Abstract
Context: There is a need for the discovery of novel natural and semi-synthetic sunscreen that is safe and effective. Piperine has a UV absorption band of 230–400 nm with high molar absorptivity. This compound has a high potential to be developed to sunscreen.
Objective: This study develops new UV protection compounds from piperine by using chemical synthesis.
Materials and methods: Piperine was isolated from Piper nigrum L. (Piperaceae) fruits, converted to piperic acid by alkaline hydrolysis, and prepared as ester derivatives by chemical synthesis. The piperate derivatives were prepared as 5% o/w emulsion, and the SPF values were evaluated. The best compound was submitted to cytotoxicity test using MTT assay.
Results: Piperic acid was prepared in 86.96% yield. Next, piperic acid was reacted with alcohols using Steglich reaction to obtain methyl piperate, ethyl piperate, propyl piperate, isopropyl piperate, and isobutyl piperate in 62.39–92.79% yield. All compounds were prepared as 5% oil in water emulsion and measured its SPF and UVA/UVB values using an SPF-290S analyzer. The SPF values (n = 6) of the piperate derivatives were 2.68 ± 0.17, 8.89 ± 0.46, 6.86 ± 0.91, 16.37 ± 1.8, and 9.68 ± 1.71. The UVA/UVB ratios of all compounds ranged from 0.860 to 0.967. Cytotoxicity of isopropyl piperate was evaluated using human skin fibroblast cells and the IC50 was equal to 120.2 μM.
Discussion and conclusion: From the results, isopropyl piperate is an outstanding compound that can be developed into a UV protection agent.
Introduction
The UV radiation from sunlight is now a major cause that is harmful to human health. Exposure to UV light from the sun can cause sunburn, premature skin aging, DNA damage, and skin cancer (Fuchs, Citation1998; Xu et al, Citation2006). The damage of the extracellular matrix integrity in skin tissue by UVA radiation can make skin wrinkle and become photoaging in many young and adult persons who have activities under the sunlight (Lavker et al., Citation1995). UVA (320–400 nm) penetrates deep into the skin and its effects are additive to the effects of UVB (280–320 nm) for inducing skin cancer (Lucas et al., Citation2006). The rising incidence rates of melanoma and non-melanoma skin cancer (NMSC) are probably caused by a combination of increased sun exposure or exposure to ultraviolet (UV) light, increased outdoor activities, changes in clothing style, increased longevity, ozone depletion, genetics, and, in some cases, immune suppression (Leiter & Garbe, Citation2008).
The use of sunscreens products which can absorb or block UV radiation is an effective approach for reducing the skin damage and has the immunosuppressive effects of sunlight (Ferrero et al., Citation2002). Sunscreen is recognized to be an effective and inexpensive method in preventing the development of skin cancers triggered by UV radiation (Kullavanijaya & Lim, Citation2005). There is a report that 10% of new case in malignance can be avoided from developing cancer if they apply the proper sunscreen continuously. Furthermore, 78% of non-melanoma skin cancer can be prevented from developing cancer by using sunscreens (Polonini et al., Citation2012).
Piperine is an alkaloid isolated from Piper nigrum L (Piperaceae) and has about 3–9% in dry fruit (Madhavi et al., Citation2009). Piperine has UV absorption ranged from 230 to 400 nm with high molar absorptivity (3.10 × 105 M−1 cm−1) at 344 nm (Zsila et al., Citation2005). This UV absorption band covers UVA and UVB regions that cause the skin damage and skin cancers. Our study focus on developing piperic acid derivatives as new UV protection compounds by using a synthetic method. These piperic acid derivatives possessed the chromophore of piperine skeleton but less irritation or harmful than piperine. Next, the piperic acid derivatives were prepared in an emulsion form and the SPF and UVA/UVB values of all compounds were evaluated. The best candidate was subjected to cytotoxicity assay using human skin fibroblast cells.
Materials and methods
Instruments and reagents
1,3-Butylene glycol was obtained from Kyowa Hakko (Tokyo, Japan). Carbopol 940 polymer was obtained from Lubrizol (Wickliffe, OH). l-Arginine was purchased from CellMark (Balmoral Plaza, Singapore). Nikkomulese 41 was obtained from Nikkol (Tokyo, Japan) while cetostearyl alcohol and caprylic/capric triglyceride were obtained from Parchem (New Rochelle, NY). Salisol 3 was purchased from Salicylates and Chemicals (Mumbai, India). Dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) were obtained from Aldrich (Howell, NJ). All other reagents and solvents were of reagent grade and used without further purification. TLC was performed on silica gel 60 GF254 (Merck, Damstadt, Germany). For column chromatography, silica gel (Merck 230–400 mesh) was used. SPF measurements were obtained from SPF-290S (Optometrics Corporation, Littleton, MA). UV spectra were performed on UV 2501 PC (Shimadzu, Nakagyo-ku, Japan). Infrared spectra were obtained from Spectrum 100 FT-IR (Perkin Elmer, Waltham, MA). NMR spectra were recorded with an Avance (1H, 300 MHz, Bruker, MA) spectrometer. Chemical shifts are reported in ppm, and coupling constants are reported in Hz. All NMR spectra were obtained in deuterated chloroform (CDCl3) or DMSO-d6 and referenced to the residual solvent peak. Mass spectra were obtained from Thermo Finigan Polaris Q (Thermo Fisher Scientific, Waltham, MA).
Plant materials
The plant material of Piper nigrum was obtained from a local drugstore in Nonthaburi province, Thailand, on March 2013. It was identified by Dr.Wandee Gritsanapan with the specimens at the Forest Herbarium, Department of National Park, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment, Bangkok. The voucher specimens of Piper nigrum (SRU 024) was deposited at Faculty of Oriental Medicine, Rangsit University, Pathumthani, Thailand.
Preparation of crude extracts
The dried fruit powder of Piper nigrum (100 g) was extracted with 95% ethanol (400 mL) by marceration at room temperature for 7 d. The extract was filtered with Whatman No. 1 and the filtrate was evaporated under reduced pressure with a rotary evaporator to obtain 9 g of finally crude dark brown extract.
Isolation of piperine
The crude extract (9 g) was dissolved in ethanol (30 mL). The mixture was added potassium hydroxide (8.0 g) and stirred until it dissolved. Then, the mixture was filtered through Whatman No. 1 and left it overnight for free evaporation at room temperature. After this process, piperine was precipitated from the mixture as a brown solid compound. The crude product was washed with hot water three times to remove the water-dissolving residues. The solid was recrystallized with isopropanol and kept at the 15 °C for 3 d. After that, the crystal was filtered through Whatman no. 1 to obtain yellow solid of 2.9591 g with a melting point of 129–130 °C. This compound was identified based on the consistency with the literature data (Berger & Sicker, Citation2009; Ikan, Citation1991; Lupina & Cripps, Citation1987).
Preparation of piperic acid
A round bottom flask (50 mL) with piperine (0.50 g) was added 20% alcoholic potassium hydroxide (30 mL). The mixture was refluxed at 70 °C for 12 h and then cooled down to room temperature. The solution was neutralized with 1 M HCl to pH 3.0 and then transferred to a separatory funnel. Dichloromethane (30 mL) was added to the separator and the aqueous layer was extracted. The extraction was repeated two times and the dichloromethane layers were collected, evaporated to obtain crude piperic acid. The crude compound was recrystallized with methanol:water (8:2) to give a crystal of piperic acid 0.3328 g (86.96% yield) with a melting point of 213–215 °C. This compound was identified based on the consistency with the literature data (Zarai et al., Citation2013). UV(MeOH): λmax 340 nm, IR: (KBr disc) 3300–2500 (broad), 1671, 1594 cm−1, 1H NMR: (300 MHz, DMSO-d6) δ [ppm], 5.92 (d, J = 15.1 Hz, 1H), 6.05 (s, 2H), 6.92–7.00 (m, 4H), 7.23 (s, 1H), 7.25–7.32 (m, 1H). 13C NMR: (75.47 MHz, DMSO-d6): 167.8, 148.2, 148.0, 144.8, 139.9, 130.6, 124.9, 123.2, 121.2, 108.6, 105.8, 101.4, and GC/MS (m/z, relative intensity) 218 [M+].
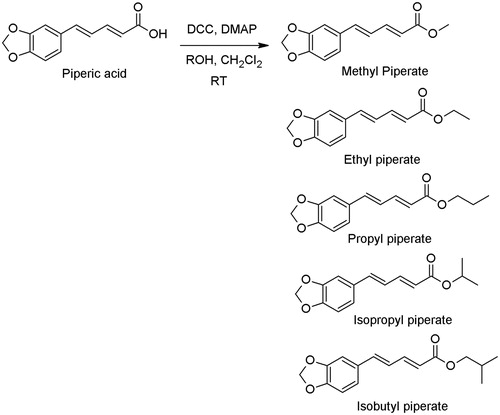
General procedure for the preparation of piperic acid derivatives 1–5, method A – preparation of methyl piperate (1)
A round bottom flask (50 mL) with piperic acid (0.0903 g, 0.46 mmol) in dry dichloromethane (15 mL) was added methanol (1.0 mL), 4-dimethylaminopyridine (DMAP) (0.044 g, 0.36 mmol), and dicyclohexylcarbodiimide (DCC) (0.114 g, 0.55 mmol) (Neises & Steglich, Citation1978). The mixture was stirred under nitrogen atmosphere for 24 h and the reaction was followed by TLC. After the reaction was completed, the reaction mixture was evaporated by using a rotary evaporator and the residue was subjected to silica gel column chromatography. The mobile phase was the mixture of hexane:ethyl acetate (7:3). The product was collected and evaporated the solvent off to obtain a pale yellow solid 0.0903 g (84.94% yield) with a melting point of 141–142 °C. This compound was identified based on the consistency with the literature data (Kijjoa et al., Citation1989). UV (MeOH): λmax 340 nm, IR: (KBr disc) 1703, 1616, 1606 cm−1. 1H NMR: (300 MHz, CDCl3) δ [ppm] 3.85 (s, 3H), 5.95 (d, 1H), 6.05 (s, 2H), 6.73 (dd, J = 11, 15 Hz, 1H), 6.81 (d, J = 8 Hz, 1H), 6.84 (d, J = 15 Hz, 1H), 6.94 (dd, J = 1.7, 8 Hz, 1H), 7.02 (d, J = 1.6 Hz, 1H), 7.45 (dd, J = 10.8, 15.3 Hz, 1H). 13C NMR: (75.47 MHz, CDCl3) 167.6, 148.6, 148.2, 144.3, 130.5, 124.4, 122.9, 119.9, 108.5, 105.9, 101.4, 51.5, and GC/MS (m/z, relative intensity) 232 [M+].
Preparation of ethyl piperate
The title compound was obtained from piperic acid (0.5003 g, 2.29 mmol), ethanol (1.0 mL), 4-dimethylaminopyridine (DMAP) (0.22 g, 1.83 mmol), and dicyclohexylcarbodiimide (DCC) (0.57 g, 2.75 mmol) as described above in method A. A pale yellow solid 0.5227 g (92.79% yield) was obtained with a melting point of 117–118 °C. UV (MeOH): λmax 342 nm, IR: (KBr disc) 1704, 1618, 1609, 1489 cm−1. 1H NMR: (300 MHz, CDCl3) δ [ppm] 1.31 (t, J = 7 Hz, 3H), 4.22 (q, J = 7 Hz, 2H), 5.93 (d, J = 15 Hz, 1H), 5.98 (s, 2H), 6.69 (dd, J = 16, 11 Hz, 1H), 6.78 (d, J = 8 Hz, 1H), 6.81 (d, J = 15 Hz, 1H), 6.91 (dd, J = 1.6, 9 Hz, 1H), 6.99 (d, J = 1.6 Hz, 1H), 7.41 (dd, J = 10.5, 15.3 Hz, 1H). 13C NMR: (75.47 MHz, CDCl3) 167.1, 148.5, 148.2, 144.6, 140.1, 130.5, 124.5, 122.9, 120.4, 108.5, 105.8, 101.3, 60.2, 14.3, and GC/MS (m/z, relative intensity) 246 [M+].
Preparation of propyl piperate
The title compound was obtained from piperic acid (0.1005 g, 0.46 mmol), n-propanol (1.0 mL), 4-dimethylaminopyridine (DMAP) (0.04 g, 0.36 mmol), and dicyclohexylcarbodiimide (DCC) (0.11 g, 0.55 mmol) as described above in method A. A pale yellow solid 0.0747 g (62.39% yield) was obtained with a melting point of 97–98 °C. UV (MeOH): λmax 342 nm, IR: (KBr disc) 1693, 1611, 1488 cm−1. 1H NMR: (300 MHz, CDCl3) δ [ppm] 0.97 (t, J = 7.4 Hz, 3H), 1.70 (sextet, J = 7.3 Hz, 2H), 4.12 (t, J = 6.8 Hz, 3H), 5.95 (d, J = 15.3 Hz, 1H), 5.98 (s, 2H), 6.70 (dd, J = 10.6, 16 Hz, 1H), 6.79 (d, J = 8 Hz, 1H), 6.81 (d, J = 15.5 Hz, 1H), 6.91 (dd, J = 1.5, 8.1 Hz, 1H), 6.99 (d, J = 1.6 Hz, 1H), 7.41 (dd, J = 10.5, 15.3 Hz, 1H). 13C NMR: (75.47 MHz, CDCl3) 167.2, 148.5, 148.2, 144.6, 140.0, 130.5, 124.5, 122.9, 120.4, 108.5, 105.8, 101.4, 65.9, 22.1, 10.4, and GC/MS (m/z, relative intensity) 260 [M+].
Preparation of isopropyl piperate
The title compound was obtained from piperic acid (1.1590 g, 5.31 mmol), isopropanol (1.0 mL), 4-dimethylaminopyridine (DMAP) (0.529 g, 4.33 mmol), and dicyclohexylcarbodiimide (DCC) (1.154 g, 5.59 mmol) as described above in method A. A pale yellow liquid 1.1683 g (84.60% yield) was obtained. UV (MeOH): λmax 343 nm, IR: (KBr disc) 1698, 1609, 1489 cm−1. 1H NMR: (300 MHz, CDCl3) δ [ppm] 1.28 (d, J = 6.2 Hz, 6H), 5.09 (sep, J = 6.3 Hz, 1H), 5.91 (d, J = 15.2 Hz, 1H), 5.98 (s, 2H), 6.68 (dd, J = 10.4, 15.4 Hz, 1H), 6.77 (d, J = 2.4 Hz, 1H), 6.81 (d, J = 9.9 Hz, 1H), 6.96 (dd, J = 1.4, 8.1 Hz, 1H), 6.99 (d, J = 1.7 Hz, 1H), 7.39 (dd, J = 10.5, 15.2 Hz, 1H). 13C NMR: (75.47 MHz, CDCl3) 166.7, 148.4, 148.2, 144.4, 139.9, 130.6, 124.5, 122.8, 120.9, 108.5, 105.8, 101.3, 67.5, 21.9, and GC/MS (m/z, relative intensity) 260 [M+].
Preparation of isobutyl piperate
The title compound was obtained from piperic acid (1.0051 g, 4.61 mmol), isopropanol (1.0 mL), 4-dimethylaminopyridine (DMAP) (0.529 g, 4.33 mmol), and dicyclohexylcarbodiimide (DCC) (1.154 g, 5.59 mmol) as described above in method A. A pale yellow semi-solid 1.1683 g (92.50% yield) was obtained. UV: λmax 344 nm, IR: (KBr disc) 1705, 1609, 1489 cm−1. 1H NMR: (300 MHz, CDCl3) δ [ppm] 0.98 (d, J = 6.7 Hz, 6H), 2.01 (sep, J = 6.3 Hz, 1H), 3.97 (d, J = 7.7 Hz, 2H), 5.98 (d, J = 15.4 Hz, 1H), 6.00 (s, 2H), 6.71 (dd, J = 10.4, 15.4 Hz, 1H), 6.80 (d, J = 4.7 Hz, 1H), 6.84 (d, J = 11.1 Hz, 1H), 6.93 (dd, J = 1.6, 8.1 Hz, 1H), 7.01 (d, J = 1.6 Hz, 1H), 7.43 (dd, J = 10.5, 15.5 Hz, 1H). 13C NMR: (75.47 MHz, CDCl3) 167.2, 148.5, 148.2, 144.6, 140.0, 130.6, 124.5, 122.8, 120.4, 108.5, 105.8, 101.3, 70.4, 27.8, 19.1, and MS (LC-MS): M+ = 274, [M+ + 23 = 297].
Preparation of piperic acid derivative emulsion and determination of SPF values
The piperic acid derivative was prepared as o/w emulsion (Nikkol Nikkomulese LC supplementary information, Citation2012). The process started with using Nikkomulesse 41, cetostearyl alcohol, caprylic/capric triglyceride, and piperic acid derivative (5% w/w) as an oil phase adding into 1,3-butylene glycol, carbopol 940, l-arginine, and water at 80 °C. The mixture was homogenized and cooled down to room temperature. Next, the Transpore Tape® was placed on an open metal frame and the emulsion was applied in small spots with the syringe over a 50 cm2 area at 2 μL/cm2. The lotion spots were spread uniformly over the surface area. The sample was kept in the dark for 15 min. The SPF measurement was performed on Optometrics SPF-290S analyzer. First, the blank Transpore Tape® was measured and the data were collected. Next, the sample was measured in six different points and the data were analyzed for SPF, UVA/UVB ratio, and Boots Star Rating values. In this test, we used salisol-3 (benzophenone 3) as a reference compound.
Cytotoxicity determination of human skin fibroblast cells
Human skin fibroblast cells (CRL-2522) were purchased from American Type Culture Center (ATTC). Typically, 6 × 104 cells were seeded in 96-well plates and cultured in Minimum Essential Medium Eagle (MEM) with Earle’s balance salt and L-glutamine, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (PS) at 37 °C under 5% CO2. After 24 h, the cells were washed with 100 μL of serum-free MEM (1% PS) twice and incubated with 100 μL of different concentrations of isopropyl piperate and salisol-3 suspensions in serum-free MEM (1% PS). After 24 h exposure, the cells were washed twice with 100 μL of serum-free MEM and incubated with 100 μL of 0.5 mg/mL methylthiazolyldiphenyl–tetrazolium bromide (MTT) containing media for 2 h at 37 °C under 5% CO2. Finally, the MTT containing media was removed and the insoluble purple formazan crystals produced by live cells were dissolved in 100 μL of dimethylsulfoxide (DMSO). The plate was placed on a rocking shaker for 20 min and then the optical density of the produced strain was measured at 570 nm (Liao et al., Citation2011). Ellipticine and doxorubicin were used as positive controls while DMSO was used as a negative control.
Computational details
In this study, we used computational approaches to support our experimental data. All molecular geometries of piperate derivatives are fully optimized using the density functional theory (DFT) method with the B3LYP/6-311 + G** level of theory in the GAUSSIAN 09 B01 program (www.gaussian.com). To locate the local minima, frequency analysis of all optimized structures was rechecked to confirm all structures with no imaginary frequencies. The difference in molecular orbital analysis structural variation among these molecules and the effect of the side chain substitution were analyzed to gain the understanding of photophysical properties of UV absorption. The different substitutions (methyl, ethyl, propyl, isopropyl, and isobutyl groups) were used to explain the behavior of the excited states of the compounds.
Results and discussion
The isolation of piperine from black pepper was a straightforward process and obtained piperine in a reasonable yield. Piperine was hydrolyzed with aqueous potassium hydroxide to give piperic acid in good yield. Next step, the esterification reactions of piperic acid with alcohols were performed by using the Steglich reaction. The esterification reactions () gave methyl piperate, ethyl piperate, propyl piperate, isopropyl piperate, and isobutyl piperate in 84.94, 92.79, 62.39, 84.60, and 92.50% yields, respectively. The UV absorptions of piperine, piperic acid and all piperic acid derivatives were measured in methanol with an UV spectrophotometer scanning from 200 to 450 nm. Each compound showed the UV absorption in the same pattern as piperine. The wavelengths of the most intense UV absorption (λmax) of all derivatives ranged from 342 to 344 nm and the molar absorptivity ranged from 16 844 to 35 855 M−1 cm−1().
Table 1. UV absorption data of piperic acid derivatives in methanol.
For SPF measurements of all compounds in this study using the Optometrics SPF-290S analyzer, the SPF values of piperine, piperic acid, methyl piperate, ethyl piperate, propyl piperate, isopropyl piperate, and isobutyl piperate ranged between 2.68 ± 0.17 and 16.37 ± 1.8 (). For the UVA/UVB ratio, all piperic acid derivatives gave high values which ranged from 0.860 to 0.967. In addition, the Boots Star Rating values ranged from 4 (superior) to 5 (ultra). In this study, salisol 3 (benzophenone 3) was used as a reference UV protection compound and showed that SPF was equal to 10.41 ± 1.74. Its UVA/UVB ratio and Boots Star Rating values were 0.525 and 2 (moderate), respectively. When the SPF values of all piperic acid derivatives were compared with their molar absorptivity values, we could not predict the SPF value from the molar absorptivity of the compound. This incident occurred because the piperic acid derivatives were mixed in o/w emulsion which was a different medium from methanol. The efficacy of sunscreens is often influenced by the solvents in which they are dissolved (Agrapidis-Paloympis et al., Citation1987). Thus the emulsion base may influences the SPF value with a unique interaction for each compound.
Table 2. The SPF values of the piperic acid derivatives (5% w/w) o/w emulsion.
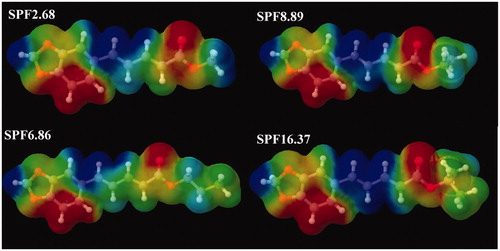
Among the synthesized compounds, isopropyl piperate demonstrated a high SPF value which could protect UVB and also showed high UVA/UVB ratio and Boots Star Rating value which should be a good protection agent for UVA radiation. There was a question why isopropyl piperate possessed a remarkable UV protection property than other derivatives. We decided to perform the molecular modeling to gain insight about this phenomenon. In this work, the calculation results showed that the structural variations of bond lengths of all compounds ranged from 1.349 to 1.449 Å for the central ring (r1–r10) and from 1.374 to 1.438 Å for the side chain (). All bond lengths were almost the same trend except for r17 of isopropyl piperate which was found to be longer than the other compounds resulting in shorter hydrogen bonding length r19 in the structure. The structure of isopropyl piperate with main chain having an intra-molecular hydrogen bonding interaction with hydrogen of substituent group () may have an advantage in the conformation of the π-bond system which enhances the effective electron delocalization.
Table 3. Molecular labeling and structural geometries of piperates compoundsa.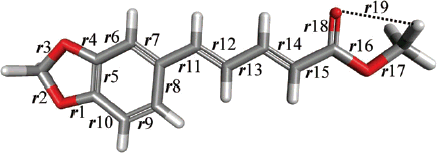
In addition, the positive electron density distribution (blue color, ) of isopropyl piperate in the conjugated double bond region covered over the larger area than other derivatives which mean the electrons were effortlessly delocalized to the oxygen atom of the carbonyl group. As results, the isopropyl piperate may stay in this conformation in the o/w emulsion which has intra-molecular hydrogen bonding and play an important role in the molecular structure concerning with the UV absorption band. This finding explains that the topological requirements of the connectivity of rings in piperate structure influence to the π-electron delocalization and UV protection properties.
For the cytotoxicity test, isopropyl piperate was subject to MTT assay with human skin fibroblast cells. The IC50 results of isopropyl piperate and salisol 3 equaled to 120.2 and 36.72 µM, respectively. The values showed that isopropyl piperate was less cytotoxic than salisol 3.
Conclusions
This research is to develop new UV protection compounds from piperine by converting piperine to piperic acid and preparing its ester derivatives in good yields. Isopropyl piperate has a high potential to be an UV protection agent with high SPF, high UVA/UVB ratio, and superior Boots Star Rating score with low cytotoxicity to human skin fibroblast cells. This is the first report on active UV protection of isopropyl piperate from piperine isolated from Piper nigrum.
Acknowledgements
The authors acknowledge WATA cluster faculty of Engineering, Kasetsart University (URL:http://www.eng.ku.ac.th) for providing computing resources that have contributed to the research results reported within this paper.
Declaration of interest
The authors report no declaration of interest. The authors would like to thank Research Institute, Rangsit University for financial support to this research.
References
- Agrapidis-Paloympis LE, Nash RA, Shaath NA. (1987). The effect of solvents on the ultraviolet absorbance of sunscreens. J Soc Cosmet Chem 38:209–21
- Berger S, Sicker D. (2009). Classic in Spectroscopy, Isolation and Structure Elucidation of Natural Product, 1st ed. Weinheim: Wiley-VCH Verlag & Co. KGaA
- Ferrero L, Pissavini S, Marguerite ZL. (2002). Sunscreen in vitro spectroscopy: Application to UVA protection assessment and correlation with in vivo persistent pigment darkening. Int J Cosmetic Sci 24:63–70
- Fuchs J. (1998). Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, l-ascorbic acid and beta-carotene in cutaneous photoprotection. Free Radical Biol Med 25:848–73
- Ikan R. (1991). Natural Products: A Laboratory Guide, 2nd ed. San Diego, CA: Academic Press
- Kijjoa A, Pinto MM, Tantisewie B, Herz W. (1989). A new linalool derivative and other constituents from Piper ribesoides. Planta Med 55:193–4
- Kullavanijaya P, Lim HW. (2005). Photoprotection. J Am Acad Dermatol 52:937–58
- Lavker RM, Gerberick GF, Veres D, et al. (1995). Cumulative effects from repeated exposures to suberythemal dose of UVB and UVA in human skin. J Am Acad Dermatol 32:53–62
- Leiter U, Garbe, C. (2008). Epidemiology of melanoma and nonmelanoma skin cancer – The role of sunlight. Adv Exp Med Biol 624:89–103
- Liao K, Lin Y, Macosko CW, Haynes CL. (2011). Cytotoxicity of graphene oxide in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces 3:2607–15
- Lucas R, McMichael T, Smith W, Armstrong B. (2006). Solar Ultraviolet Radiation: Global Burden of Disease from Solar Ultraviolet Radiation. Geneva, Switzerland: World Health Organization
- Lupina T, Cripps H. (1987). UV spectrophotometric determination of piperine in pepper preparation: Collaborative study. J Assoc Off Anal Chem 70:112–3
- Madhavi BB, Nath AR, Banji D, et al. (2009). Extraction, identification, formulation and evaluation of piperine in alginate beads. Int J Pharm Pharm Sci 1:156–61
- Neises B, Steglich W. (1978). Simple method for the esterification of carboxylic acids. Angew Chem Int Ed 17:522–4
- Nikkol Nikkomulese LC Supplementary Information. Lecture Note Distributed in Phytocosmetic ORM 624 at Rangsit University, Thailand on 19 January 2013
- Polonini HC, Gomes TBB, Goncalves KM, et al. (2012). In vitro efficacy of photoprotection in sunscreens: A comparison between methods. Lat Am J Pharm 31:353–61
- Xu Y, Shao Y, Voorhees JJ, Fisher GJ. (2006). Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocyte. J Biol Chem 281:27389–97
- Zarai Z, Boujelbene E, Salem NB, et al. (2013). Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci Tech 50:634–41
- Zsila F, Hazal E, Sawyer L. (2005). Binding of the pepper alkaloid piperine to bovine β-lactoglobulin: Circular dichroism spectroscopy and molecular modeling study. J Agric Food Chem 53:10179–85