Abstract
Context: The content of withanolides in the roots of Withania somnifera (L.) Dunal (Solanaceae) is important for therapeutic application. Earlier studies have shown that the deficiency of macro- and micronutrients affects the growth of W. somnifera. Therefore, we examined the effect of these deficiencies on the withanolides content of the roots.
Objective: To examine the effect of molybdenum accretion in nitrogen-, phosphorus-, calcium- and potassium-deficient soils on the accumulation of withanolides in the roots of W. somnifera. Different withanolides have different therapeutic applications hence major bioactive withanolides assume importance.
Materials and methods: Methanol extracts of the roots were subjected to HPTLC and individual withanolides were identified by comparing their Rf values with those of the authentic samples. Molybdenum was quantified by atomic absorption spectroscopy. Free radical scavenging activity was monitored by the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay.
Results: Molybdenum content in roots of nitrogen-, phosphorus-, calcium-, potassium-deficient, and control plants were 7.02 ± 2.1, 13.1 ± 1.6, 17.1 ± 0.9, 33.5 ± 3.3, and 33.9 ± 1.6 ppm, respectively. Levels of withaferine A increased with the increase in the Mo content in roots from 7.79 ± 2.2 mg/g to 12.57 ± 3.4 mg/g. Antioxidant activity of nitrogen-deficient plants was the lowest (24.7 ± 2.2%) compared to other groups.
Discussion and conclusion: It was observed that nitrogen metabolism-dependent molybdenum uptake influences the withanolides accumulation in the roots.
Introduction
Withania somnifera (L.) Dunal (Solanaceae), commonly known as Ashwagandha, Winter Cherry and Indian ginseng, is considered to be one of the most important medicinal plants of Indian Ayurveda for over 3000 years (Gupta & Rana, Citation2007; Kulkarni & Dhir, Citation2008; Mishra et al., Citation2000). It is used in the forms of decoctions, infusions, ointments, powder, and syrups. Withania somnifera is located mainly in arid zones of Baluchistan, Pakistan, Afghanistan, Sri Lanka, Congo, South Africa, Egypt, Morocco, Jordan, and India. In India, it is widely grown in the states of Madhya Pradesh, Uttar Pradesh, Punjab, Gujarat, and Rajasthan. Withania somnifera has several therapeutic activities, e.g., antioxidant, antimicrobial, antifungal (Mehrotra et al., Citation2011), adaptogenic (Bhattacharya & Muruuganandam, Citation2003), nootropic (Dhuley, Citation2001), cardioprotective (Gupta et al., Citation2004), anticancer (Senthilnathan et al., Citation2006), neuroprotective (Kulkarni & Dhir, Citation2008), anticonvulsant (Kulkarni et al., Citation2008), immunomodulatory (Ziauddin et al., Citation1996), apoptic (Senthil et al., Citation2007), diuretic (Dhabheliya et al., Citation2010), hepatoprotective (Elberry et al., Citation2010), and anti-inflammatory (Pawar et al., Citation2011; Singh et al., Citation2011). These therapeutic activities are attributed mainly to the withanolides present in the roots (Ali et al., Citation1997). Biosynthesis of withanolides occurs by de novo pathway in roots as well as in leaf (Chatterjee et al., Citation2010; Mirjalili et al., Citation2009; Sangwan et al., Citation2008).
Nitrogen (N), phosphorous (P), potassium (K), and calcium (Ca) are the major macronutrients for plant growth while molybdenum (Mo) is a micronutrient which governs biosynthesis and transportation of secondary metabolites (Hamlin, Citation2006). It may be expected that variations in macro- and micronutrients concentration in soil can influence the growth of plants and also biosynthesis/-transport of withanolides. In fact, our earlier studies have shown that deficiency of the macro- and micronutrients adversely affects the growth of W. somnifera (Suryapujary et al., Citation2010). In view of this, it is of interest to find out if and how the biosynthesis/contents of withanolides are also influenced under these conditions.
Material and methods
Chemicals
Glacial acetic acid, ferric chloride hexahydrate, orthophosphoric acid, sulfuric acid, methanol, nitric acid, hydrochloric acid, 1,1-diphenyl-2-picryl-hydrazyl, dichloromethane, toluene, acetone, diethyl ether, cholesterol, and pre-coated silica gel plates [0.2 mm thickness, 60F254 (20 cm ×10 cm)] were procured from Merck Ltd., Mumbai, India. The standards: withanoside V (WS V), withaferine A (WF A), 1,2-deoxywithastramonolide (1,2-DWM), withanolide A (WN A), and withanolide B (WN B) were procured from Natural Remedies Pvt. Ltd., Bangalore, India.
Plant material
Withania somnifera was cultivated in Vidarbha region of Maharashtra State, India. Based on the characteristic symptoms (Suryapujary et al., Citation2010) healthy (control), calcium-deficient (-Ca), potassium-deficient (-K), phosphorus-deficient (-P), and nitrogen-deficient (-N) W. somnifera fresh plants were identified and collected in the month of November and December 2010 from different areas of Vidarbha and were authenticated by Dr. Manasi Deshpande, Professor and Head, Department of Ayurvedic Materia Medica, Ayurved College, Bharati Vidyapeeth Deemed University, Pune, Maharashtra, India, prior to analysis. In each group, 15 plant samples were used.
Preparative procedures
Preparation of color reagent for total withanolide determination
Stock solution (21.5 g ferric chloride hexahydrate dissolved in 100 ml orthophosphoric acid) (8 ml) was diluted to 100 ml with conc. sulfuric acid.
Preparation of root extracts for withanolide and molybdenum analysis
(i) Withanolide analysis: The roots were dried in the shade and pulverized to a coarse powder which was subjected to Soxhlet extraction using methanol. The methanol extract was concentrated under vacuum, dried in vacuum desiccator and redissolved in methanol at a concentration of 20 mg/ml and used for further analysis.
(ii) Molybdenum analysis: 1 g root samples were subjected to acid digestion with 5 ml of concentrated HNO3. To the digested sample, 2 ml concentrated HCl was added and the samples were diluted to 25 ml with deionized water.
Preparation of standard solutions
(i) Withanolide: Stock solutions (1 mg/ml) were prepared in methanol. 4–24 µl of working standards (50 ng/ml made in methanol) were applied to the HPTLC plate to obtain six point calibration curves. The methanol extracts of the roots were subjected to HPTLC analysis for the quantification of the individual withanolides.
(ii) Molybdenum (Mo): Stock solution 1 g Mo was dissolved in a minimum amount of deionized water and HNO3, and 8 ml HC1 was added and was diluted with deionized water to 1 l. Working Mo standards 25, 50, and 100 ppm were prepared by diluting the stock solution.
Estimation of free radical scavenging activity by the DPPH method
Free radical scavenging activity of methanol extracts of W. somnifera was measured by the DPPH method with some modifications (Shimada et al., Citation1992, Yuan et al., Citation2008). 0.1 mM solution of DPPH in methanol (1 ml) was added to 0.5 ml of 100 μg methanol extract of all groups. After 30 min, the absorbance was measured at 517 nm. The percentage of DPPH inhibition was calculated using the equation:
where A0 is the absorbance of the blank and A1 is the absorbance of 100 μg of methanol root extract of Withania somnifera.
Determination of molybdenum
Determination of the Mo content was carried out by atomic absorbance spectroscopy (AAS) in a Thermofisher Scientific AA201 Atomic Absorbance Spectrometer (Thermofisher Scientific, Waltham, MA) (Hanlon, Citation1998). The Mo content in roots was calculated using the following equation:
Determination of total withanolides (TW) content
A modified spectrometric method was used to determine total withanolides content in roots. For this purpose, 1 ml methanol extract was used for the development of color by adding 2 ml of glacial acetic acid and 21 ml of color reagent (Mishra, Citation1994). After keeping for 5 min in an ice bath, the optical density was recorded on a spectrophotometer at 540 nm. The concentration of withanolides was calculated using cholesterol as standard.
Quantification of major bioactive withanolides (MW)
This was carried out by HPTLC chromatography using dichloromethane:toluene:methanol:acetone:diethyl ether (7.5:7.5:3:1:1 v/v) as the mobile phase. The chromatogram was run up to 80 mm. Quantitative evaluation of the plate was performed in the absorption reflection mode at 235 nm, tungsten (W) lamp and deuterium (D2), using slit width: 4.00 × 0.30 mm, data resolution 100 µm/step, scanning speed: 20 mm/s, and base line correction was used (Devkar et al., Citation2012, Citation2014).
Statistical analysis
The data were processed by standard computer program: Excel (2003) and statistical analysis was carried out by one-way ANOVA followed by the post hoc Bonferroni test using Graphpad Prism 5.00 (GraphPad Software, San Diego, CA). The results are expressed as mean ±SD. The p values < 0.05 were considered as significant.
Results
A clear cut separation of major withanolides can be noted in . shows the concentration-dependant HPTLC pattern of standard withanolides as well as the withanolides pattern for the root extract from the experimental samples. WS V, WF A, 1, 2 DWM, WN A, and WN B were identified on the basis of their respective Rf values of 0.7, 0.58, 0.61, 0.68, and 0.79.
Figure 1. HPTLC chromatogram at 235 nm of withanolides, namely withanoside V (WE V), withaferine A (WF A), 1,2-deoxy-withastramonolide (1,2-DWM), withanolide A (WN A), and withanolide B (WN B).
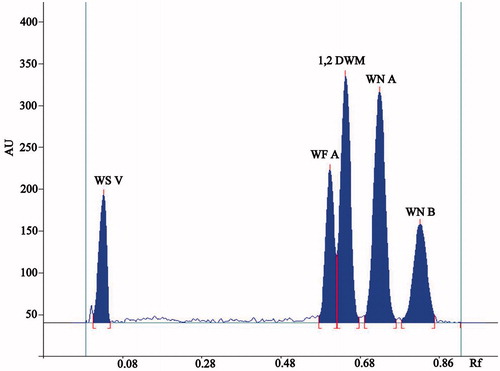
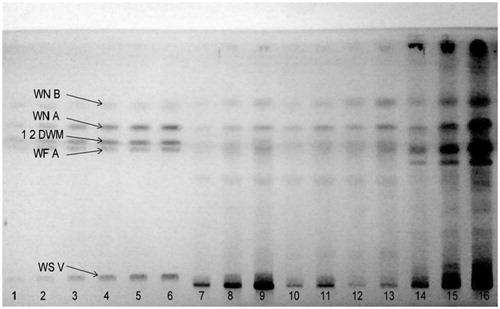
Figure 2. Image of HPTLC plate of W. somnifera at 235 nm. 1–6 tracks for standards mixture; 9, 16 (Control); 8, 15 (-Ca); 7, 14 (-K); 11, 13 (-P), and 10, 12 (-N).
summarizes the effect of macronutrient deficiency on withanolide levels and molybdenum content in the roots of W. somnifera. It can be noted that deficiency of Ca, K, or P had only marginal effect on the content of all withanolides. However, deficiency of N had significant deleterious effect. Thus the content of WS V is totally abolished while there was an overall 36–37% reduction in the MW and TW. Also, the free radical scavenging activity was decreased by 30%. The decrease in antioxidant activity seems to correlate with the reduction in total withanolide content in various deficiency conditions ().
Table 1. Effect of macronutrient deficiency on withanoside V (WE V), withaferine A (WF A), 1, 2 deoxy-withastramonolide (1,2-DWM), withanolide A (WN A), withanolide B (WN B), major withanolides (MW), total withanolides (TW), molybdenum (Mo) content and antioxidant activity of healthy (control), calcium-deficient (-Ca), potassium-deficient (-K), phosphorus-deficient (-P), and nitrogen-deficient (-N) W. somnifera plant.
It can also be noted that the deficiency of Ca and P lowered the Mo content by 50 and 60%, respectively, while deficiency of N caused a significant 80% reduction. It is of interest to note here that despite 50 and 60% reduction in the Mo content in Ca and P deficiency, there was no significant decrease in the individual and total withanolide contents. However, when the concentration of Mo was 7 ppm in -N plants, the levels were affected significantly as pointed have shown above. Hence, we suggest that Mo levels of up to 13 ppm or so may be an absolute requirement for maintaining normal withanolide concentration; levels below this lead to adverse effect.
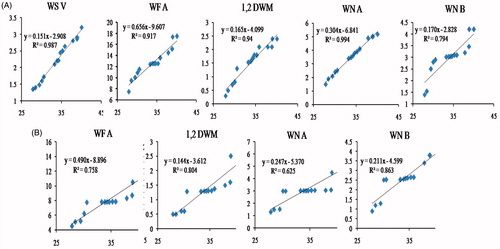
With a view to elaborating the dependence of withanolide contents on Mo, we plotted the concentration of individual withanolides against Mo concentration from different 15 independent observations for control and -N groups. These plots are shown in . It is clear that for the control group a good correlation between withanolide content and Mo concentration was obtained; regression values (R2) ranged from 0.758 to 0.994 (). Even for -N group R2 values ranged from 0.625 to 0.987, implying good correlationship ().
Discussion
N, P, K, and Ca are essential macronutrients for normal growth of the plants (Brady & Weil, Citation1999); 45 kgN ha−1 and 26 kgP ha−1 have been reported to be optimum for normal growth of W. somnifera (Nasira et al., Citation2012). In our previous communication, we have shown that the soils were depleted by about 49% for N, 88% for P, 88% for K and 38% for Ca deficiency (Suryapujary et al., Citation2010). Root length and root diameter are important selection criteria for economic yield (Das et al., Citation2011). The medicinal properties of W. somnifera are attributed to the withanolides content in the roots which are used in several formulations (Ali et al., Citation1997). Thus, critical concentration of macronutrients (N, P, K, and Ca) and also possibly of Mo are required for the proper growth as well as withanolides content for commercial and therapeutic application. Hence, the aim of the present studies was to investigate how these nutrient deficiencies influence the contents of withanolides in the roots.
It is clear from the data presented () that deficiency of Mo had drastic effect on withanolide contents; deficiency of other macronutrients had a marginal effect. The regression analysis studies () showed that there was a positive correlationship between withanolide contents with the Mo content in the roots in control as well as –N condition. Mo is the cofactor of important plant enzymes involved in redox processes: nitrate reductase, xanthine dehydrogenase, aldehyde oxidase, and probably sulfite oxidase (Zimmer & Mendel Citation1999). There is no known transporter for Mo. It is believed that Mo is transported by sulfate () transporter. This assumption is based on the fact that Mo has a structural similarity to
. Transport of
is an energy-dependent process (Ferrari & Renosto, Citation1972). In other words, it is a process of active transport. Therefore, one would presume that Mo transport is also an energy-dependent active transport process. This would mean that the plants will accrete Mo by an energy-dependent process; concentration-dependent passive transport is not involved. In other words, the plants will acquire the requisite quantity of Mo irrespective of its concentration in the soil. In this context, it is of interest to note that the concentration of Mo in soil shows a wide range of variation of 0.5–5 ppm; a 10-fold variation (Sharma & Chatterjee, Citation1997).
It has been shown that uptake of Mo is interlinked with N metabolism. Especially, the role of nitrate reductase is emphasized (Axler et al., 1980; Hamlin, Citation2006). Results of our present studies are consistent with the above. The results would imply that only a severe deficiency of N would impair the withanolide content of the roots together with the Mo content. The content of withanolides in roots is the most important criterion for therapeutic use. Hence, it may be suggested that the soils deficient in N may be fortified with manures rich in N, e.g., urea.
Conclusions
Therefore, it is to be concluded that it is important to maintain normal levels of macronutrients N, P, K, and Ca and especially of N for not only getting a good yield of roots but also for getting the desired amount of withanolides in the roots. It is also evident from the present work that N metabolism-dependent Mo uptake influences the WF A accumulation, which is a major pharmaceutically important withanolide.
Acknowledgements
We thank Dr. Shivajirao Kadam, Vice Chancellor, Bharati Vidyapeeth Deemed University, for his support and encouragement.
Declaration of interest
There is no conflict of interest. We are also thankful to Indian Council of Agriculture Research (ICAR) for the research grant to Dr. M. V. Hegde under National Agriculture Innovation Project (NAIP).
References
- Ali M, Shuaib M, Ansari SM. (1997). Withanolides from the stem bark of Withania somnifera. Phytochemistry 44:1163–8
- Axler RP, Gersberg RM, Goldman CR. (1980). Stimulation of nitrate uptake and photosynthesis by molybdenum in Castle Lake, California. Can J Fish Aquat Sci 37:707–12
- Bhattacharya S, Muruganandam A. (2003). Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol Biochem Behav 75:547–55
- Brady NC, Weil RR. (1999). The Nature and Properties of Soils, 12th ed. Upper Saddle River, USA: Pearson Prentice Hall, Pearson Education Inc
- Chatterjee S, Shrivastava S, Khalid A, et al. (2010). Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Photochemistry 71:1085–94
- Das A, Datta AK, Ghose S, Bhattacharyya A. (2011). Genetic analysis in Poshita and Jawahar 22 varieties of Withania somnifera. Plant Arch 11:59–62
- Devkar ST, Badhe YS, Jagtap SD, Hegde MV. (2012). Quantification of major bioactive withanolides in Withania somnifera (Ashwagandha) roots by HPTLC for rapid validation of Ayurvedic products. JPC-Modern TLC 25:290–4
- Devkar ST, Jagtap SD, Katyare SS, Hegde MV. (2014). Estimation of antioxidant potential of individual components present in complex mixture of Withania sominfera (Ashwagnadha) root fraction by TLC-DPPH method. JPC-Modern TLC 27:157–61
- Dhabheliya J, Khan SA, Joshipura M, et al. (2010). Diuretic potential of aqueous extract of fruits of Withania coagulans Dunal in experimental rats. Int J Pharm Sci 2:51–3
- Dhuley JN. (2001). Nootropic like effect of Ashwagandha (W. somnifera L.) in mice. Phytother Res 15:524–8
- Elberry AA, Harraz FM, Ghareib SA, et al. (2010). Antihepatotoxic effect of Marrubium vulgare and W. somnifera extracts on carbon tetrachloride-induced hepatotoxicity in rats. J Basic Clin Pharm 1:247–54
- Ferrari G, Renosto F. (1972). Regulation of sulfate uptake by excised barley roots in the presence of selenate. Plant Physiol 49:114–16
- Gupta G, Rana A. (2007). Withania somnifera (Ashwagandha): A review. Pharmacogn Rev 1:129–36
- Gupta SK, Mohanty I, Talwar KK, et al. (2004). Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem 260:39–47
- Hamlin RL. (2006). Molybdenum. In: Barker AV, David PJ, eds. Handbook of Plant Nutrition. New York: CRC Press, Taylor & Francis, 375–94
- Hanlon EA. (1998). Elemental determination by atomic absorption spectrophotometer. In: Kalra YP, ed. Handbook of Reference Methods for Plant Analysis. New York: Soil and Plant Analysis Council, Inc., CRC Press, Taylor & Francis, 157–64
- Kulkarni S, Dhir A. (2008). Review article, Withania somnifera: An Indian ginseng. Prog Neuro-Psychopharmacol Biol Psychiatry 32:1093–105
- Kulkarni SK, Akula KK, Dhir A. (2008). Effect of Withania somnifera Dunal root extract against pentylenetetrazol seizure threshold in mice: Possible involvement of GABAergic system. Indian J Exp Biol 46:465–9
- Mehrotra V, Mehrotra S, Kirar V, et al. (2011). Antioxidant and antimicrobial activities of aqueous extract of Withania somnifera against methicillin-resistant Staphylococcus aureus. J Microb Biot 1:40–5
- Mirjalili MH, Moyano E, Bonfill M, et al. (2009). Review, steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14:2373–93
- Mishra SN. (1994). Colorimetric method for estimation of total withanaloids. 10th All India Workshop Report on Medicinal and Aromatic Plants 379–381
- Mishra LC, Singh BB, Dagenais S. (2000). Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Altern Med Rev 5:334–46
- Nasira S, Masroor M, Khana A. (2012). Critical dose of nitrogen and phosphorus enhanced growth, yield and alkaloid content in Withania somnifera. J Plant Nutr 35:1705–24
- Pawar P, Gilda S, Sharma S, et al. (2011). Rectal gel application of Withania somnifera root extract expounds antiinflammatory and mucorestorative activity in TNBS induced inflammatory bowel disease. BMC Complement Altern Med 11:1–9
- Sangwan RS, Chaurasiya ND, Lal P, et al. (2008). Withanolide A is inherently de novo biosynthesized in roots of the medicinal plant Ashwagandha (Withania somnifera). Physiol Plant 133:278–87
- Senthil V, Ramadevi S, Venkatakrishnan V, et al. (2007). Withanolides induces apoptosis in HL-60 leukemia cells via mitochondrial mediated cytochrome C release and caspase activation. Chem Biol Interact 167:19–30
- Senthilnathan P, Padmavati R, Banu SM, Sakthisekaran D. (2006). Enhancement of antitumor effect of paclitaxel in combination with immunomodulatory Withania somnifera on benzo(a)pyrene induced experimental lung cancer. Chem Biol Interact 159:180–5
- Sharma CP, Chatterjee C. (1997). Molybdenum availability in alkaline soils. In: Gupta UC, ed. Molybdenum in Agriculture. USA: Cambridge University Press, 131–49
- Shimada K, Fujikawa K, Yahara K, Nakamura T. (1992). Antoxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–8
- Singh A, Saharan VA, Garg R, Gupta VB. (2011). Effect of time on extraction of Ashwagandha in various hydro alcoholic compositions and their anti-inflammatory activity. Int J Green Pharm 5:69–74
- Suryapujary SM, Deotale RD, Devkar ST, et al. (2010). Studies on nutrient deficiency symptoms in Ashwagandha (Withania somnifera L.). J Soils and Crops 20:39–41
- Yuan JP, Li X, Xu SP, et al. (2008). Hydrolysis kinetics of secoisolariciresionl diglucoside oligomers from flaxseed. J Agric Food Chem 56:10041–7
- Ziauddin M, Phansalkar N, Patki P, et al. (1996). Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol 50:69–76
- Zimmer W, Mendel R. (1999). Molybdenum metabolism in plants. Plant Biol 1:160–8