Abstract
Context: Mangiferin (2-C-β-d-gluco-pyranosyl-1,3,6,7-tetrahydroxyxanthone) is a well-known natural antioxidant distributed in various plants of the Anacardiaceae and Gentianaceae families. Mangiferin can inhibit carcinogen-induced lung or colon tumor formation in experimental animals. However, the molecular mechanisms of its chemopreventive activity remain unexplored.
Objective: This study aimed to investigate the effects of mangiferin on chemical carcinogen-induced DNA damage and Nrf2-ARE signaling in hematopoietic cells.
Materials and methods: Mononuclear cells (MNCs) were isolated from human umbilical cord blood (hUCB). DNA damage was evaluated by comet and micronucleus assays. The expression of Nrf2 and NQO1 was examined by immunofluorescence and western blotting. An electrophoretic mobility shift assay (EMSA) was used to detect the binding activity of Nrf2 with NQO1-ARE sequences.
Results: We found that mangiferin treatment significantly reduced DNA damage in etoposide-treated MNCs, which was verified by decreased olive tail moment (OTM) and micronucleus (MN) frequency. Mangiferin treatment significantly promoted Nrf2 translocation into the nucleus and increased nuclear Nrf2 expression. Moreover, NQO1, an Nrf2 signaling target, was significantly upregulated by mangiferin treatment, and the binding activity of Nrf2 with NQO1-ARE sequences was elevated after mangiferin treatment.
Discussion and conclusion: Mangiferin activated Nrf2 signaling, upregulated NQO1 expression, and significantly reduced etoposide-induced DNA damage. Thus, mangiferin is a potential cytoprotective agent for hematopoietic cells.
Introduction
Oxidative stress evoked by environmental insults such as pollutants, chemicals, and natural toxins is closely associated with the etiology of malignant diseases including leukemia (Li & Kong, Citation2009; Zhou et al., Citation2010). The environmental insults usually activate the antioxidative functions in cells and induce the expression of antioxidants and phase II detoxifying enzymes, such as NAD(P)H:quinone oxidoreductase 1 (NQO1) and glutathione S-transferase (GST). Thus, cells can resist the detrimental effects of oxidative injury and protect themselves from subsequent DNA damage (Sun et al., Citation2007; Velichkova & Hasson, Citation2005).
Pivotal to the antioxidative response is the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a member of the leucine zipper protein family. The transcriptional activity of Nrf2 is repressed in part by Kelch-like ECH-associated protein-1 (Keap-1) under homeostatic conditions. It can be activated by oxidative stress and some dietary compounds or synthetic chemicals, which results in the accumulation of Nrf2 protein in the nucleus. Nrf2 forms a heterodimer with musculo-aponeurotic fibrosarcoma (Maf) protein, interacts with the cis-acting enhancer sequence called antioxidant response element (ARE), and subsequently initiates transcription of phase II detoxifying enzymes (Eggler et al., Citation2008; Kundu & Surh, Citation2010). Phase II detoxifying enzymes modulate the metabolism of carcinogens and inhibit carcinogenesis in animal models (Irigaray & Belpomme, Citation2010). Mice lacking Nrf2 are more susceptible to carcinogen-induced carcinogenesis, while Nrf2 inducers/activators have been shown to prevent carcinogenesis in animal models (Yates, Citation2006; Yates et al., Citation2009). Therefore, Nrf2-ARE antioxidant signaling has been considered a promising target for cellular protection and chemoprevention.
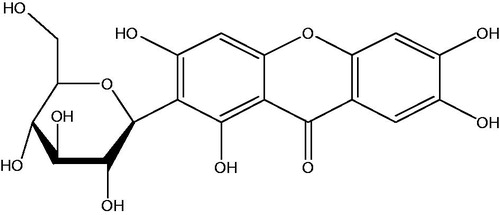
The chemical structure of mangiferin (2-C-β-d-gluco-pyranosyl-1,3,6,7-tetrahydroxyxanthone) is shown in . It is a recently discovered pharmacologically active phytochemical, which possesses antioxidative properties (Andreu et al., Citation2005; Muruganandan et al., Citation2002). This compound is widely distributed in different plants of the Anacardiaceae and Gentianaceae families, including Mangifera indica L. (mango), especially in the leaves and bark of plants (Andreu et al., Citation2005). Mango juice and juice extracts can inhibit chemical-induced neoplastic transformation of mammalian cell lines, suggesting their chemopreventive activities (Percival et al., Citation2006). Moreover, mangiferin inhibited chemical carcinogen-induced lung or colon cancer in experimental animals, indicating that it is a naturally occurring chemopreventive agent (Rajendran et al., Citation2008; Yoshimi et al., Citation2001). However, it is unclear whether mangiferin has an inhibitory effect on leukemia and its related molecular mechanisms. Although it has been demonstrated that mangiferin treatment can upregulate the expression of detoxification enzymes and reduce DNA damage in a benzo(a)pyrene-induced lung carcinogenesis animal model (Rajendran et al., Citation2008), it is still unclear whether the inhibitory effect of mangiferin treatment on carcinogenesis is achieved by Nrf-ARE signaling and detoxification enzymes.
As an important chemotherapeutic agent, etoposide has been shown to cause multiple forms of DNA damage, including DNA single-strand breaks, DNA double-strand breaks, and DNA-protein cross-links, which are responsible for the drug cytotoxicity (Montecucco & Biamonti, Citation2007; Wozniak & Ross, Citation1983). Moreover, previous studies have explored the association of etoposide with acute myeloid leukemia (AML) by using human umbilical cord blood (hUCB) cells (Libura et al., Citation2005; Vlasova et al., Citation2011). In the present study, we investigated the effects of mangiferin on the Nrf-ARE signaling pathway and etoposide-induced DNA damage in mononuclear cells (MNCs) from hUCB. Here, we observed that mangiferin activated Nrf2-ARE signaling and induced the expression of NQO1 in MNCs.
Materials and methods
Reagents
Mangiferin (C19H18O11, molecular weight 422.34 g/mol, Sigma, St. Louis, MO) was dissolved in dimethylsulfoxide (DMSO) and stored at −20 °C. Etoposide was obtained from Jiangsu Hengrui Medicine (Lianyungang, China). Rabbit polyclonal antibody against human Nrf2 (C-20), mouse monoclonal antibody against human NQO1 (A180), horseradish peroxidase (HRP)-labeled goat-anti-rabbit antibody, HRP-labeled goat-anti-mouse antibody, and mouse monoclonal antibody against human β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody against human lamin B was from Biosynthesis Biotechnology (Beijing, China). Fluorescein isothiocyanate (FITC)-conjugated goat-anti-rabbit antibody was purchased from Pierce (Rockford, IL). RPMI 1640 cell culture medium was purchased from Gibco (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from Hangzhou Sijiqing Biological Engineering Materials (Hangzhou, China). Hoechst 33258 and propidium iodide (PI) were purchased from Sigma (St. Louis, MO).
Isolation and culture of MNCs from hUCB
Samples of hUCB were obtained from women at full-term delivery with informed consent. This study was approved by the Institutional Ethics Review Committee of Tongji Medical College, Huazhong University of Science and Technology. After removing erythrocytes with 6% hydroxyethyl starch, the MNC fraction in heparin-treated hUCB was isolated by Ficoll–Paque density gradient centrifugation. MNCs were cultured in RPMI 1640 supplemented with 20% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin, in a humidified incubator at 37 °C and 5% CO2.
Comet assay
To investigate the effect of mangiferin on etoposide-induced DNA damage, MNCs were treated with 0, 1, 10, or 100 μg/ml etoposide for 2 h (etoposide group), or preincubated with 50 μM mangiferin for 4 h and then treated with 0, 1, 10, or 100 μg/ml etoposide for 2 h (mangiferin + etoposide group). DNA damage was evaluated by a comet assay, which was performed according to the published protocol with minor modifications (Balasubramanyam et al., Citation2009). Briefly, 200 µl of low-melting agarose [1% (v/v) in phosphate-buffered saline (PBS, pH 7.4)] at 37 °C mixed with 10 µl of PBS containing MNCs was transferred onto a precoated [0.5% (v/v) normal melting agarose in PBS (pH 7.4)] slide. Electrophoresis was conducted at 25 V (1.25 V/cm) and 300 mA for 30 min at 4 °C. The slides were stained with PI (25 μg/ml). Comet images were examined using an Olympus fluorescence BX51 microscope (Olympus Corporation, Tokyo, Japan) equipped with an excitation filter of 515–535 nm and a barrier filter of 590 nm, at 200 × and 400 × magnifications. Comet images were captured by an on-line charge-coupled device (CCD) camera (Zeiss, Jena, Germany) and analyzed using Cometscore software (Tritek, Sumerduck, VA). Fifty randomly chosen and nonoverlapping comets in one gel were analyzed, and three gels were analyzed per data point. The results were reported as olive tail moment (OTM), which is an index that takes into account both the migration of the genetic material and the relative amount of DNA in the tail.
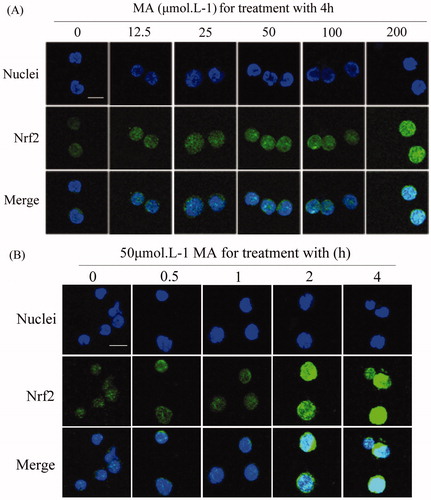
Figure 2. Mangiferin induced the nuclear accumulation of Nrf2 in MNC hUCB cells in confocal microscopy. (A) Cells were treated with 0, 12.5, 25, 50, 100, or 200 μM mangiferin for 4 h in dose–response studies or (B) incubated with 50 μM mangiferin for 0, 0.5, 1, 2, or 4 h, respectively, in time–response studies. Subcellular localization of Nrf2 was determined by confocal microscopy using FITC-conjugated Nrf2 antibody. The nuclei were visualized by Hoechst staining. Merging the Nrf2 and nuclei images confirmed the nuclear localization of Nrf2 (400 × magnification). Scale bars=10 μm.
Micronucleus assay
In the etoposide treatment group, MNCs were treated with 0, 0.025, 0.05, or 0.1 μg/ml etoposide for 24 h. In the mangiferin + etoposide treatment group, MNCs were preincubated with 50 μM mangiferin for 4 h before etoposide treatment. Approximately 1 × 106 cells were collected by centrifugation (500 rpm, 5 min, 4 °C) after treatment and resuspended in 30 µl of heat-inactivated FBS. Aliquots (10 µl) were applied to slides precoated with 3% polylysine. Air-dried slides were submerged in absolute methanol for 10 min and then stored in a dark room until staining with diluted Giemsa solution. MNCs were viewed and captured using an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan) at 1000 × magnification. For each data point, 3000 mononucleated, nonapoptotic, and nonnecrotic cells were analyzed for the presence of a MN (1000 cells per slide × 3 duplicate slides). To be scored as an MN-containing cell, the MN event(s) had to be approximately round in shape, exhibit similar staining characteristics as the main nucleus, be less than one-third of the size of the main nucleus (Bryce et al., Citation2007), and could touch but not overlap with the main nucleus. The occurrence of MNCs was presented as the mean frequency permillage.
Confocal microscopy
To study the effect of mangiferin on the Nrf signaling pathway, MNCs were treated with 0, 12.5, 25, 50, 100, or 200 μM mangiferin for 4 h or with 50 μM mangiferin for 0, 0.5, 1, 2, or 4 h. Nuclear localization of Nrf2 was detected with confocal microscopy. The mangiferin-treated cells were fixed with 4% paraformaldehyde and then carefully seeded on poly-l-lysine-coated coverslips. Cells were permeabilized with 0.3% Triton X-100, blocked with 3% bovine serum albumin, and incubated with Nrf2 antibody at 4 °C overnight. Then the cells were incubated with FITC-conjugated goat-anti-rabbit antibody, and their nuclei were stained with Hoechst 33258. Confocal microscopy was performed, and the images were captured by an Olympus IX71 laser scanning confocal microscope system (Olympus Corporation, Tokyo, Japan) fitted with an AxioCam MRc color CCD (Zeiss, Jena, Germany). The images were analyzed using the Olympus Fluoview Version 4.3 FV500 Tiempo software (Olympus Corporation, Tokyo, Japan). Control cells were processed identically except for the mangiferin treatment. Normal rabbit IgG was used as a negative control (Kim et al., Citation2007).
Western blotting
MNCs were treated with 50 μM mangiferin for 0, 0.5, or 2 h. For immunoblotting, whole-cell lysates were prepared by using a buffer composed of 150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA, 1% (v/v) Nonidet P40, 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 25 μg/ml leupeptin for 30 min at 4 °C (Zhao et al., Citation2010). Nuclear and cytoplasmic extracts were obtained using the Nuclear Extract Popper kit (Pierce, Rockford, IL) (Trotter & Archer, Citation2004). Protein concentration was determined by a BCA Protein Assay Kit (Beyotime Institute Biotechnology, Shanghai, China) (Adilakshmi & Laine, Citation2002). After denaturation, an equal amount of protein extracts was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with buffer containing 20 mM Tris-HCl (pH 7.5), 500 mM NaCl, and 5% nonfat milk for 1 h at room temperature. It was subsequently incubated with Nrf2 antibody or NOQ1 antibody for 24 h at 4 °C, followed by incubation with HRP-labeled secondary antibody after three washes. These blots were developed using an Enhanced Chemiluminescent Detection System (Pierce, Rockford, IL). After stripping, the membrane was reprobed with human β-actin antibody or lamin B antibody as a control for equal protein loading and protein integrity.
Electrophoretic mobility shift assay
MNCs were treated with 50 μM mangiferin or no treatment (control) for 4 h. Nuclear extracts were made using the Nuclear Extract Popper kit (Pierce, Rockford, IL) as described previously (Trotter & Archer, Citation2004; Yamada & Farber, Citation2003). EMSA was performed using the oligomer 5′-AAATCGCAGTCACAGTGACTCAGCAGAATCTGAGCCTAG-3′, which contained the human NQO1-ARE sequences. This oligomer was end-labeled with biotin (Invitrogen China, Shanghai, China) and annealed with its antisense oligomer to yield a double-stranded probe. All EMSA experiments were performed on 6% polyacrylamide gels in Tris-borate-EDTA buffer (45 mM Tris-borate and 1 mM EDTA). Each EMSA reaction mixture contained 500 ng of poly(dI-dC), 1 × LightShift EMSA kit binding buffer (Pierce, Rockford, IL), 1 × LightShift loading dye (Pierce, Rockford, IL), and appropriate amounts of the biotin-labeled human NQO1-ARE probes and protein preparations. Competition reactions were done with 170-fold or 340-fold excess of unlabeled oligonucleotides. Supershift experiments were conducted by incubating nuclear extracts with 0.4 μg of Nrf2 antibody for 2 h on ice. EMSA gels were electroblotted onto a nylon membrane. Signal development was carried out by following the LightShift Chemiluminescent EMSA kit protocol (Pierce, Rockford, IL) with BioMax films (Kodak, Rochester, NY) for luminescence detection (Kumar et al., Citation2008; Newcomb et al., Citation2007).
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from at least three independent experiments and processed by SPSS 15.0 statistical software for Windows (SPSS, Chicago, IL). One-way analysis of variance and the Student–Newman–Keuls test were applied for comparisons between each group. A value of p < 0.05 was considered statistically significant.
Results
Mangiferin activates Nrf2-mediated signaling in hUCB MNCs
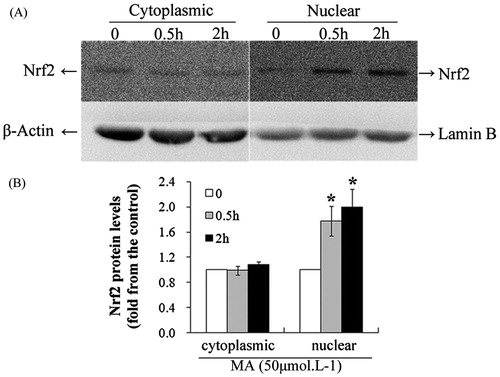
To explore whether mangiferin activates the Nrf2-mediated antioxidant signaling pathway in hUCB MNCs, the nuclear accumulation of Nrf2 was examined by confocal microscopy and western blotting. The location and integrity of the nucleus were assessed by Hoechst 33258 staining. The overlapping of Nrf2 and Hoechst 33258 staining confirmed the nuclear localization of Nrf2. Nrf2 protein was mainly located in the nuclear area in control cells. After mangiferin treatment, the fluorescence intensity in the nucleus was obviously enhanced in a time-dependent manner, indicating the nuclear accumulation of Nrf2 (). Likewise, significant upregulation of Nrf2 protein in the nucleus after mangiferin treatment was verified by western blotting (). These results indicate that mangiferin promotes the nuclear accumulation of Nrf2, thus activating Nrf2 signaling.
Figure 3. Mangiferin increased the nuclear accumulation of Nrf2 in MNC hUCB cells in western blotting. (A) Cells were treated with 50 μM mangiferin for 0, 0.5, or 2 h. Subcellular expression of Nrf2 was determined by western blotting. β-Actin or lamin B was examined as the control for equal protein loading and protein integrity. (B) Nrf2 expression determined by western blotting was quantified by densitometry. Data represented the mean ± SD of at least three independent experiments (*p < 0.05).
Mangiferin increases the binding of Nrf2 with ARE in hUCB MNCs
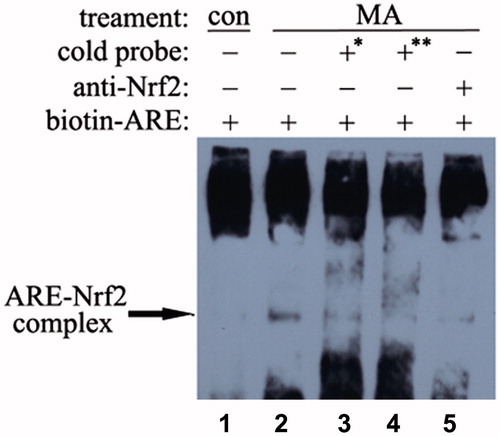
In the nuclear area, Nrf2 protein can bind to ARE and initiate a series of antioxidant/detoxifying enzymes such as NQO1. The EMSA results revealed that mangiferin enhanced the binding of the human NQO1-ARE DNA sequence with Nrf2 protein (). To test the binding specificity of Nrf2 to the human NQO1-ARE-containing DNA probe, a competition assay was performed with a 170-fold and 340-fold excess of cold probes (unlabeled probes) (, lanes 3 and 4). Compared with lane 2 (neither with cold probe nor Nrf2 antibody), the cold probe reduced (170-fold) or nearly abolished (340-fold) the formation of the DNA-protein complex. This study also verified the presence of Nrf2 in the DNA-protein complex by a supershift assay. In addition, preincubation with Nrf2 antibodies markedly reduced the formation of the ARE–protein complex (, lanes 5, compared with lane 2).
Figure 4. Effects of mangiferin on Nrf2-ARE binding in MNC hUCB cells. The interaction of Nrf2 with ARE was analyzed by EMSA. Lane 1, control cells without stress. Lane 2, cells were treated with 50 μM mangiferin for 4 h, then the nuclear extracts were incubated with biotin-labeled human NQO1-ARE probes. Lane 3 or 4, cells were treated with 50 μM mangiferin for 4 h, then the nuclear extracts were incubated with 170-fold or 340-fold excess of cold probes (unlabeled human NQO1-ARE probes). Lane 5, cells were treated with 50 μM mangiferin for 4 h, after that the nuclear extracts were pre-incubated with 0.4 μg Nrf2 antibodies and then incubated with biotin-labeled human NQO1-ARE probes. The arrow indicates the position of the ARE-Nrf2 complexes. *170-fold excess of cold probes and **340-fold excess of cold probes.
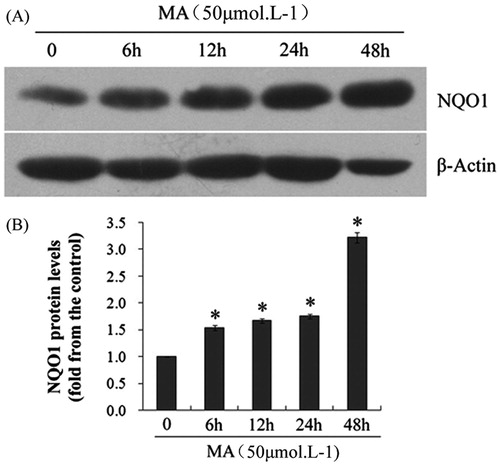
Mangiferin increases the NQO1 protein level in hUCB MNCs
The effect of mangiferin on NQO1 protein expression was examined by western blotting. hUCB MNCs were treated with 50 μM mangiferin for 0, 6, 12, 24, or 48 h. Compared with the control group, the NQO1 level was significantly elevated after mangiferin treatment in a dose-dependent manner ().
Figure 5. Effects of mangiferin on NQO1 expression in MNC hUCB cells. (A) After treating with 50 μM mangiferin for 0, 6, 12, 24, or 48 h, total cell lysates were subjected to western blotting with NQO1 antibodies. β-Actin was examined as the control for equal protein loading and protein integrity. (B) NQO1 expression was quantified by densitometry. Data represented the mean ± SD of at least three independent experiments (*p < 0.05).
Mangiferin reduces etoposide-induced DNA damage in hUCB MNCs
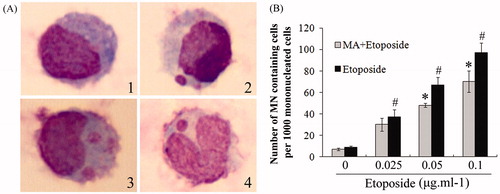
The inhibitory activity of mangiferin on the genotoxicity of etoposide in hUCB MNCs was assessed by a comet assay and an MN assay. hUCB MNCs showed obvious DNA damage in a dose-dependent manner after etoposide treatment. When treated with 100 μg/ml etoposide, the OTM value was significantly increased by 26.3-fold compared with the control (p < 0.05) (). The MN frequency was also significantly increased to 97% with the treatment of 0.1 μg/ml etoposide, compared with 9% in control cells (p < 0.05) (). However, etoposide-induced DNA damage was significantly alleviated when the cells were preincubated with 50 μM mangiferin for 4 h. The OTM was only increased by 13.4-fold in the 50 μM mangiferin plus 100 μg/ml etoposide treatment group, which was only 50.8% of that in the 100 μg/ml etoposide group (p < 0.05) (). The MN frequency was significantly reduced after mangiferin treatment (). These results indicate that the antioxidant mangiferin effectively inhibits etoposide-induced DNA damage in hUCB MNCs.
Figure 6. Mangiferin reduced etoposide-induced DNA damage in MNC hUCB cells in comet assay. (A) Typical comet images (400 × magnification). In etoposide treatment groups, cells were treated with 0, 1, 10, or 100 μg/ml etoposide for 2 h. In MA and etoposide combination treatment groups, cells were pre-incubated with 50 μM mangiferin for 4 h before etoposide treatment. (B) OTM values. (C) %DNA in tail values. Data represented the mean ± SD of at least three independent experiments. (*p < 0.05 compared with the group treating with same concentration of etoposide. #p < 0.05 compared with the nontreated control group).
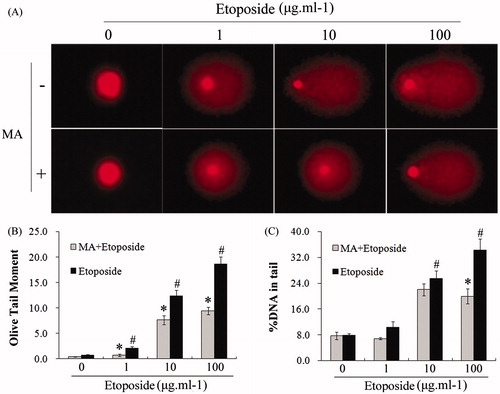
Figure 7. Mangiferin decreased etoposide-induced MN in MNC hUCB cells in MN assay. (A) Typical images (1000 × magnification): (1) normal mononucleated cell; (2) mononucleated cell containing one MN; (3) mononucleated cell containing two MNs; (4) “V” type rhabditiform nuclei cell containing one MN. (B) MN frequency in each group. In etoposide treatment groups, cells were treated with 0, 0.025, 0.05, or 0.1 μg/ml etoposide for 24 h. In MA and etoposide combination treatment groups, cells were pre-incubated with 50 μM mangiferin for 4 h before etoposide treatment. Data represented the mean ± SD of at least three independent experiments. (*p < 0.05 compared with the group treating with same concentration of etoposide. #p < 0.05 compared with the nontreated control group).
Discussion
In this study, we demonstrated that the natural antioxidant mangiferin activated Nrf2-ARE signaling. Recent reports have found that Nrf2 is predominantly localized in the nucleus of various cells, including HepG2, H4IIEC3, and human umbilical vein endothelial cells, in the absence of any stress inducers (Nguyen, Citation2005; Nguyen et al., Citation2009). In the present study, Nrf2 was mainly localized in the nucleus of nontreated hUCB MNCs, which is consistent with these literature reports. It is also documented that Keap1 has nucleocytoplasmic shuttling activity under nonstressed conditions, transiently enters the nucleus, and targets Nrf2 for ubiquitylation in the nuclear compartment (Nguyen, Citation2005; Sun et al., Citation2007; Velichkova & Hasson, Citation2005). Thus, activation of Nrf2 is most likely a result of its stabilization, leading to its accumulation in the nucleus (Hayes et al., Citation2010; Stewart et al., Citation2003). Here, we demonstrated that mangiferin increased the accumulation of Nrf2 in hUCB MNCs, especially in the nuclei, which indicates that mangiferin activates Nrf2. We also showed that mangiferin increased the binding of Nrf2 to ARE and enhanced the expression of the ARE downstream antioxidant enzyme NQO1. Therefore, our results provide evidence that mangiferin can activate Nrf2-ARE signaling in hematopoietic cells.
In this study, we also presented that the natural antioxidant mangiferin alleviated etoposide-induced DNA damage in hematopoietic cells. The mechanisms underlying the cytoprotective activity of mangiferin remain unexplored. However, one of the explanations could be that mangiferin activates Nrf2-ARE signaling. Nrf2 activation by synthetic triterpenoids protects colonic epithelial cells against ionizing radiation-induced damage, in part, by enhancing DNA damage response signaling (Kim et al., 2012). A recent study has reported that the dysfunctional inhibitor of Nrf2 (INrf2) caused decreased etoposide-mediated DNA fragmentation (Niture & Jaiswal, Citation2012). Thus, the finding that mangiferin reduced etoposide-induced DNA damage may be caused by the activation of the Nrf2-ARE signaling pathway.
Moreover, the role of mangiferin as an “Nrf2 activator” could also be one of the possible explanations for chemopreventive activity. Recent studies have found that phytochemicals from dietary plants can protect cells against exogenous insults, preventing carcinogenesis by exerting anti-oxidative stress effects through activation of Nrf2-ARE signaling (Hayes et al., Citation2010; Kwak & Kensler, Citation2010; Shu et al., Citation2010). In the present study, mangiferin significantly reduced etoposide-induced DNA damage. Furthermore, etoposide is also known to cause secondary malignancies including therapy-related AML (Felix, Citation2001). Etoposide-induced DNA damage leads to chromosomal breakages and translocations involving both 5q31 and 11q23, which are specific chromosome regions of significance in leukemogenesis (Escobar et al., Citation2007; Felix et al., Citation2006). Therefore, mangiferin may present chemopreventive activity against therapy-related AML, which needs further study.
NQO1 is one of the most important Nrf2-dependent antioxidant response genes, whose defects result in increasing the susceptibility of DNA damage and leukemogenesis (Bauer et al., Citation2003; Iskander & Jaiswal, Citation2005). It has been reported that the NQO1*2 variant enzyme (codon 609 C→T, encoding a proline to serine substitution) predisposes one to not only de novo AML but also therapy-related leukemia/myelodysplastic syndrome (Malik et al., Citation2006). NQO1 gene expression enhances the clearance of oxidant metabolites in vivo, eases DNA damage induced by carcinogens, reduces gene mutation and chromosomal aberration, and prevents tumorigenesis (Yates & Kensler, Citation2007). Here, we observed that mangiferin treatment significantly upregulated the expression of NQO1 and dramatically activated the binding activity of Nrf2 with NQO1 ARE sequences. This evidence further supports that mangiferin may play an important role in protecting hematopoietic cells from leukemogenesis through Nrf2-ARE signaling. Interestingly, we observed the basal expression of NQO1 in nontreated hUCB MNCs. However, previous reports suggest that NQO1 is present in freshly isolated human bone marrow stromal cells but not in MNCs, although NQO1 expression can be induced by benzene metabolites (Ross, Citation2005). In our study, the same antibody against NQO1 (A180) was used in the immunoblotting assay. Possible explanations for this discrepancy could be stress of delivery, susceptibility of circulating hematopoietic cells to external insults, or differences between hUCB MNCs and bone marrow MNCs. Moreover, it has been recognized recently that Nrf2 can be recruited to the chromatin constitutively to drive basal expression of NQO1 (McMahon et al., Citation2001; Nguyen, Citation2005). Here, we also confirmed the nuclear localization of Nrf2 in nontreated cells. Therefore, the basal expression of NQO1 in hUCB MNCs could be the result of transcriptional activity of Nrf2 under homeostatic conditions.
However, according to certain recent studies, the cytoprotective activity of the Nrf2 activator is controversial. Just as Nrf2 protects normal cells, studies have shown that Nrf2 may also protect cancer cells from chemotherapeutic agents and facilitate cancer progression (Jaramillo & Zhang, Citation2013; Sporn & Liby, Citation2012). While reduction of DNA caused by etoposide might eventually prevent a second malignancy, mangiferin may also reduce the primary therapeutic effects of etoposide against leukemia. Nevertheless, our recent research has found that mangiferin does not reduce the sensitivity of AML cells to etoposide (Zhang et al., Citation2013). One of the possible explanations may be that mangiferin induces apoptosis through suppressing NF-κB signaling in AML cells (Shoji et al., Citation2011). The antitumor activity of mangiferin may neutralize its adverse effects on chemosensitivity. Therefore, mangiferin may be a natural Nrf2 activator that protects normal cells but not tumor cells, which deserves further study.
Conclusion
This study demonstrated that the phytochemical mangiferin effectively activated Nrf2-ARE signaling and reduced etoposide-induced DNA damage in hUCB MNCs. Thus, mangiferin can be considered as a potential cytoprotective agent for hematopoietic cells.
Acknowledgements
The authors would like to thank the Department of Central Laboratory, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, for offering relevant experimental facilities and technical support.
Declaration of interest
The authors declare no conflict of interest. This work was supported by grants from the National Natural Sciences Foundation of China (Nos. 30900632 and 81070429).
References
- Adilakshmi T, Laine RO. (2002). Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J Biol Chem 277:4147–51
- Andreu GL, Delgado R, Velho JA, et al. (2005). Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition. Arch Biochem Biophys 439:184–93
- Balasubramanyam A, Sailaja N, Mahboob M, et al. (2009). In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test. Mutagenesis 24:245–51
- Bauer AK, Faiola B, Abernethy DJ, et al. (2003). Genetic susceptibility to benzene-induced toxicity: Role of NADPH: Quinone oxidoreductase-1. Cancer Res 63:929–35
- Bryce SM, Bemis JC, Avlasevich SL, Dertinger SD. (2007). In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity. Mutat Res 630:78–91
- Eggler AL, Gay KA, Mesecar AD. (2008). Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res 52:S84–94
- Escobar PA, Smith MT, Vasishta A, et al. (2007). Leukaemia-specific chromosome damage detected by comet with fluorescence in situ hybridization (comet-FISH). Mutagenesis 22:321–7
- Felix CA. (2001). Leukemias related to treatment with DNA topoisomerase II inhibitors. Med Pediatr Oncol 36:525–35
- Felix CA, Kolaris CP, Osheroff N. (2006). Topoisomerase II and the etiology of chromosomal translocations. DNA Repair (Amst) 5:1093–108
- Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. (2010). Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 13:1713–48
- Irigaray P, Belpomme D. (2010). Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis 31:135–48
- Iskander K, Jaiswal AK. (2005). Quinone oxidoreductases in protection against myelogenous hyperplasia and benzene toxicity. Chem Biol Interact 153 and 154:147–57
- Jaramillo MC, Zhang DD. (2013). The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27:2179–91
- Kim YJ, Ahn JY, Liang P, et al. (2007). Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res 67:546–54
- Kim SB, Pandita RK, Eskiocak U, et al. (2012). Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci USA 109:2949–55
- Kumar S, Sun X, Wedgwood S, Black SM. (2008). Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol 292:L370–7
- Kundu JK, Surh YJ. (2010). Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res 27:999–1013
- Kwak MK, Kensler TW. (2010). Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol 244:66–76
- Li W, Kong AN. (2009). Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48:91–104
- Libura J, Slater Dj, Felix Ca, Richardson C. (2005). Therapy-related acute myeloid leukemia-like MLL rearrangements are induced by etoposide in primary human CD34 + cells and remain stable after clonal expansion. Blood 105:2124–31
- Malik E, Cohen SB, Sahar D, et al. (2006). The frequencies of NAD(P)H quinone oxidoreductase (NQO1) variant allele in Israeli ethnic groups and the relationship of NQO1*2 to adult acute myeloid leukemia in Israeli patients. Haematologica 91:956–9
- McMahon M, Itoch K, Yamamoto M, et al. (2001). The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res 61:3299–307
- Montecucco A, Biamonti G. (2007). Cellular response to etoposide treatment. Cancer Lett 252:9–18
- Muruganandan S, Gupta S, Kataria M, et al. (2002). Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176:165–73
- Newcomb M, Chen CY, Wu JH. (2007). Induction of the celC operon of Clostridium thermocellum by laminaribiose. Proc Natl Acad Sci USA 104:3747–52
- Nguyen T. (2005). Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem 280:32485–92
- Nguyen T, Nioi P, Pickett CB. (2009). The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–5
- Niture SK, Jaiswal AK. (2012). Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem 287:9873–86
- Percival SS, Talcott ST, Chin ST, et al. (2006). Neoplastic transformation of BALB/3T3 cells and cell cycle of HL-60 cells are inhibited by mango (Mangifera indica L.) juice and mango juice extracts. J Nutr 136:1300–4
- Rajendran P, Ekambaram G, Sakthisekaran D. (2008). Protective role of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol Pharm Bull 31:1053–8
- Ross D. (2005). Functions and distribution of NQO1 in human bone marrow: Potential clues to benzene toxicity. Chem Biol Interact 153 and 154:137–46
- Shoji K, Tsubaki M, Yamazoe Y, et al. (2011). Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-kappaB in HL-60 cells. Arch Pharm Res 34:469–75
- Shu L, Cheung KL, Khor TO, et al. (2010). Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev 29:483–502
- Sporn MB, Liby KT. (2012). NRF2 and cancer: The good, the bad and the importance of context. Nat Rev Cancer 12:564–71
- Stewart D, Killeen E, Naquin R, et al. (2003). Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem 278:2396–402
- Sun Z, Zhang S, Chan JY, Zhang DD. (2007). Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol 27:6334–49
- Trotter KW, Archer TK. (2004). Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol 24:3347–58
- Velichkova M, Hasson T. (2005). Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol 25:4501–13
- Vlasova II, Feng WH, Goff JP, et al. (2011). Myeloperoxidase-dependent oxidation of etoposide in human myeloid progenitor CD34 + cells. Mol Pharmacol 79:479–87
- Wozniak AJ, Ross WE. (1983). DNA damage as a basis for 4′-demethylepipodophyllotoxin-9-(4,6-O-ethylidene-beta-d-glucopyranoside) (etoposide) cytotoxicity. Cancer Res 43:120–4
- Yamada NA, Castro A, Farber RA. (2003). Variation in the extent of microsatellite instability in human cell lines with defects in different mismatch repair genes. Mutagenesis 18:277–82
- Yates MS. (2006). Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res 66:2488–94
- Yates MS, Kensler TW. (2007). Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect 20:109–17
- Yates MS, Tran QT, Dolan PM, et al. (2009). Genetic versus chemoprotective activation of Nrf2 signaling: Overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30:1024–31
- Yoshimi N, Matsunaga K, Katayama M, et al. (2001). The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett 163:163–70
- Zhang BP, Zhao J, Li SS, et al. (2013). Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro: A novel Nrf2 activator without a “dark side”. Acta Pharmacol Sin 35:257–66
- Zhao F, Chen Y, Li R, et al. (2010). Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology 267:70–9
- Zhou FL, Zhang WG, Wei YC, et al. (2010). Involvement of oxidative stress in the relapse of acute myeloid leukemia. J Biol Chem 285:15010–15