma & Srivastava, Citation2001). Thus, neural cells and/or brain regions are likely to respond differentially to changes in metabolic rates associated with the generation of reactive oxygen species ROS (Hussain et al., Citation1995). Indeed, there is abundant evidence invoking regional sensitivity to oxidative stress that is dependent on cellular and regional redox status (Baek et al., Citation1999). Cerebral cortex is more susceptible to oxidative damage relative to other brain regions (Mohamadin et al., Citation2010; Noda et al., Citation1983).
The present study investigated the toxicity of compounds present in the extract of fish organs collected in autumn by mouse bioassay and in Wistar rats that daily received extracts from the different organs of S. salpa by gastric gavage, for 7 d, to mimic human consumption. To evaluate the toxicity of the treatment, clinical symptoms and mortality were recorded. Since some marine toxins had been found to induce an oxidative stress (Davies, Citation1987; Saoudi et al., Citation2009), we also investigated lipid peroxidation and changes in the activity of antioxidant enzymes in rat cerebral cortex.
Materials and methods
Fish collection
The study was conducted off the Island of Kerkennah (Gulf of Gabes, southeast Tunisia). This archipelago is characterized by extensive P. oceanica sea grass meadows (Hamza et al., Citation2000). Sarpa salpa were collected between 2008 and 2010 in autumn season. Immediately after capture, the fish were dissected: the flesh, brain, liver, and viscera (except liver) were removed, rinsed with ice-cold saline, and stored at −80 °C until further analysis.
Lipid-soluble extracts preparations from fish organs and LD50% determination
Samples for toxicity assay were prepared as follows: the fish flesh or organ (50–100 g) was thawed and cooked at 70 °C for 15 min in a water bag to denature proteins in order to enhance extraction efficiency during homogenization. Samples were cooled to room temperature, minced, and extracted twice with acetone (3 L/kg flesh) using an explosion-proof homogenizer. The acetone filtrate was dried in a rotor evaporator at 55 °C water bath and 556 mbar vacuum, re-dissolved in 90% of aqueous methanol (0.5 L/kg flesh) and extracted twice with hexane (1:1, v:v) to remove impurities from the aqueous methanol phase. The aqueous methanol portion was dried in a rotor evaporator at 55 °C water bath and 337 mbar vacuum, re-dissolved again in 25% of aqueous ethanol (0.5 L/kg flesh) and extracted with diethyl ether (1:1, v:v) three times. The extraction of lipophilic toxin from fish tissues was performed by subsequent liquid–liquid partitioning (separator funnel) as described by Lewis (Citation2003). Diethyl ether extracts were concentrated by using a rotor evaporator, re-dissolved in a known volume of chloroform:methanol (97:3, v:v) for quantification and dried under nitrogen gas. The protein extracts were stored at −80 °C prior to testing.
In order to study the toxicity of marine toxin expected to be present in the samples, lipid-soluble extracts of the samples were analyzed using a mouse bioassay that was previously described by Vernoux (Citation1994) and Lewis (1995, Citation2003). Mouse bioassay experiments were carried out using seven groups of male mice weighing 18–22 g (eight animals per group) purchased from the Central Pharmacy of Tunisia (SIPHAT, Tunisia). Animals were housed in a controlled environment (22 ± 3 °C, 54–56% humidity, a 12 h light/dark cycle). Mice were fed with a commercial balanced diet (SICO, Sfax, Tunisia) and drinking water was offered ad libitum. The body weight (BW) of the mice at the start of the experiment was measured. The ether-soluble extract was suspended in 1% Tween 60/0.9% saline at different concentrations, sonicated at 37 °C for 5–10 min, 0.8 mL (0.04 mL/1 g of mouse) was administered by intraperitoneal (i.p) injection and assayed in duplicate. Control mice were administered the same volume of 1% Tween 60/0.9% saline only. The mice were closely monitored at 1 h interval for 3–5 h after sample injection. Symptoms of intoxication including hypothermia (rectal body temperature below 33 °C), diarrhea, reduced locomotor activity, and time of death of the mice (if this occurred within the first 24 h) were recorded. Symptoms or signs of intoxication in mice, other than the above mentioned, were rejected in this experiment to avoid subjective bias (Hoffman et al., 1983; Lewis, 1995). The diethyl ether extract containing marine toxin was quantified using the principle of the dose versus time-to-death relationship equation log (MU) = 2.3 log (1+1/T), where MU is the number of mouse units (one MU = LD50 dose for a 20 g mouse) and T is the survival time in hours of each mouse (Lewis & Sellin, 1993; Lewis, 1995, Citation2003).
Evaluation of clinical and functional parameters following exposure to marine toxin(s) in a rat model
Animals
Male Wistar rats weighing about 130–135 g were purchased from the Central Pharmacy of Tunisia (SIPHAT, Tunisia). They were housed at 22 ± 3 °C with light/dark periods of 12 h and a minimum relative humidity of 40%.
The animals had free access to commercial pellet diet (SICO, Sfax, Tunisia) and water ad libitum. The general guidelines for the use and care of living animals in scientific investigations were followed (Council of European communities, Citation2010). The handling of the animals was approved by the Tunisian Ethical Committee for the Care and Use of Laboratory Animals.
Experimental protocols
After acclimatizing to the laboratory conditions for 1 week, 45 rats were divided into five groups that were daily received by gastric gavage; for the controls group (C) with 0.3 mL of a 1% Tween 60/0.9% saline solution/100 g of body weight (BW); for the flesh treated group (FT) with 0.3 mL of flesh extract/100 g BW; for the brain-treated group (BT) with 0.3 mL of brain extract/100 g BW; for the liver-treated group (LT) 0.3 mL of liver extract/100 g BW; and for the viscera-treated group (VT) with 0.3 mL of viscera extract/100 g BW. The dose of ciguatoxin extracts of viscera, liver, brain, and flesh of S. salpa administered to rats were as follows: 172, 313, 2050, and 2660 mg/kg BW, respectively. The doses of extract organs 0.3 mL/100 g of BW, chosen in our experiment, represent 1/7 of LD50% for each extract organs.
At the end of treatment, six animals from each group were rapidly sacrificed by cervical decapitation to avoid stress. Serum samples were obtained by centrifugation at 4000 × g for 15 min and stored at −80 °C until analysis. The cerebral cortex tissue was immediately removed and dissected over ice-cold glass slides. The brains were collected and a part was homogenized (10% w/v) with an Ultra Turrax homogenizer in ice-cold, 1.15% KCl–0.01 M sodium, potassium phosphate buffer. Homogenates were centrifuged at 10 000 × g for 20 min at 4 °C. The resulting supernatants were used for immediate lipid peroxidation and protein oxidation determination. Homogenate aliquots were stored at −80 °C for further biochemical assays. Other parts of these cerebral cortex tissues were fixed in 10% buffered formalin and processed for paraffin sectioning and histological studies.
Biochemical determinations
Protein quantification
Protein contents in cerebral cortex were measured by the method of Lowry et al. (Citation1951) using bovine serum albumin as standard.
Lipid peroxidation
The cerebral cortex malondialdehyde concentrations, marker of lipid peroxidation, were determined spectrophotometrically according to Draper and Hadley (Citation1990). Briefly, an aliquot of cerebral cortex extract supernatant was mixed with 1 mL of 5% trichloroacetic acid and centrifuged at 2500 × g for 10 min. An amount of 1 mL of thiobarbituric acid reagent (0.67%) was added to 500 μL of supernatant and heated at 90 °C for 15 min. The mixture was then cooled and measured for absorbance at 532 nm using a spectrophotometer (Jenway UV-6305, Essex, England). The malondialdehyde values were calculated using 1,1,3,3-tetraethoxypropane as a standard and expressed as nmoles of malondialdehyde/mg of protein.
Determination of cerebral cortex antioxidant enzyme activities
Catalase (CAT) activity was measured according to Aebi (Citation1984). A total of 20 µL cerebral cortex homogenate (about 1.5 mg proteins) were added to 1 mL phosphate buffer (0.1 M, pH 7) containing 100 mM H2O2. The rate of H2O2 decomposition was followed by measuring the decrease in absorbance at 240 nm for 1 min. The enzyme activity was calculated using an extinction coefficient of 0.043 mM−1 cm−1 and expressed in international units (I.U), i.e., in µmoles H2O2 destroyed/min/mg protein, at 25 °C.
Superoxide dismutase (SOD) activity was assayed by measuring its ability to inhibit the photoreduction of nitroblue terazolium (NBT) (Beyer & Fridovich, Citation1987). In this assay, one unit of SOD is defined as the amount required inhibiting the photo reduction of NBT by 50%. Riboflavin (0.26 mM final concentration) was added to start the reaction and the absorbance was recorded at 560 nm for 20 min. The activity was expressed as units/mg protein, at 25 °C.
Glutathione peroxidase (GPx) activity was determined using a commercial kit (Catalog no. RS 505; Randox, Ltd [Crumlin, United Kingdom]). GPx catalyzes the oxidation of GSH by cumene hydroperoxide. In the presence of GSH reductase and NADPH, the oxidized GSH is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm was measured (Randox, Citation1996). The enzyme activity was expressed as nmoles of GSH oxidized/min/mg protein.
Determination of AChE activity in plasma and cerebral cortex
AChE activity was measured in plasma and homogenates according to the method of Ellman et al. (Citation1961), using acetylthiocholine iodide as a substrate. The reaction mixture was composed as follows: phosphate buffer (0.1 M; pH = 8) and 0.01 M DTNB. The hydrolysis rate of acetylthiocholine iodide was measured at 412 nm through the release of the thiol compound which, when reacted with DTNB, produced the color-forming compound TNB. The reaction was initiated by adding 0.075 M acetylthiocholine iodide. Activities were expressed as nmoles of substrate/min/mg protein.
Histological studies
Cerebral cortex samples for histological examination by light microscopy were fixed in 10% of formalin and processed in a series of graded ethanol solutions. They were then embedded in paraffin, serially sectioned at 5 μm and stained with hematoxylin–eosin.
TUNEL assay
After deparaffinization and rehydratation, tissue sections were incubated with 0.1% (v/v) Triton X-100 for 2 min on ice, followed by washing of the slides twice in PBS (0.8 mM CaCl2 · 2H2O, 2.6 mM KCl, 1.4 mM KH2PO4, 0.4 mM MgCl2 · 6H2O, 136 mM NaCl, 8 mM Na2HPO4, pH 7.2). The specimens were then incubated for 1 h at 37 °C in a solution consisting of 1 mM cobalt chloride, 140 mM sodium cacodylate, and terminal deoxyribonucleotidyl transferase (TdT) at a final concentration of 0.1 U/μL to insert biotin-16-dUTP at the 3′-ends of DNA fragments. A streptavidin-peroxidase complex and 3-amino-9-ethylcarbazole served as the detection system for biotin. Sections were lightly counterstained with hematoxylin and mounted in glycerin jelly. Negative control included omission of TdT from the labeling mixture.
Statistical analysis
The data were analyzed using the statistical package program Stat view 5 Soft Ware for Windows (SAS Institute, Berkley, CA). Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test as a post hoc test for comparison between groups. All values were expressed as mean ± SD. Differences were considered significant if p < 0.05.
Results
LD50% determination of marine toxin accumulated in the organs of S. salpa
After extraction with diethyl ether, we obtained the crude ciguatoxins (neurotoxins) from 50 g of fish sample: viscera (viscera except liver) 1.16 ± 0.20 g (mean ± SE); liver 1.02 ± 0.15 g (mean ± SE); brain 0.5 ± 0.11 g (mean ± SE); and flesh (including dark muscle) 0.69 ± 0.20 g (mean ± SE).
For the LD50% determination, we used six experimental groups and one control group, each of the eight mice. Affected mice exhibited typical signs of neurotoxicity disorders including hypothermia (rectal body temperature <33 °C; trembling), a significantly reduced locomotor activity during the first 2 h and failure breathing and no evident signs of gastrointestinal problems (e.g., diarrhea). Results are given in .
Table 1. Concentration of toxicity expressed in mouse units per 100 g tissue and microgrammes per kg tissue, estimated in fish organs of S. salpa collected from the Island of Kerkennah (Gulf of Gabes; South East Tunisia) during autumn (2008–2010).
The toxin concentrations in the liver, viscera, and brain extracts were significantly different (p < 0.05) compared with the flesh extract. There was also a significant difference between the liver and the viscera organ. Concentrations of toxins in organs can, therefore, be ranked in ascending order: flesh, brain, liver, and viscera.
Evaluation of clinical symptoms and body, brain weights following exposure to marine toxins in a rat model
No death and a significantly reduced locomotor activity during the first 2 h and failure breathing and no evident signs of gastrointestinal problems (e.g., diarrhea) were observed in rats treated with extracts from the organs of S. salpa. After 7 d of treatment, the total body and cerebral cortex weights of rats treated with liver extracts LT and those treated with viscera extract VT were found to be lower than that of control (). In the groups of FT and BT rats, no significant change of the weight of the organs was observed. Only the body weight was reduced, as compared with control, but to a lesser extent than in the LT and VT groups.
Table 2. Effects of tissue extracts of flesh, liver, brain, and viscera of S. salpa (0.3 mL/100 g, v/w) on body and cerebral cortex weights of control and treated rats after 7 d of treatment.
Estimation of lipid peroxidation levels (TBARS)
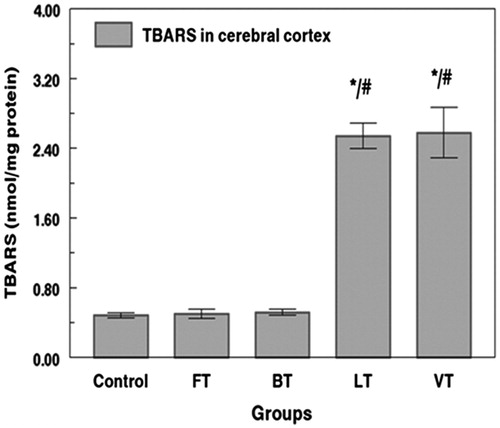
TBARS concentration increased by 425% and 433% in cerebral cortex tissue of LT and VT rats. The increase of the lipid peroxidation appeared generally to be higher in VT and LT rats, in the cerebral cortex. Conversely, no significant changes were observed in cerebral cortex TBARS levels of FT and BT rats as compared with the control animals ().
Figure 1. Effects of tissue extracts of flesh, brain, liver, and viscera of S. salpa (0.3 mL/100 g, v/w) on the cerebral cortex TBARS level of treated rats versus control rats. FT, flesh-treated group; BT, brain-treated group; LT, liver-treated group; VT, viscera-treated group. FT-, BT-, LT-, and VT-treated groups compared with the control group: *p < 0.05. FT and BT groups compared with LT and VT: #p < 0.05.
Analysis of SOD, CAT, and GPx activities in cerebral cortex tissues
As shown in , extracts of liver and viscera of S. salpa induced a significant decrease (p < 0.05) of SOD activity in cerebral cortex of LT and VT rats by 13% and 16%, respectively, as compared with the control animals. Moreover, the treatment also leads to a decrease of CAT activity by 25%, 26% and GPx activity by 25%, 27%, in cerebral cortex of LT and VT rats, respectively. Conversely, extracts of flesh and brain of S. salpa induced an increase of SOD, CAT, and GPx activities, in cerebral cortex of FT and BT rats by 26%, 28%; 23%, 24%; and 44%, 46%, respectively.
Table 3. Effects of tissue extracts of flesh, brain, liver, and viscera of S. salpa (0.3 mL/100g, v/w) on the cerebral cortex SOD, CAT, and GPx activities of treated rats versus control group.
Plasma and cerebral cortex AChE activity
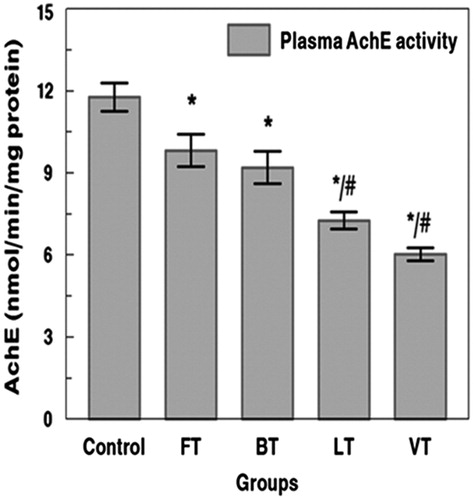
Exposure to marine toxins was estimated using AChE. Rats treated with FT, BT, LT, and VT showed a significant inhibition of AChE activity by 27%, 34%, 58%, and 78%, respectively, when compared with those of controls (). Moreover, rats treated with FT, BT, LT, and VT showed a significant inhibition of AChE activity in plasma by 16%, 21%, 38%, and 48%, respectively, when compared with those of controls (). The inhibition of AChE appeared generally to be higher in VT and LT rats, in the plasma and cerebral cortex, when compared with those of FT and BT groups.
Figure 2. Effects of tissue extracts of flesh, brain, liver, and viscera of S. salpa (0.3 mL/100 g, v/w) on the cerebral cortex AchE activity of treated rats versus control rats. FT, flesh-treated group; BT, brain-treated group; LT, liver-treated group; VT, viscera-treated group. FT-, BT-, LT- and VT-treated groups compared with the control group: *p < 0.05. FT and BT groups compared with LT and VT: #p < 0.05.
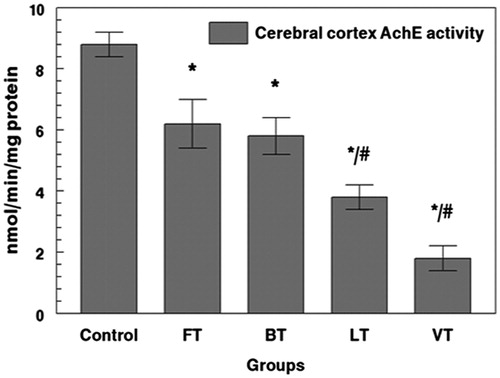
Figure 3. Effects of tissue extracts of flesh, brain, liver, and viscera of S. salpa (0.3 mL/100 g, v/w) on the plasma AchE activity of treated rats versus control rats. FT, flesh-treated group; BT, brain-treated group; LT, liver-treated group; VT, viscera-treated group. FT-, BT-, LT-, and VT-treated groups compared with the control group: *p < 0.05. FT and BT groups compared with LT and VT: #p < 0.05.
Histological observations
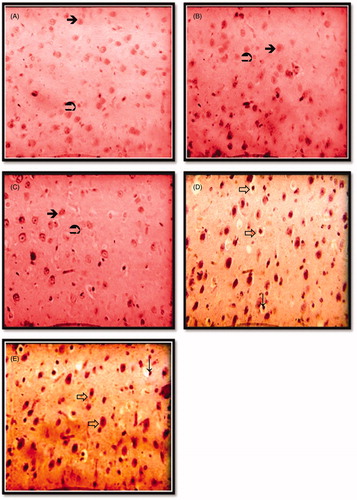
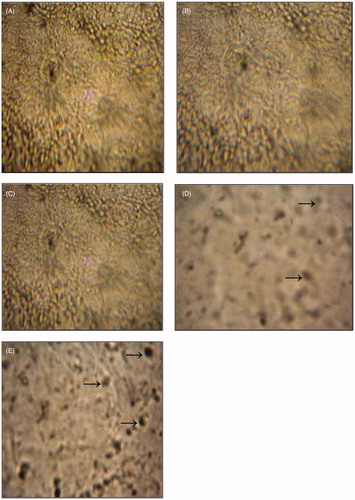
Upon histological examination of control rats, cerebral cortex tissue presented normal histoarchitecture (). Similar observations were registered in FT and BT rats (). The hemorrhage and vacuolated spaces in cerebral cortex cells were clearly observed in all animals LT and VT rats () when compared with control.
Figure 4. Photomicrographs showing histological changes in cerebral cortex in different groups; control group (A); flesh- and brain-treated groups (B); (C) and liver- and viscera-treated groups (D); (E) several abnormalities (indicated by arrows). (A) Histological picture showing normal cerebral cortex tissue. Hematoxylin and eosin staining, ×400. ![]()
TUNEL observations
Nick end-labeling for the detection of apoptotic neural cells and/or brain regions revealed a high percentage of TUNEL-positive cells in cerebral cortex following liver and viscera extracts of S. salpa received by gastric gavage (). The density of the apoptotic cells was more significant in VT than LT. Conversely, FT and BT rats did not show apoptotic cells in cerebral cortex ().
Figure 5. Photomicrographs of section of cerebral cortex in different groups; control group (A); flesh- and brain-treated groups (B) and (C) and liver- and viscera-treated groups (D) and (E) stained by TUNEL technique (magnification ×400). Cerebral cortex was fixed by direct immersion in a 4% paraformaldehyde in 0.1 M phosphate buffer. Serial sections (5 μm) were mounted on gelatin-coated glass slides cut and stained using the TUNEL technique (see section Materials and methods). →Apoptotic cells (arrows).
Discussion
The fish S. salpa is widely used for human consumption, however, it can pose a threat to their health which was shown to be seasonal dependent (Bellassoued et al., Citation2012). For this study, the collection of S. salpa was, therefore, done in autumn, the season when the toxicity of this animal is the highest. We used the rat as a model to evaluate potential toxicity of S. salpa organs and the incidence of oxidative stress and neurotoxicity. After 7 d of treatment, the total body and cerebral cortex weight of rats receiving liver extracts LT and those receiving viscera extract VT were found to be lower than that of control. Changes in body weight are a valuable indicator in evaluating the toxicity of extract organs of S. salpa. The slight drop in weight after 7 d of treatment in all the treated groups could be a normal physiological and adaptations responses (mediated by the extract of S. salpa organs), thus decreasing the appetite and thereby lowering caloric intake by the animals.
The brain tissue has a high rate of oxidative metabolism, consuming about 20% of the cardiac output. Since it is rich in polyunsaturated fatty acids (PUFAs), the brain is especially vulnerable to oxidation (Wagner et al., Citation1994). Some regions are highly enriched in non-heme iron, which is catalytically involved in the production of oxygen free radicals (Hill & Switzer, Citation1984) thus increasing the risk of neurodegenerative diseases. According to Mandavilli and Rao (Citation1996), cortex and hippocampus are more susceptible to oxidative damage when compared with the cerebellum. The present study concludes that treatment of rats with the extract of viscera and liver of S. salpa causes cerebral cortex damage and toxicity.
The increase in antioxidant enzyme activities (SOD, CAT, and GPx) without accompanied changes in TBARS levels in cerebral cortex of rats treated with flesh and brain extracts can originate from a low accumulation of ciguatoxins originated from epiphytic toxic phytoplanktons that live on the P. oceanica leaves to the fish organs by grazing (Bellassoued et al., Citation2012). It is more plausible that the toxic effects on humans are especially due to flesh and sometimes to brain consumption. Cellular antioxidant systems have demonstrated a great adaptation to oxidative stress in order to counteract the excessive ROS production (Alvarez & Boveris, Citation1993; Sureda et al., Citation2004). ROS may also act as messenger molecules to activate adaptive responses through redox-sensitive signaling pathways to maintain cellular oxidant–antioxidant homeostasis. Activation of NF-kappaβ signaling pathway has been shown to elevate the gene expression of antioxidant enzymes (Ji et al., Citation2006). The toxicity in the flesh and brain of S. salpa was lower than the toxicity in the viscera and liver (Bellassoued et al., Citation2012). Consequently, the difference between this result and the decrease in antioxidant enzyme activities (SOD, CAT, and GPx) with a concomitant increase in TBARS levels after liver and viscera extract’s treatment. Our results showed that the increase of the lipid peroxidation appeared generally to be higher in VT and LT rats in the cerebral cortex. These effects are not specific, since they are reported in numerous toxicity studies (Davies et al., Citation1987; Saoudi et al., Citation2009), but they confirm the presence of ciguatoxins in the S. salpa extracts.
The standardized mouse bioassay is used to test specimens for neurotoxicity. The bioassay is based on the time until death of mice injected intraperitoneally with crude ciguatoxins residues extracted from fish organs. Relative toxicity is expressed in mouse units. Any detectable level of toxin per 100 g of shellfish tissue is considered potentially unsafe for human consumption, in practice residue toxicity >20 MU was adopted as the guidance level for the prohibition of shellfish harvesting (Dickey et al., Citation1999). We noticed that the cytotoxic compounds present in different organs of S. salpa can pose a threat to human health and is a source of intoxication especially in the visceral part.
In addition to these oxidative stress biomarkers, assessment of the activity of the enzyme AChE is also considered a very valuable tool (Ferrari et al., Citation2007). Changes in AChE activity are frequently used as a biomarker for neurotoxicity (Mahmood & Carmichael, Citation1987). AChE is an enzyme that breaks down the neurotransmitter acetylcholine at the synaptic cleft. During our experimental study, AChE was decreased in cerebral cortex and plasma for all groups treated and especially in LT and VT groups. We hypothesized that these effects could be related to ciguatoxins accumulation among organs of S. salpa. It is known that ciguatoxins provokes changes in neural systems that are based on the complex formation of neurotoxins with acetylcholine (Hyde & Carmichael, Citation1991), and thus the inhibition of AChE activity. The cyanotoxins, the ones that have the greatest influence on AChE activity, are the group of neurotoxins, such as anatoxins, which act by inhibiting the activity of AChE (Monserrat et al., Citation2001). Moreover, Araoz et al. (Citation2009) confirmed the decrease of AChE activity among rats exposed to influence of neurotoxins. The changes in the activity of AChE may be induced indirectly by ROS, formed by xenobiotic reactions (Ferrari et al., Citation2007).
Damage to the protein part of AChE may be the result of a direct interaction of neurotoxins or an indirect effect of oxidative stress generated by this ciguatoxins as well as with changes in membrane fluidity as reported by Watanabe et al. (Citation1988) and by Laurent et al. (Citation2008).
Ciguatoxins accumulate in the muscle of fish and are carried up throughout the food chain. They also occur in the liver and viscera at concentrations estimated to be 10 times higher than in the muscle (Vernoux, Citation1994). Generally, most of the consumption of P. oceanica (approximately 75%) has been attributed to S. salpa (Cebrián et al., Citation1996), although the relative importance of grazing by fish varies strongly both spatially and temporally (Alcoverro et al., Citation1997; Prado et al., Citation2008; Peirano et al., Citation2001; Tomas et al., Citation2005). The interaction between herbivores and sea grass can be mediated by epiphytes (Young et al., Citation2005), at least in part, because seagrasses do not appear to be an attractive food source (Hereu, Citation2006) as the presence of phenolics in them proves to be a source of chemical deterrents (McMillan, 1984). The proliferation in the Gulf of Gabes of unwanted microalgae has been widely shown to be an increasing problem in both coastal and estuarine environments (Leong &Taguchi, Citation2005; Smayda, Citation1997), causing significant overfishing of demersal resources, thus degrading benthic habitats (Turki et al., Citation2006) and shellfish poisoning (Matsuoka et al., Citation2003). In Tunisia (1994), toxic blooms cause the death of fish in the Gulf of Gabes (Hansen et al., Citation2004), inciting the authorities to launch a regular monitoring in shellfish areas.
Histopathological studies also provided important evidence for the biochemical analysis. Under microscopic examination, severe distortions in cellular architecture were observed in cerebral cortex of LT and VT rats, which were characterized by exhibited hemorrhage and vacuolated spaces in the affected area. This can be due to reactive oxygen species which resulting from ciguatoxins administered in rats. These observations indicated marked changes in the cerebral cortex overall histoarchitecture in response to ciguatoxin, which could be due to their toxic effects. Similar changes in the cerebral cortex tissue of Wistar rats have been reported by previous findings of Pulido (Citation2008). The presence of cells death in the cerebral cortex proves the effect of ciguatoxins or neurotoxins found in the organs of S. salpa as a pro-apoptotic compound of cell death. Numerous marine natural products are potent modulators of programmed cell death. Cyanobacterial toxins secreted in the water column induce adverse effects in animals and humans (Codd et al., Citation2005; Ince & Codd, Citation2005). Cyanotoxins cause neuronal degeneration by an excitoxic mechanism (Copani et al., Citation1990, Citation1991).
Conclusion
Overall, the findings of the current study indicate that the toxicity levels observed in fish organs are decreasing in the following order: viscera, liver, brain, and flesh. Moreover, the viscera, liver, and edible parts of S. salpa collected along the Island of Kerkennah can be contaminated with cytotoxic compounds able to induce an oxidative stress at the level of cerebral cortex. The histological findings confirmed the biochemical results. The danger of using this fish as food will depend on the amount of fish ingested with ciguatoxins accumulated among organs. Hence, the liver and viscera of this fish can pose a threat to human health and consumption should for this reason be dissuaded. The results of these animal experiments are indicative but additional studies are needed with regard to extrapolating the data to humans.
Declaration of interest
The authors have no conflict of interests. This research was supported by the Tunisian Ministry of Higher Education and Scientific Research and the Tunisian Ministry of Public Health.
References
- Aebi H. (1984). Catalase in vitro. Meth Enzymol 105:121–6
- Alcoverro T, Duarte CM, Romero J. (1997). The influence of herbivores on Posidonia oceanica epiphytes. Aquat Bot 130:93–104
- Alvarez S, Boveris A. (1993). Induction of antioxidant enzymes and DT diaphorase in human blood mononuclear cells by light stress. Arch Biochem Biophys 305:247–51
- Araoz R, Molgo J, De Marsac NT. (2009). Neurotoxic cyanobacterial toxins. Toxicon 56:813–28
- Armitage AR, Frankovich TA, Fourqurean JW. (2006). Variable responses within epiphytic and benthic microalgal communities to nutrient enrichment. Hydrobiologia 569:423–35
- Baek BS, Kwon HJ, Lee KH, et al. (1999). Regional difference of ROS generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch Pharm Res 22:361–6
- Bellassoued K, Hamza A, Van Pelt J, Elfeki A. (2012). Seasonal variation of Sarpa salpa fish toxicity, as related to phytoplankton consumption, accumulation of heavy metals, lipids peroxidation level in fish tissues and toxicity upon mice. Environ Monit Assess 185:1137–50
- Ben Brahim M, Hannechi I, Hamza A, et al. (2010). Variability in the structure of epiphytic assemblages of Posidonia oceanica in relation to human interferences in the Gulf of Gabes, Tunisia. Mar Environ Res 70:411–21
- Beyer Jr WF, Fridovich I. (1987). Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–66
- Cebrián J, Duarte CM, Marbà N, et al. (1996). Herbivory on Posidonia oceanica: Magnitude and variability in the Spanish Mediterranean. Mar Ecol Prog Ser 130:147–55
- Chateau-Degat ML. (2003). Marine toxins: Appearance of health problem. Vertigo 4:1–11
- Chevaldonne P. (1990). Ciguatera and the saupe, Sarpa salpa, in the Mediterranean: A possible misinterpretation. J Fish Biol 37:503–4
- Codd GA, Morrison LF, Metcalf JS. (2005). Cyanobacterial toxins: Risk management for health protection. Toxicol Appl Pharm 1203:206–72
- Copani A, Canonico PL, Catania MV, et al. (1991). Interaction between beta-N-methylamino-l-alanine and excitatory amino acid receptors in brain slices and neuronal cultures. Brain Res 558:79–86
- Copani A, Canonico PL, Nicoletti F. (1990). Beta-N-methylamino-l-alanine (l-BMAA) is a potent agonist of ‘metabolotropic’ glutamate receptors. Eur J Pharmacol 181:327–8
- Council of European Communities. (2010). Council instructions about the protection of living animals used in scientific investigations. Off J Eur Commun (JO 10/63/CEE) L276:1–33
- Davies KJA, Delsignore ME, Lin SW. (1987). Protein damage and degradation by oxygen radicals. Modification of amino acids. J Biol Chem 262:9902–7
- Dickey R, Jester E, Granade R, et al. (1999). Monitoring brevetoxins during a G. breve red tide: Comparison of a sodium channel specific cytotoxicity assay and mouse bioassay for determination of neurotoxic shellfish toxins in shellfish extracts. Nat Toxins 7:157–65
- Draper HH, Hadley M. (1990). Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 86:421–31
- Ellman GL, Courtney KD, Andres V, Featherstone R. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Ferrari A, Venturino A, Péchen de D'Angelo AM. (2007). Muscular and brain cholinesterase sensitivities to azinphos methyl and carbaryl in the juvenile rainbow trout Oncorhynchus mykiss. Comp Biochem Physiol 146C:308–13
- Frankovich TA, Armitage AR, Wachnicka AH, et al. (2009). Nutrient effects on sea grass epiphyte community structure in Florida Bay. J Phycol 45:1010–20
- Giovannetti E, Montefalcone M, Morri C, et al. (2010). Early warning response of Posidonia oceanica epiphyte community to environmental alterations (Ligurian Sea, NW Mediterranean). Mar Pollut Bull 60:1032–9
- Halstead BW. (1988). Poisonous and Venomous Marine Animals. Princetown: Darwin Press Inc, 683–6
- Hamza A, Bouain A, EL Abed A. (2000). Observations sur la floraison et la fructification de la phanérogame marine Posidonia oceanica (Linneaus) Delile sur les côtes du Golfe de Gabès (Tunisie). Mésogée 58:93–9
- Hamza-Chaffai A, Amiard JC, Cosson RP. (1999). Relationship between metallothionein and metals in a natural population of clam Ruditapes decussates from Sfax coast, a non-linear model using Box-Cox transformation. Comp Biochem Physiol 123:153–63
- Hansen G, Erard-Le Denn E, Daugbjerg N, Rodriguez F. (2004). Karenia selliformis responsible for the fish-kills in the Gulf of Gabes, Tunisia 1994. Communication Ifremer: Poster présenté à la Conférence Internationale sur le phytoplancton toxique
- Hereu B. (2006). Depletion of palatable algae by sea urchins and fishes in a Mediterranean subtidal community. Mar Ecol Prog Ser 313:95–103
- Hill JM, Switzer RC. (1984). The regional distribution and cellular localization of iron in the rat brain. Neuroscience 11:595–603
- Hoffman PA, Granade HR, Mcmillan JP. (1983). The mouse ciguatoxin bioassay: A dose-response curve and symptomatology analysis. Toxicon 21:363--9
- Hussain S, Slikker Jr W, Ali SF. (1995). Age-related changes in antioxidant enzymes, superoxide dismutase, catalase, glutathione peroxidase and glutathione in different regions of mouse brain. Int J Dev Neurosci 13:811–17
- Hyde EG, Carmichael WW. (1991). Anatoxin-a(s), a naturally occurring organophosphate, is an irreversible active site-directed inhibitor of acetylcholinesterase (EC 3.1.1.7). J Biochem Toxicol 6:195–201
- Ince PG, Codd GA. (2005). Return of the cycad hypothesis – Doses the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol Appl Neurobiol 31:345–53
- Ji LL, Gomez-Cabrera MC, Vina J. (2006). Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci 1067:425–35
- Laurent D, Kerbrat AS, Taiana Darius H, et al. (2008). Are cyanobacteria involved in ciguatera fish poisoning-like outbreaks in New Caledonia. Harmful Algae 7:827–38
- Leong SCY, Taguchi S. (2005). Optical characteristics of the harmful dinoflagellate Alexandrium tamarense in response to different nitrogen sources. Harmful Algae 4:211–19
- Lewis RJ. (1995). Detection of ciguatoxins and related benthic dinoflagellate toxins: In vivo and in vitro methods. In: Hallegraeff GM, Anderson DM, Cembella AD, eds. Manual on Harmful Marine Microalgae IOC Manuals and Guides. Vol. 33. France: UNESCO, 135--61
- Lewis RJ. (2003). Detection of toxins associated with ciguatera fish poisoning. In: Hallegraeff GM, Anderson DM, Cembella AD, eds. Manual on Harmful Marine Microalgae, IOC Manuals and Guides. Vol. 33. France: UNESCO, 267–77
- Lewis RJ, Sellin M. (1993). Recovery of ciguatera from fish flesh. Toxicon 311:1333--6
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with Folin phenol reagent. J Biol Chem 193:265–75
- Mahmood NA, Carmichael WW. (1987). Anatoxin-a(s), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon 25:1221–7
- Mandavilli BS, Rao KS. (1996). Neurons in the cerebral cortex are more susceptible to DNA damage in ageing rat brain. Int J Biochem Mol Biol 40:507–14
- Matsuoka K, Joyce LB, Kotani Y, Matsuyama Y. (2003). Modern dinoflagellate cysts in hypertrophic coastal waters of Tokyo Bay, Japan. J Plankton Res 25:1441–70
- McMillan C. (1984). The condensed tannins (Proanthocyanidins) in seagrasses. Aquatic Botany 20:351--7
- Meric S, Nicola ED, Iaccarino M, et al. (2005). Toxicity of leather tanning wastewater effluents in sea urchin early development and in marine microalgae. Chemosphere 61:208–17
- Mohamadin AM, Sheikh B, Abd El-Aal AA, et al. (2010). Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pestic Biochem Physiol 98:128–34
- Monserrat JM, Yunes JS, Bianchini A. (2001). Effects of Anabaena spiroides (cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ Toxicol Chem 20:1228–35
- Noda Y, McGeer PL, McGeer EG. (1983). Lipid peroxide distribution in brain and the effect of hyperbaric oxygen. J Neurochem 40:1329–32
- Peirano A, Niccolai I, Mauro R, Bianchi CN. (2001). Seasonal grazing and food preference of herbivores in a Posidonia oceanica meadow. Scientia Marina 65:367–74
- Prado P, Farina S, Tomas F, et al. (2008). Marine protection and meadow size alter fish herbivory in sea grass ecosystems. Mar Ecol Prog Ser 371:11–21
- Pulido OM. (2008). Domoic acid toxicologic pathology: A review. Mar Drugs 6:180–219
- Raikhlin-Eisenkraft B, Finkelstein Y, Spanier E. (1989). Ciguatera like poisoning in the Mediterranean. Vet Hum Toxicol 30:352–3
- Randox Laboratories Ltd. (1996). Radicales Libres. Crumlin, United Kingdom, 1–16
- Saoudi M, Allegui MS, Abdelmouleh A, et al. (2009). Protective effects of aqueous extract of Artemisia campestris puffer fish Lagocephalus lagocephalus extract induced oxidative damage in rats. Exp Toxicol Pathol 62:601–5
- Smayda TJ. (1997). Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr 42:1137–53
- Sureda A, Batle JM, Tauler P, et al. (2004). Neutrophil tolerance to oxidative stress induced by hypoxia/reoxygenation. Free Radical Res 38:1003–9
- Tomas F, Turon X, Romero J. (2005). Seasonal and small-scale spatial variability of herbivory pressure on the temperate sea grass Posidonia oceanica. Mar Ecol Prog Ser 301:95–107
- Turki S, Harzallah A, Sammari C. (2006). Occurrence of harmful dinoflagellates in two different Tunisian ecosystems: The lake of Bizerte and the Gulf of Gabes. Cah Biol Mar 47:253–60
- Vaillant V, Caumes E, DeValk H, et al. (2001). Intoxication alimentaire à la ciguatera: Savoir l’évoquer même en l’absence de voyage. Bull Épidémiol Hebd 38:187
- Velimirov B. (1984). Grazing of Sarpa salpa L. on Posidonia oceanica and utilization of soluble compounds. In: Boudouresque CF, Grissac AJD, Olivier J, eds. I International Workshop on Posidonia oceanica Beds. Marseille: GIS Posidonie, 381–7
- Verma RS, Srivastava N. (2001). Chlorpyrifos induced alterations in levels of thiobarbituric acid reactive substances and glutathione in rat brain. Indian J Exp Biol 39:174–7
- Vernoux JP. (1994). The Mouse Ciguatoxin Bioassay: Directions for use to Control Fish for Consumption. Memoirs of the Queensland Museum, pp 625–9
- Wagner BA, Buettner GR, Burna GP. (1994). Free radical mediated peroxidation in cells: Oxidability is a function of cell lipid bis-allylic hydrogen content. Biochemistry 33:4449–53
- Watanabe T, Lockey RF, Krzanowski JJ. (1988). Airway smooth muscle contraction induced by Ptychodiscus brevis (red tide) toxin as related to a trigger mechanism of bronchial asthma. Immunol Allergy Pract 10:185–92
- Young EB, Lavery PS, Van Elven B, et al. (2005). Nitrate reductase activity in macroalgae and its vertical distribution in macroalgae epiphytes of sea grass. Mar Ecol Prog Ser 288:103–14

