Abstract
Context: Elevated oxidative stress plays a key role in diabetes-associated vascular disease. Excessive production of reactive oxygen species via advanced glycation end products (AGEs) activates peroxisome proliferator-activated receptor gamma (PPARγ) and the transcription factor nuclear factor-kB (NF-κB) in aortic vascular smooth muscle cells (VSMCs). Apocynin, a drug with an antioxidant effect, has also been proposed as a therapeutic agent for atherosclerotic disease.
Objectives: This work investigates the effects of apocynin on the PPARγ and NF-κB protein expression evoked by AGEs in cultured VSMCs from Goto–Kakisaki (GK) rats, a non-obese insulin model of both insulin resistance and type 2 diabetes.
Materials and methods: VSMCs, isolated from aortas of GK and non-diabetic rats, were cultured. The expression of proteins was evaluated by Western blot. The blood glucose concentration was measured with a blood glucose test meter. The diabetes of GK rats was controlled by blood glucose and insulin determinations (non-fasting values). The serum insulin concentration was determined by radioimmunoassay.
Results: In VSMCs from non-diabetic and GK rats, apocynin (1 and 10 µM) abolished the protein overexpression of NF-κB induced by glycated bovine serum albumin (AGEs-BSA) incubation. However, apocynin (1 and 10 µM) enhanced the expression of PPARγ protein in the presence of AGEs-BSA (100 μg/mL) in VSMCs from non-diabetic, but not from GK rats.
Conclusion: These findings suggest that apocynin decreases the incidence of alterations in VSMCs induced by AGEs through the reduction of NF-κB and may represent an attractive therapeutic approach to treat diabetes mellitus.
Introduction
Hyperglycemia and advanced glycation end products (AGEs) play an important role in accelerated atherosclerosis in diabetes. However, the mechanism by which diabetes is linked to vascular complications remains uncertain and is likely to be complex. The interaction of AGEs with their cell surface receptors (RAGEs) in vascular smooth muscle cells (VSMCs) has been shown to generate reactive oxygen species (ROS) and to activate the redox-sensitive transcription factor NF-κB. The activation of NF-κB one controls the gene transcription of mediators involved in inflammatory responses, especially tumor necrosis factor α, interleukin 1β, vascular cell adhesion molecule, and intercellular adhesion molecule. This process is associated with abnormal proliferation of VSMCs, including effects that lead to aggravation of atherosclerosis in patients with type 2 diabetes via inflammation (Brownlee, Citation1995; Schmidt & Stern, Citation2000; Yerneni et al., Citation1999).
NADPH oxidase has been implicated as the major source of ROS generation in the vasculature in response to high glucose and AGEs. Considering the presence of the NADPH oxidase in several tissues and their clear linkage with inflammatory diseases, several substances with antioxidant property have been tested, as inhibitors of the generation and/or scavengers of the ROS produced via NADPH oxidase activation (Guzik & Harrison, Citation2006; Jones et al., Citation1996). Apocynin has been described as a reversible inhibitor of NADPH oxidase activity that impedes assembly of the p47phox subunit with the membrane complex. It has been proposed as a potential therapeutic agent in the treatment of atherosclerotic disease (Meyer & Schmitt, Citation2000). It was noticed that apocynin markedly decreased mRNA expression of renal cortical gp91phox, p22phox, p47phox, and p67phox NADPH subunits in Dahl salt-sensitive rats (Tian et al., Citation2008). Furthermore, a recent study demonstrated that apocynin suppressed NADPH oxidase subunits in diabetic rats and rat mesangial cells exposed to 30 mM glucose (Xu et al., Citation2009).
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that have been shown to mediate anti-inflammatory actions in VSMCs. Activators of PPARα (lipid-lowering fibrate derivatives) and PPARγ (antidiabetic thiazolidinediones) can inhibit NADPH oxidase activity by inhibiting the expression of Nox subunits (Diep et al., Citation2002; Hwang et al., Citation2005) and can promote the production of nitric oxide in endothelial cells (Calnek et al., Citation2003; Polikandriotis et al., Citation2005). Accordingly, PPARs agonists diminish ROS production in angiotensin II-infused rats and decrease p22phox expression and improve endothelial function in diabetic rats (Diep et al., Citation2002; Hwang et al., Citation2007). To our knowledge, however, no data are available about the action of apocynin on the PPARγ protein expression in VSMCs in type 2 diabetes and especially in Goto–Kakisaki (GK) rats, a non-obese model of both insulin resistance and type 2 diabetes. Therefore, the aim of present work was to evaluate the effects of apocynin on the NF-κB protein expression and PPARγ protein expression caused by glycated bovine serum albumin (AGEs-BSA) in cultured aortic VSMCs from GK rats and non-diabetic rats.
Materials and methods
Materials
The antibodies used for immunohistochemical analysis were the rabbit polyclonal antibodies anti-PPARγ-1 and 2 (Calbiochem, San Diego, CA); mouse monoclonal anti-p65 NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-β-actin (Sigma-Aldrich, St Louis, MO). Horseradish peroxidase-conjugated secondary anti-mouse IgG were from Jackson Immuno Research Laboratories (Bar Harbor, ME). 15-Deoxy prostaglandin J2 (15 d PGJ2) was obtained from Calbiochem. Dulbecco modified Eagle’s medium/Ham’s Nutrient Mixture F-10 (DMEM/F-10), fetal calf serum (FCS), and all other reagents for cell cultures were from Eurobio (Les Ulis, France). Unless otherwise stated, the chemical reagents were from Sigma-Aldrich (St Louis, MO).
Methods
Animals
Male GK rats (12–15 weeks old) were used in the present study. They were inbred in our laboratory (progenitors issued from a colony from M&B A/S, Ry, Denmark) and spontaneously developed diabetes without obesity at 4 weeks of age. Non-diabetic male Wistar rats (Iffa Credo, L’Arbresle, France) were used as controls. All animals had free access to water and standard laboratory rat chow in an air-conditioned room with a 12-h light/dark cycle. All animals were cared for compliance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication 85-23, revised 1996) and all procedures were in accordance with institutional guidelines for animal experimentation.
For the VSMCs cultures, body weight was 352 ± 15 (n = 8) and 350 ± 10 g (n = 8) for GK and control rats, respectively. Animals were anesthetized with intraperitoneal injection of sodium pentobarbital (60 mg/kg). Intracardiac blood samples were taken and thoracic aortas were harvested for VSMC preparation. Control of glycemic status of GK rats was performed by blood glucose and insulin determinations (non-fasting values). Blood glucose concentration was measured with a blood glucose test meter (Glucotrend, Boehringer-Mannheim, Mannheim, Germany), and was significantly (p < 0.05) enhanced in GK rats (14.3 ± 0.6 mmol/L) compared with control ones (7.0 ± 0.2 mmol/L). Likewise, serum insulin concentration which was determined by radioimmunoassay (Dia Sorin, Vercelli, Italy) was significantly (p < 0.05) increased in GK rats (241.7 ± 10.2 pmol/L) compared with control rats (83.5 ± 4.2 pmol/L).
Preparation of AGEs-BSA
AGEs-BSA was prepared in vitro by incubating 50 g/L BSA with 0.5 mol/L d-glucose in phosphate buffered saline (PBS) (pH 7.4) for 6 weeks under sterile conditions in 5% CO2 and 95% O2 at 37 °C. Control non-glycated BSA (50 g/L) was prepared in the same manner without D-glucose supplementation. AGEs-BSA and control BSA were dialyzed for 24 h against PBS overnight to remove the unincorporated glucose. AGE content was estimated by fluorescence spectroscopy (excitation at 390 nm and emission at 460 nm), which revealed an 11-fold increase in fluorescence for AGEs-BSA compared with control BSA. These solutions were stored at 7 °C until use. The AGEs-BSA preparation used herein can be considered as a model for AGEs whereas the commercial AGEs-BSA (Sigma, St Louis, MO) is a model of early glycation Amadori-modified protein (1–5 mole of hexose/albumin molecule as fructosamine).
Cell preparation and culture
VSMCs were isolated from the thoracic aortas of GK (n = 8) and control (n = 8) rats by enzymatic dissociation as previously described (Parés-Herbuté et al., Citation1996). The isolated cells were plated at a density of 20 × 103 cells/cm2 and cultured in DMEM/F-10 containing 5 mmol/L glucose, and supplemented with 2 mmol/L l-glutamine, 25 mmol/L HEPES, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% (v/v) FCS (Eurobio). The culture plates were incubated in a humidified incubator at 37 °C in 5% CO2 and 95% O2. Secondary cultures were established by trypsinization of primary cultures and replated at 20 × 103 cells/cm2. The cells were allowed to grow to subconfluence for the experiments and were then pre-incubated in DMEM containing 0.5% FCS for 48 h. In line with the protocol used, VSMCs from GK and control rats were incubated for 24 h in culture medium-10% FCS in the presence of AGEs-BSA (100 μg/mL) with or without apocynin (1 and 10 µM).
SDS-PAGE and Western blot analysis
Expression of NF-κB and PPARγ proteins was studied using Western blot analysis. The medium was removed and the cells were rinsed three times with PBS, lysed with 0.5 mL of ice cold RIPA buffer (PBS, 1% nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing freshly prepared 1 mmol/L PMSF, 2 mg/L aprotinin, 10 mmol/L pesptatin A, 2 mg/L leucopeptin, and 1 mmol/L sodium orthovanadate, and scraped with a cell scraper. Using a syringe fitted with a 21-gauge needle, the lysate was transferred to a microcentrifuge tube. After centrifugation, the protein concentration in supernatants was determined by the method of Lowry (Protein Dc, Bio-Rad Laboratories, Marne la Coquette, France). A 20 μg aliquot of total proteins was analyzed by 10% SDS-PAGE. The proteins were transferred to polyvinylideneluoride (PVDF) membrane (Immobilon, Millipore, Molsheim, France) by electro-transfer. Non-specific IgGs were blocked with 5% fat-free milk powder in Tris-buffered saline (TBS) and 0.02% Tween-20, for 1 h at room temperature. Then, membranes were incubated with antibodies for NF-κB p65 (1:200) or PPARγ (1:2000) and β-actin (1:5000) for 60 min at room temperature. After washing (TBS), transfers were developed with horseradish peroxidase-conjugated second antibody (1:5000). Bound antibodies were detected using the enhanced chemiluminescence procedure (ECL, Amersham Biosciences EuropeGmbH, Orsay, France). The NIH (National Institutes of Health) Image program, version 1.6, was used for quantitative densitometric scanning of films. Results are expressed in an arbitrary unit as the ratio of NF-κB or PPARγ to β-actin expression.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance (p < 0.05) of the results was performed using the unpaired Student’s t-test and one-way analysis of variance (ANOVA) followed by Bonferroni's multiple test when appropriate.
Results
NF-κB protein expression in VSMCs from rat aortas stimulated by AGEs-BSA in presence and absence of apocynin
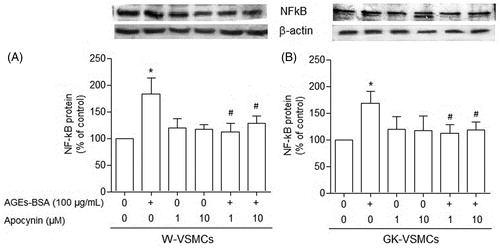
When VSMCs from non-diabetic () and GK () rats were incubated for 24 h with lower (10, 50, and 100 μg/mL), but not higher (500 and 1000 μg/mL) concentrations of AGEs-BSA, the expression of NF-κB protein was nearly 45% higher than in the absence of AGEs-BSA (p < 0.05). In the absence of apocynin, AGEs-BSA (100 μg/mL) significantly increased the expression of NF-κB protein in VSMCs from both GK () and non-diabetic () rats. However, in the presence of apocynin (1 and 10 µM), the stimulation of NF-κB protein expression evoked by AGEs-BSA (100 μg/mL) in VSMCs from both non-diabetic and GK rats was abolished (p < 0.05; and , respectively).
Figure 1. Western blot analysis of NF-κB expression, expressed as percentage in relation to control, in vascular smooth muscle cells (VSMCs) from non-diabetic rats (panel A) and Goto–Kakisaki (GK) rats (panel B). We performed immunoblotting to compare the protein content of NF-κB in VSMCs stimulated by AGE-BSA at the concentrations indicated. Values represent the NF-κB/β-actin ratio. Data are expressed as mean ± SEM of three independent experiments per group, each performed in quadruplicate. *p < 0.05 versus bar one (one-way ANOVA followed by Bonferroni's test).
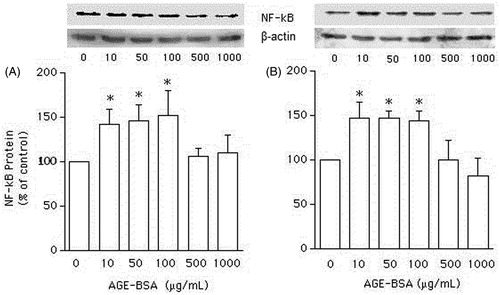
Figure 2. NF-κB expression in vascular smooth muscle cells (VSMCs) from non-diabetic (W) rats (panel A) and Goto–Kakisaki (GK) rats (panel B) stimulated by AGEs-BSA in the presence or absence of apocynin. The influence of AGEs-BSA (100 μg/mL) on NF-κB expression, expressed as percentage in relation to control, in the presence of apocynin (1 and 10 µM) was investigated by Western blot analysis. Values represent the NF-κB/β-actin ratio. Data are shown as mean ± SEM of three independent experiments per group, each performed in quadruplicate. *p < 0.05 versus bar one (one-way ANOVA followed by Bonferroni's test), #p < 0.05 versus bar two (one-way ANOVA followed by Bonferroni's test).
Stimulation of PPAR-γ protein expression by AGEs-BSA
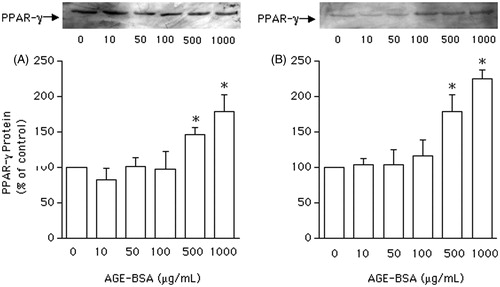
To investigate the effect of AGEs on PPARγ protein expression, VSMCs from non-diabetic and GK rats were incubated with AGEs-BSA at 10, 50, 100, 500, and 1000 μg/mL for 24 h. In cells from both groups, the expression of PPARγ protein was not altered in the presence of AGEs-BSA at 10–100 μg/mL ( and ). However, with higher AGEs-BSA concentrations (500 and 1000 μg/mL), PPARγ protein expression was significantly (p < 0.05) enhanced by 62 and 100%, respectively, in VSMCs from GK rats (), and by 50% and 75%, respectively, in VSMCs from non-diabetic ().
Figure 3. Western blot analysis of peroxisome proliferator-activated receptor gamma (PPARγ) expression, expressed as percentage in relation to control, in vascular smooth muscle cells (VSMCs) from non-diabetic rats (panel A) and Goto–Kakisaki (GK) rats (panel B). Immunoblot was performed to compare the protein content of PPARγ expression in VSMCs stimulated by AGEs-BSA at the concentrations indicated. Values represent the PPARγ/β-actin ratio. Data are expressed as mean ± SEM of three independent experiments per group, each performed in quadruplicate. *p < 0.05 versus bar one (one-way ANOVA followed by Bonferroni's test).
Influence of AGEs-BSA on PPAR-γ protein expression in the absence and presence of apocynin in VSMCs from GK and non-diabetic rats
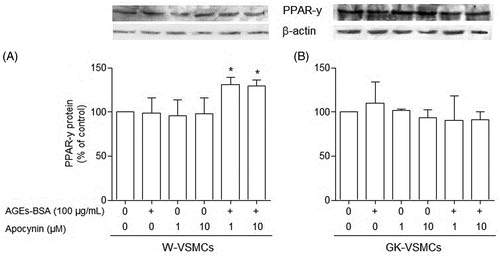
In the absence of apocynin, AGEs-BSA (100 μg/mL) was unable to alter the PPAR-γ protein expression in VSMCs from both GK () and non-diabetic () rats. However, in the presence of apocynin (1 and 10 µM), the expression of PPARγ protein was significantly (p < 0.05) enhanced (by 30%) by AGEs-BSA (100 μg/mL) in VSMCs from non-diabetic rats (), but remained unchanged in VSMCs from GK rats ().
Figure 4. Peroxisome proliferator-activated receptor gamma (PPARγ) expression in vascular smooth muscle cells (VSMCs) from non-diabetic (W) rats (panel A) and Goto–Kakisaki (GK) rats (panel B) stimulated by AGEs-BSA in the presence or absence of apocynin. The influence of AGEs-BSA (100 μg/mL) on PPARγ expression, expressed as percentage in relation to control, in the presence of apocynin (1 and 10 µM) was investigated by Western blot analysis. Values represent the PPARγ/β-actin ratio. Data are shown as the mean ± SEM of three independent experiments per group, each performed in quadruplicate. *p < 0.05 versus bar two (one-way ANOVA followed by Bonferroni's test).
Discussion
It is known that NF-κB activation participates in vascular remodeling and plays a role in genesis of stress oxidative too. Indeed, NF-κB increases during the early stages of insulin resistance, occurring before hyperglycemia sets in (Faure et al., Citation2001). Here, we compared the effects of apocynin, a reversible inhibitor of NADPH oxidase activity, on the NF-κB protein expression in VSMCs from non-diabetic and diabetic rats. We found that apocynin has an inhibitory effect on the activation of NF-κB protein expression by AGEs-BSA in VSMCs from non-diabetic and GK rats. Significant responses to apocynin stimulation have also been observed in other models (Satriano & Schlondorff, Citation1994) such as endothelial cells, with the use of submicromolar concentrations (Csiszar et al., Citation2006).
Previously, we demonstrated that RAGE and NF-κB protein expressions were more than three-fold higher in aortas from GK rats than from non-diabetic rats (de Oliveira Silva et al., Citation2008). This overexpression was associated with higher plasma AGEs, aortic production of superoxide anion, and advanced oxidation protein products (de Oliveira Silva et al., Citation2008), as was observed in the present study (data not shown). These findings do validate the model of GK aorta used herein which fits the present understanding of insulin resistance and type 2 diabetes. The GK rats are a non-obese model of NIDDM that have elevated fasting glucose, impaired response to glucose, and increased HbA1c levels at an early age (Goto et al., Citation1976; Yagihashi et al., Citation2002; Yasuda et al., Citation2002). It has been suggested that impaired pancreatic mitochondrial function may partially explain the depressed insulin release (Oliveira et al., Citation2001, Citation2002). Whether this is related to a shift in mitochondrial antioxidative capacity resulting in accelerated apoptosis is unclear (Ferreira et al., Citation1999). More recently, Rosengren et al. (Citation2010) identified a polymorphism in the GK that resulted in overexpression of the α2A-adrenergic receptor leading to depressed insulin sensitivity. This polymorphism was also associated with decreased insulin sensitivity in humans supporting the concept that decreased insulin sensitivity may be one contributing cause of NIDDM, further linking the GK model to the human condition (Rosengren et al., Citation2010).
To our knowledge, however, no data are available about the effects of apocynin on the PPARγ protein expression induced by AGEs-BSA in VSMCs in type 2 diabetes and especially in GK rats. In the absence of apocynin, an increase in PPARγ protein expression was observed in VSMCs from GK and non-diabetic rats stimulated only with higher (≥ 500 µg/mL) concentrations of AGEs-BSA. However, in the presence of apocynin, PPARγ protein expression was significantly enhanced even by a lower concentration (100 µg/mL) of AGEs-BSA in VSMCs from non-diabetic, but not GK rats. This is the first report showing the ability of apocynin to up-regulate PPARγ protein levels in the presence of AGEs-BSA. In primary cultures of bovine retinal endothelial cells, the inhibition of NADPH oxidase by apocynin or deletion of Nox-2 restores normal levels of retinal PPARγ (Tawfik et al., Citation2009). Such results seem to reinforce the idea of the launching therapeutic trials with PPARγ agonists at early stages of disease, long before AGEs cellular concentrations reach deleterious levels.
In our model using VSMCs from GK rats, apocynin (1 and 10 µM) was unable to up-regulate PPARγ expression in the presence of AGEs-BSA (100 μg/mL). This finding may suggest that the suppression of PPARγ expression is involved in the pathogenesis of diabetes and NADPH oxidase could be an upstream mediator of these changes. Most of the studies performed with vascular cells (Liu et al., Citation2007; Martín et al., Citation2012; Zhang et al., Citation2006) have used higher apocynin concentrations. Those might be required owing to the short duration of the in vitro experiments (only some when using cells cultures, instead of several years of stress oxidative in diabetes pathology). The micro-environment of the cells during in vitro experiments and cell isolation procedures could also be involved.
Conclusion
In summary, our findings support the hypothesis that apocynin improves the perturbations induced by AGEs-BSA through inhibition of NF-κB protein expression in VSMCs from GK rats. The long-term effect of apocynin on the AGEs-induced PPAR-γ protein expression deserves further investigation in VSMCs from GK rats to elucidate whether there is a link between NADPH oxidase and PPARγ that leads to vascular dysfunction.
Acknowledgements
This study is part of a Dissertation developed by C. O. Silva in order to obtain a Ph.D. degree in Biological Sciences while she was student at the Laboratoire de Biochimie, Hôpital Lapeyronie et Université Montpellier, Montpellier, France.
Declaration of interest
This study was supported by the French Research-National Education Ministry (LNHA 2993) and the CAPES-COFECUB (France-Brazil research cooperation) project number 296/99-I.
References
- Brownlee M. (1995). The pathological implications of protein glycation. Clin Invest Med 18:275–81
- Calnek DS, Mazzella L, Roser S, et al. (2003). Peroxisome proliferator-activated receptor gamma ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol 23:52–7
- Csiszar A, Smith K, Labinskyy N, et al. (2006). Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: Role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291:1694–9
- de Oliveira Silva C, Delbosc S, Araïs C, et al. (2008). Modulation of CD36 protein expression by AGEs and insulin in aortic VSMCs from diabetic and non-diabetic rats. Nutr Metab Cardiovas Dis 1:23–30
- Diep QN, Amiri F, Touyz RM, et al. (2002). PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension 40:866–71
- Faure P, Ramon O, Favier A. (2001). Glucose and redox sensitive transcription factors: Consequences on the insulin receptor. J Soc Biol 195:383–5
- Ferreira FM, Palmeira CM, Matos MJ, et al. (1999). Decreased susceptibility to lipid peroxidation of Goto–Kakizaki rats: Relationship to mitochondrial antioxidant capacity. Life Sci 65:1013–125
- Goto Y, Kakizaki M, Masaki N. (1976). Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119:85–90
- Guzik TJ, Harrison DG. (2006). Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov 11:524–33
- Hwang J, Kleinhenz DJ, Lassègue B, et al. (2005). Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol 288:C899–905
- Hwang J, Kleinhenz DJ, Rupnow HL, et al. (2007). The PPARgamma ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vasc Pharmacol 46:456–62
- Jones SA, O'Donnell VB, Wood JD, et al. (1996). Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271:1626–34
- Liu S, Ma X, Gong M, et al. (2007). Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med 42:852–63
- Martín A, Pérez-Girón JV, Hernanz R, et al. (2012). Peroxisome proliferator-activated receptor-γ activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress. J Hypertens 30:315–26
- Meyer JW, Schmitt ME. (2000). A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett 472:1–4
- Oliveira PJ, Rolo AP, Seiça R, et al. (2001). Decreased susceptibility of heart mitochondria from diabetic GK rats to mitochondrial permeability transition induced by calcium phosphate. Biosci Rep 21:45–53
- Oliveira PJ, Rolo AP, Seiça R, et al. (2002). Impact of diabetes on induction of the mitochondrial permeability transition. Rev Port Cardiol 21:759–66
- Parés-Herbuté N, Hillaire-Buys D, Etienne P, et al. (1996). Adenosine inhibitory effect on enhanced growth of aortic smooth muscle cells from streptozotocin-induced diabetic rats. Br J Pharmacol 118:783–9
- Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. (2005). Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma dependent mechanisms. Arterioscler Thromb Vasc Biol 25:1810–16
- Rosengren AH, Jokubka R, Tojjar D, et al. (2010). Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327:217–20
- Satriano J, Schlondorff D. (1994). Activation and attenuation of transcription factor NF-κB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3′:5′-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest 94:1629–36
- Schmidt AM, Stern DM. (2000). Hyperinsulinemia and vascular dysfunction: The role of nuclear factor kappa B yet again. Circ Res 87:722–4
- Tawfik A, Sanders T, Kahook K, et al. (2009). Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: Role of NADPH oxidase. Invest Ophthalmol Vis Sci 50:878–84
- Tian N, Moore RS, Phillips WE, et al. (2008). NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J physiol Regul Integr Comp Physiol 295:1858–65
- Xu M, Dai DZ, Dai Y. (2009). Normalizing NADPH oxidase contributes to attenuating diabetic nephropathy by the dual endothelin receptor antagonist CPU0213 in rats. Am J Nephrol 29:252–6
- Yagihashi S, Goto Y, Kakizaki M, Kaseda N. (2002). Thickening of glomerular basement membrane in spontaneously diabetic rats. Diabetologia 15:309–12
- Yasuda K, Nishikawa W, Iwanaka N, et al. (2002). Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto–Kakizaki rats. Clin Exp Pharmacol Physiol 29:1001–8
- Yerneni KK, Bai W, Khan BV, et al. (1999). Hyperglycemia-induced activation of nuclear transcription factor kB in vascular smooth muscle cells. Diabetes 48:855–64
- Zhang M, Kho AL, Anilkumar N, et al. (2006). Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: Involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 113:1235–43

