Abstract
Context: The fruit pulp of Luffa cylindrica Roemer (Cucurbitaceae) (LC) has been used to induce hemostasis, resolve phlegm and clear fever in traditional Korean medicine. However, the efficacy of LC has not been examined in atopic dermatitis (AD).
Objective: A 70% ethanol extract of LC was evaluated to determine anti-inflammation and anti-AD effects in vitro and in vivo.
Materials and methods: The inhibitory effects of LC on the production of PGE2 and histamine were respectively measured in lipopolysaccharide-treated (1 μg/mL) RAW264.7 macrophages and phorbol-12 myristate 13-acetate (50 nM) and A23187 (1 µM)-stimulated HMC-1 mast cells. The production of AD-related chemokines (RANTES, TARC, and MDC) were evaluated in IFN-γ and TNF-α-stimulated (10 ng/mL, each) HaCaT keratinocytes. LC (10 mg/mouse/d) was topically applied to the dorsal skin and ears of Dermatophagoides farina (Pyroglyphidae)-sensitized Nc/Nga mice for 4 weeks.
Results: The IC50 values of LC on PGE2 and histamine production were 16.89 and 139.9 μg/mL, individually. The production of TARC and RANTES were inhibited 20% and 12% by LC (50 μg/mL) in HaCaT cells, respectively (p < 0.05). In sensitized-NC/Nga mice, the plasma levels of IgE and histamine were suppressed 36% and 41% by LC, respectively (p < 0.05). LC also reduced hemorrhage, hypertrophy, and hyperkeratosis of the epidermis and infiltration of mast cells in the dorsal skin and ear.
Discussion and conclusion: LC can inhibit AD-like skin lesions and reduce the generation of IgE via inhibition of the inflammatory responses. LC has potential as a therapeutic agent to treat allergic diseases, including AD.
Introduction
The fruit pulp of Luffa cylindrica Roemer (Cucurbitaceae), loofa sponge, has been used to promote menstruation, hemostasis, resolve phlegm, invigorate the network vessels, clear fever, and invigorate blood in traditional Korean medicine. Most often, the fibers are used for scrubbing and exfoliating dead skin in bathrooms, and the extracts of this pulp are used as a cosmetics supplement for moistening and whitening skin care. It has been reported that the ethanol and ethyl acetate extracts of L. cylindrica inhibit the inflammatory response through inhibiting production of inflammatory mediators, including nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokine (IL-6) in murine macrophage cell lines (Kao et al., Citation2012). The fruits and seeds of L. cylindrica contain bioactive compounds such as luffin P1 (anti-HIV-1 activity; Ng et al., Citation2011), α-luffin (antitumor activity) (Liu et al., Citation2010) and sapogenins (oleanolic acid and echinicystic acid), which stimulate immune responses such as proliferation of lymphocytes and phagocytosis of macrophages (Khajuria et al., Citation2007). Bryonolic acid, isolated from cultured cells of L. cylindrica, is reported to inhibit homologous passive cutaneous anaphylaxis and delayed hypersensitivity when administered to a murine model intraperitoneally (Tanaka et al., Citation1991). However, the efficacy of the pulp of L. cylindrica has not been evaluated for treating dermatologic diseases.
Atopic dermatitis (AD; atopic eczema) is defined as an inflammatory, pruritic, chronic, or chronically relapsing skin disease that is often the first step in the development of other atopic diseases as rhinitis and/or asthma (Ring et al., Citation2012a). The elevation of IgE level in serum occurs in most AD patients (Hanifin et al., Citation2004). IgE mediates mast cell activation, which results in the release of preformed mediators, such as histamine and inflammatory mediators, including cytokines (IL-4 and IL-13) and chemokines. These cytokines promote Th2-polarized T cell responses. The sensitized mast cells can stimulate keratinocytes to express numerous signals for T-cells, including proinflammatory cytokines and chemokines such as those regulated upon activation, normal T-cell expressed, and secreted (RANTES/CCL5) and thymus and activation-regulated chemokine (TARC/CCL17) (Liu et al., Citation2011; Pastore et al., Citation2006). Topical corticosteroids (TCs) and topical calcineurine inhibitors (TCIs, such as tacrolimus and pimecrolimus) have been used as first- and second-line therapies for AD, respectively. However, there are numerous risks of adverse effects with both drug classes. The chronic use of TCs can induce skin atrophy, treatment resistance, rebound dermatitis, telangiectasis, dyspigmentation, systemic toxicity, hypothalamic-pituitary-adrenal axis suppression, and growth retardation. TCIs can induce transient skin burning and increase the risk of immune system-mediated malignancies (Aoyama et al., Citation1995; Boguniewicz et al., Citation1998; Carr, Citation2013; Tomi & Luger, Citation2003).
Currently, there is an increasing use of complementary and alternative medicine, including herbal remedies, as therapeutic agents, which are recognized as safer than conventional medicines for treating AD. However, the efficacy and safety of most of these herbal remedies remain unclear. In the present study, a 70% ethanol extract from the fruit pulps of L. cylindrica (LC) was evaluated to determine its inhibitory effect on AD-like responses with in vitro and in vivo systems.
Materials and methods
Preparation of L. cylindrica extract (LC)
Luffa cylindrica pulp was purchased from Kwangmyungdang Medicinal herbs (Ulsan, Korea) in September 2009 and confirmed taxonomically by Professor Je-Hyun Lee of Dongguk University, Korea. A voucher specimen (2009-EBM9) has been deposited at the Herbal Medicine Formulation Research Group at the Korea Institute of Oriental Medicine. A total of 300 g of pulp was extracted with 70% EtOH (3 L × 3) by sonication for 60 min. The extract solution was filtered through a filter paper and evaporated to dryness using a rotary evaporator. The yield of the 70% EtOH extract was 14.93% (44.78 g).
Measurements of NO and PGE2 production in RAW264.7 cells
Murine RAW264.7 macrophage cells were purchased from the American Type Culture Collection (Rockville, MD) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL., Grand Island, NY) containing 5.5% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco BRL., Grand Island, NY), 100 U/mL penicillin, and 100 µg/mL streptomycin (P&S, Gibco BRL., Grand Island, NY). The cells were plated at a density of 2.5 × 105 cells/well in a 48-well plate for the NO and PGE2 assays. After incubation for 18 h, the cells were stimulated with 1 μg/mL of LPS in the presence and absence of LC extracts (10, 20, and 50 μg/mL) for 18 h. NG-monomethyl-l-arginine (NMMA; Sigma-Aldrich Inc., St. Louis, MO) and indomethacin (Sigma-Aldrich Inc., St. Louis, MO) were used as positive controls to inhibit production of NO and PGE2, respectively. The concentrations of NO and PGE2 in culture supernatants were measured with a Griess reagent system (Promega Co., Fitchburg, WI) and a PGE2 EIA kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer’s protocols, respectively.
Measurement of histamine production in HMC-1 cells
Human HMC-1 mast cells were kindly provided by Prof. Hyunsoo Bae (Kyunghee University, Seoul, Korea) and maintained in Iscove’s modified Dulbecco’s medium (Gibco BRL., Grand Island, NY) supplemented with 10% (v/v) heat-inactivated FBS and P&S. The cells were plated in 48-well plates (2 × 105 cell/well) and stimulated with phorbol-12-myristate 13-acetate (PMA, Sigma-Aldrich Inc., St. Louis, MO) and A23187 (a calcium ionophore, Sigma-Aldrich Inc., St. Louis, MO) in the presence and absence of LC extracts (50, 100, and 200 µg/mL) for 24 h. The levels of histamine in the culture medium were quantified using an EIA kit (Oxford Biomedical Research, Rochester Hills, MI) according to the manufacturer’s protocol. There was no cytotoxicity in the RAW264.7 and HMC-1 cells with LC treatment up to 50 and 200 µg/mL, respectively.
Measurements of chemokines production in HaCaT cells
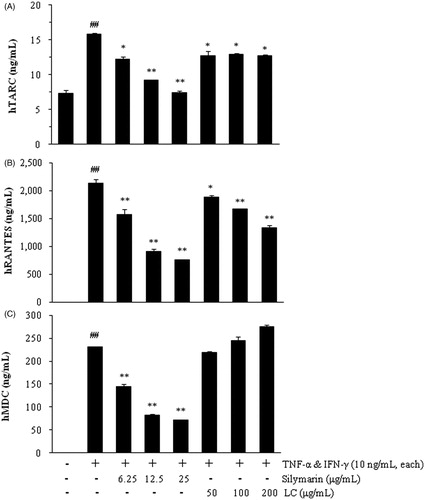
HaCaT, human keratinocytes (Boukamp et al., Citation1988), were obtained from CLS Cell Lines Service GmbH (Eppelheim, Germany) and cultured in DMEM with 10% (v/v) heat-inactivated FBS and P&S. HaCaT cells were seeded at a density of 1.0 × 106 cells/well in 6-well plates for the chemokine assay. After 24 h, the cells were washed and incubated with 1 mL of serum-free medium containing TNF-α and IFN-γ (TI, each 10 ng/mL, R&D Systems Inc., Minneapolis, MN) in the presence and absence of LC extracts (50, 100, or 200 μg/mL) for 24 h. Silymarin (Sigma-Aldrich Inc., St. Louis, MO, 20 and 50 μg/mL) was used as a positive control. The LC extract did not induce cytotoxicity at up to 200 µg/mL in the cells. In the culture medium, the quantities of chemokines, including TARC, MDC, and RANTES, were measured using EIA kits (R&D Systems Inc., Minneapolis, MN) according to the manufacturers’ protocols.
Animals and sensitization
Eight week-old male Nc/Nga mice were purchased from Central Lab. Animal Inc. (Seoul, Korea) and were allowed to familiarize for 2 weeks before the experiments were initiated. The mice were housed at 24 ± 2 °C and 55 ± 15% relative humidity with a 12 h light/12 h dark cycle room and given ad libitum access to food and water. All animal care and experimental procedures were in accordance with the guidelines of the NIH and approved by the Institutional Animal Care and Use Committee (IACUC) at the Korea Institute of Oriental Medicine (the approval number: 10–052). AD-like skin lesions were induced in the mice using Dermatophagoides farinae (Pyroglyphidae) extract ointment (Biostir-AD, Biostir Co., Ltd., Kobe, Japan) according to our previous report (Lee et al., Citation2012). The mice were grouped randomly into four groups with seven mice per group as follows: untreated (normal, 200 µL of 70% EtOH/mouse/d), D. farinae-sensitized (control, 70% EtOH), D. farinae-sensitized plus Protopic® ointment-treated (Protopic, 50 mg/mouse/d), and D. farinae-sensitized plus LC extract-treated (LC, 10 mg/mouse/d) groups. For sensitization, 50 mg of Biostir-AD was topically applied to the upper dorsal skin and the back of the ears twice weekly for 4 weeks. The LC extract was dissolved in 70% EtOH and topically applied every day during sensitization. Protopic® ointment (0.1% tacrolimus, Astellas, New York, NY) was used as a positive control.
Dermatitis score
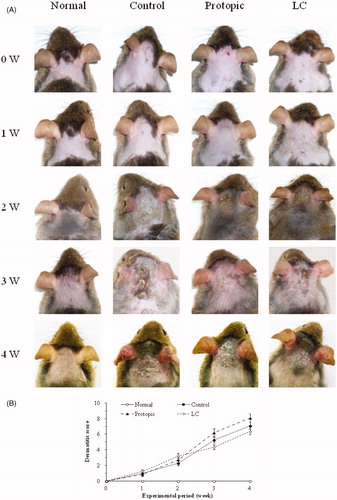
The dermatitis scores were assessed by evaluating the dorsal skins and ears every week for 4 weeks. The score was defined as the sum of the scores for erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness and individual scores were graded as follows: no symptoms, 0; mild, 1; moderate, 2; and severe, 3. The maximum score was 12 when the scores were summarized for each evaluation item.
Histological observation
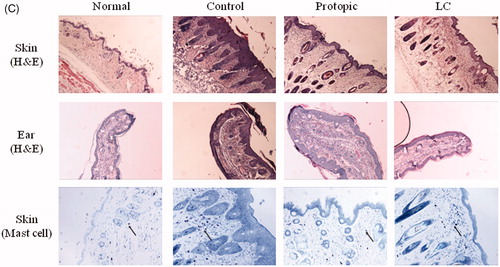
The mice were anesthetized by pentobarbital sodium (Entobar inj., Hanlim Pharm. Co., Ltd., Yubang-dong, Korea) injection (50 mg/kg body weight, i.p.). Blood samples were taken and the animals were sacrificed by exsanguinations from the aorta. A complete gross observation was performed on all terminated animals. The blood samples were collected in a microtainer (Becton, Dickinson and Company, Franklin Lakes, NJ) containing K2-EDTA. Plasma samples were collected after centrifugation at 10 000 rpm and stored at −80°C until they were further assayed. The dorsal skin and one ear of each mouse were removed and fixed in 10% (v/v) neutral buffered formalin for 24 h. The tissues were embedded in paraffin and then sectioned at a 4 μm thickness. The tissue sections were then stained with hematoxylin and eosin (H&E) or toluidine blue to estimate epidermal inflammation (hypertrophy and infiltration by inflammatory cells) and mast cell counts, respectively. The dermal mast cell content was quantified by counting the numbers of toluidine-blue positive cells in randomly selected high-power fields for each specimen.
Plasma levels of IgE and histamine
The plasma levels of histamine (Oxford Biomedical Research, Rochester Hills, MI) and IgE (Bethyl Laboratories Inc., Montgomery, TX) were measured using EIA kits in accordance with the manufacturer’s instructions.
Statistical analysis
The data are expressed as the mean ± SEM and were analyzed using one-way ANOVA followed by the Bonferroni multiple comparison method. A p value <0.05 was defined as statistically significant. All statistical analyses were performed using the SYSTAT® 8.0 program (SYSTAT Inc., Evanston, IL).
Results
Inhibitory effects of LC on NO, PGE2 and histamine production in vitro
In RAW264.7 cells, LC (20 and 50 μg/mL) significantly inhibited LPS-stimulated PGE2 production in a dose-dependent manner as compared with treatment with LPS alone (, p < 0.01). At a concentration of LC 50 μg/mL, the inhibitory effect of LC on PGE2 production (76.2% inhibition) was similar to that of the positive control, indomethacin, at 5 μM (74.4% inhibition). However, there was no difference in LPS-induced NO production in the presence and absence of LC (data not shown).
Figure 1. Luffa cylindrica extract (LC) inhibited the production of PGE2 (A) and histamine (B) in LPS-stimulated RAW 264.7 and PMA- and A23187-treated HMC-1 cells, respectively. ##p < 0.01 compared with non-stimulated cells. *p < 0.05 and **p < 0.01 compared with stimulated cells, respectively.
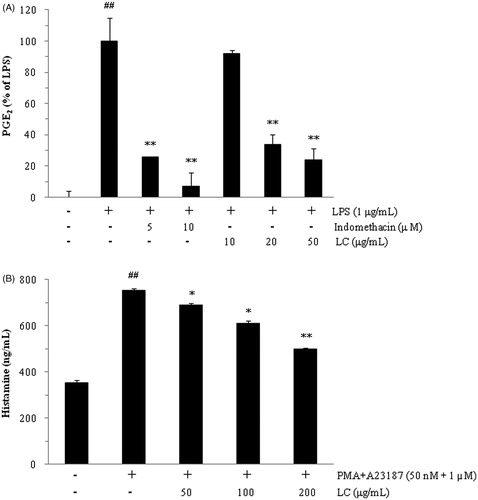
Histamine production was stimulated by PMA and A23187 in HMC-1 cells (, p < 0.01). Histamine production was suppressed by LC in a dose-dependent manner, and the IC50 value of LC was 139.9 μg/mL.
Inhibitory effects of LC on chemokine productions in HaCaT cells
The release of TARC was increased 2.16-fold by treatment with TNF-α and IFN-γ (10 ng/mL each; TI) for 24 h in HaCaT cells compared with non-stimulated cells (p < 0.01). LC (50, 100, and 200 μg/mL) reduced TARC induction approximately 20% in TI-treated cells (, p < 0.05). RANTES and MDC were not detected in the culture medium of non-stimulated HaCaT cells. The releases of RANTES and MDC were stimulated by TI in HaCaT cells (2142 ± 68 ng/mL and 230.1 ± 0.7 ng/mL, respectively; p < 0.01). LC inhibited RANTES release in TI-stimulated cells in a dose-dependent manner (p < 0.05), and the maximum inhibition rate of LC was 37.4 ± 2.8% at 200 μg/mL (, p < 0.01). However, there was no significant difference of MDC levels in the presence of LC (). Silymarin was used as a positive control to inhibit chemokine release.
Dermatitis scores and histological observations in Nc/Nga mice
Macroscopically, the AD-like skin lesions were severely developed on the back skin and ears by D. farinae sensitization for 4 weeks in Nc/Nga mice. In the LC and Protopic groups, the skin lesions were more suppressed than in the control group (). However, the dermatitis scores were not significantly different between the LC and control groups for 4 weeks ().
Figure 3. Luffa cylindrica extract (LC) ameliorated the development of atopic dermatitis-like skin lesions in D. farinae-sensitized Nc/Nga mice. Macroscopic observations (A), the dermatitis scores (B), histological features and mast cell infiltrations (C) in the skin and ears of the mice. The arrows indicate infiltrated mast cells in the dermis.
The D. farinae-sensitized control group displayed significant dorsal skin and ear lesions and hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal region. In the LC groups, these changes were ameliorated on the dorsal skin and ears. In the Protopic group, the skin lesions were reduced, whereas hypertrophy of the epidermis in the ear was not (). Infiltration of mast cells on dermis in the dorsal skin was suppressed by topical LC and Protopic treatments (, the arrows indicate mast cells).
Plasma levels of histamine and IgE in Nc/Nga mice
D. farinae-sensitization in Nc/Nga mice induced the release of histamine in plasma as follows: 28.56 ± 2.56 ng/mL in the normal group and 39.85 ± 5.44 ng/mL in the control group. The histamine levels were significantly reduced in the LC (24.33 ± 2.11 ng/mL) and Protopic (25.54 ± 1.93 ng/mL) groups compared with the control group (, p < 0.05).
Figure 4. The plasma levels of histamine (A) and IgE (B) in Nc/Nga mice. ## p < 0.01 compared with the normal group. *p < 0.05 compared with the D. farinae-sensitized group (control).
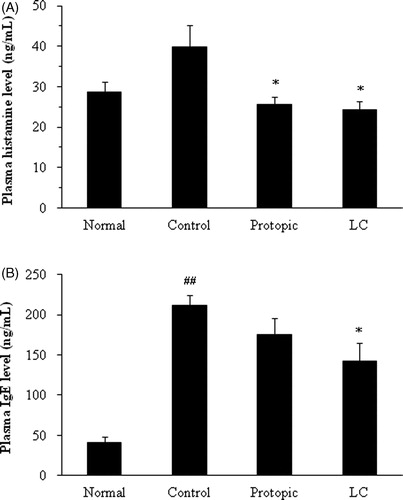
The plasma levels of IgE were significantly increased in the control group (211.3 ± 13.4 ng/mL) compared with the normal group (41.56 ± 7.02 ng/mL, p < 0.01). Topical LC treatment suppressed increase of plasma IgE levels (142.2 ± 22.4 ng/mL) compared with the controls (p < 0.05), whereas the Protopic group level was not significantly different (175.4 ± 20.4 ng/mL, ).
Discussion
Herbal remedies are used either orally or topically for skin diseases, mainly because of their anti-inflammatory and itch-relieving capacity (Ring et al., Citation2012b). AD is often accompanied by allergic inflammation, which is initiated by the activation of the adaptive immune response. The development of inflammation in AD is biphasic. An initial Th2 phase leads to a chronic phase associated with Th0 and Th1 cells (Terui, Citation2009). Mast cell mediators such as tryptase and histamine contribute to the induction of pruritus in AD (Liu et al., Citation2011). In the present study, the administration of LC extract in the ranges of concentration without any cytotoxic effects inhibited inflammatory and allergic responses through inhibitions of the release of PGE2 and histamine in RAW264.7 macrophages and HMC-1 mast cells, respectively. PGE2, as an index of cyclooxygenase (COX) activity, is one of the mediators of inflammatory response (Leung, Citation1995). AD patients have elevated Th2- and decreased Th1-expressing cells in the peripheral blood (Nakazawa et al., Citation1997). Histamine, which is mainly produced in mast cells, regulates Th1 and Th2 balance. The stimulated mast cells release histamine, and then released histamine stimulates keratinocytes to produce various proinflammatory cytokines, including RANTES, and Th2 chemokines, including TARC in AD skin (Pastore et al., Citation2006). IFN-γ and TNF-α induce the expression of AD-related chemokines such as TARC, RANTES and MDC in keratinocytes (Lee et al., Citation2012). TARC is expressed in AD keratinocytes as well as being mainly found on microvascular endothelial cells in AD lesions, and RANTES is strongly expressed in AD keratinocytes and can be found in the serum of AD patients (Pastore et al., Citation2006). The LC extract also inhibited the production of TARC and RANTES in HaCaT keratinocytes without cytotoxicity. In vitro, these results suggest that LC extract acts as a suppressor of activity and/or expression of these proinflammatory molecules, and Th2 chemokines might inhibit chronic skin inflammation, including AD. Therefore, LC was applied on an AD-like skin lesion-induced animal model in the present study.
House dust mite allergens are the most important allergens associated with human AD, and D. farinae is a common house dust mite present in the environment. Nc/Nga mice are characterized by AD-like skin lesions and display elevated levels of blood IgE. The skin changes that developed in the Nc/Nga mice closely mimic human AD, which conventionally develops following infection with mites (Fukuyama et al., Citation2011). In the present study, Biostir® ointment containing D. farinae extract induced AD-like skin lesions on the back skin and ears and increased the plasma levels of IgE and histamine in Nc/Nga mice after 4 weeks of topical application. The topical application of LC extract reduces AD-like skin lesions compared with vehicle in the D. farinae-sensitized Nc/Nga mice AD model. LC-treated mice displayed improvement of histological features such as epidermal hyperplasia and infiltration of inflammatory cells in the dermis, as compared with vehicle-treated mice. The inductions of plasma histamine and IgE were significantly suppressed by 100% and 41%, respectively. As with conventional medications that treat AD, the effects of TCs are associated with inhibiting the activation of Langerhans’ cells to activate T-cells, whereas TCIs have immunosuppressive characteristics mediated through inhibiting the activation of T-cells, which thereby decreases the release of the various proinflammatory cytokines, including IL-4, IL-5, and IL-13 (Carr, Citation2013). In the Protopic group, which served as a positive control, there was no significant difference in the plasma level of IgE compared with the control group. As one of the adverse effects of Protopic ointment, the patients experienced skin irritation and burning at the site of application (Ashcroft et al., Citation2005). Therefore, we expected that the relatively high levels of plasma IgE in the Protopic group were caused by the inflammatory response and increased itching behaviors at the application area of the ointment. The level of the anti-AD effect of LC was more than or similar to that of the topical Protopic ointment treatment as used as a positive control in our AD-like mouse model.
Conclusions
These results suggest LC inhibits the production of AD-related chemokines (TARC and RANTES) in TNF-α/IFN-γ-stimulated HaCaT cells, as well as the production of PGE2 as an inflammation marker in LPS-treated RAW264.7 cells. In addition, the topical application of LC has been demonstrated to be effective in suppressing the plasma levels of IgE and histamine and AD-like skin lesions in D. farinae-sensitized Nc/Nga mice. In conclusion, LC might be a novel approach for treating allergic diseases, including AD.
Authors’ contributions
H. H. designed the study and performed the experimental work and data analyses. H. S. L., M. Y. L., I. S. S., W. Y. J., and J. H. K. performed the experiments. H. H. and H. S. supervised the experimental work. All authors read and approved the final manuscript.
Declaration of interest
The authors report no declarations of interest. This research was supported by a grant for “Construction of Scientific Evidence for Herbal Medicine Formulas (K13030)”, from the Korea Institute of Oriental Medicine (KIOM).
References
- Aoyama H, Tabata N, Tanaka M, et al. (1995). Successful treatment of resistant facial lesions of atopic dermatitis with 0.1% FK506 ointment. Br J Dermatol 133:494–6
- Ashcroft DM, Dimmock P, Garside R, et al. (2005). Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: Meta-analysis of randomised controlled trials. Br Med J 330:516–22
- Boguniewicz M, Fiedler VC, Raimer S, et al. (1998). A randomized, vehicle-controlled trial of tacrolimus ointment for treatment of atopic dermatitis in children. Pediatric Tacrolimus Study Group. J Allergy Clin Immunol 102:637–44
- Boukamp P, Petrussevska RT, Breitkreutz D, et al. (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–71
- Carr WW. (2013). Topical calcineurin inhibitors for atopic dermatitis: Review and treatment recommendations. Paediatr Drugs 15:303–10
- Fukuyama T, Tajima Y, Hayashi K, et al. (2011). Prior or coinstantaneous oral exposure to environmental immunosuppressive agents aggravates mite allergen-induced atopic dermatitis-like immunoreaction in Nc/Nga mice. Toxicology 289:132–40
- Hanifin JM, Cooper KD, Ho VC, et al. (2004). Guidelines of care for atopic dermatitis. J Am Acad Dermatol 50:391–404
- Kao TH, Huang CW, Chen BH. (2012). Functional components in Luffa cylindrica and their effects on anti-inflammation of macrophage cells. Food Chem 135:386–95
- Khajuria A, Gupta A, Garai S, Wakhloo BP. (2007). Immunomodulatory effects of two sapogenins 1 and 2 isolated from Luffa cylindrical in Balb/C mice. Bioorg Med Chem Lett 17:1608–12
- Lee H, Lee JK, Ha H, et al. (2012). Angelicae Dahuricae Radix inhibits dust mite extract-induced atopic dermatitis-like skin lesions in Nc/Nga mice. Evid Based Complem Alternat Med 2012:743075
- Leung DY. (1995). Atopic dermatitis: The skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol 96:302–18
- Liu FT, Goodarzi H, Chen HY. (2011). IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 41:298–310
- Liu L, Wang R, He W, et al. (2010). Cloning and soluble expression of mature alpha-luffin from Luffa cylindrica and its antitumor activities in vitro. Acta Biochim Biophys Sin (Shanghai) 42:585–92
- Nakazawa M, Sugi N, Kawaguchi H, et al. (1997). Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol 99:673–82
- Ng YM, Yang Y, Sze KH, et al. (2011). Structural characterization and anti-HIV-1 activities of arginine/glutamate-rich polypeptide Luffin P1 from the seeds of sponge gourd (Luffa cylindrica). J Struct Biol 174:164–72
- Pastore S, Mascia F, Girolomoni G. (2006). The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol 16:12–31
- Ring J, Alomar A, Bieber T, et al. (2012a). Guidelines for treatment of atopic eczema (atopic dermatitis). Part I. J Eur Acad Dermatol Venereol 26:1045–60
- Ring J, Alomar A, Bieber T, et al. (2012b). Guidelines for treatment of atopic eczema (atopic dermatitis). Part II. J Eur Acad Dermatol Venereol 26:1176–93
- Tanaka S, Uno C, Akimoto M, et al. (1991). Anti-allergic effect of bryonolic acid from Luffa cylindrica cell suspension cultures. Planta Med 57:527–30
- Terui T. (2009). Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: Overview of the pathophysiology of atopic dermatitis. J Pharmacol Sci 110:232–6
- Tomi NS, Luger TA. (2003). The treatment of atopic dermatitis with topical immunomodulators. Clin Dermatol 21:215–24