Abstract
Context: 1,8-Cineole, a terpene, characterized as a major constituent occurring in the essential oils of several aromatic plants. It is widely used in pharmaceutical industry, as a food additive and for culinary purposes.
Objective: This study investigates the inhibitory effect of 1,8-cineole on transit time and diarrhea in animal models.
Materials and methods: Acute toxicity and lethality of 1-8-cineole was determined by Lork’s guidelines. The antidiarrheal effect of 1,8-cineole was investigated by determining the intestinal transit and enterpooling in rats. In all experiments, different doses of 1,8-cineole (20–120 mg/kg), atropine, and loperamide were administered orally.
Results: The LD50 of 1,8-cineole for oral administration was estimated to be 1280 mg/kg. 1,8-Cineole (20–120 mg/kg) did not show a significant decrease in small intestine transit (p > 0.05); however, the highest dose displayed a significant decrease in comparison with atropine (p < 0.05). This substance decreased the peristaltic index value to 68 ± 0.36% at a dose of 120 mg/kg compared with the control group (85.22 ± 4.31%) in the castor oil transit test. 1,8-Cineole significantly delayed the onset of diarrhea to −142.33 ± 6.08 min at 120 mg/kg, while the time was 103.66 ± 20.73 min for the control and >240 min for the loperamide. Moreover, 1,8-cineole significantly decreased intestinal fluid accumulation (p < 0.05).
Conclusions: This study demonstrated antispasmodic and antisecretory activities of 1,8-cineole and rationalized the traditional use of the plant containing various levels of this terpene in the treatment of gastrointestinal complains such as diarrhea.
Introduction
Despite improving trends in mortality rates, diarrhea still causes 15% of all deaths in children less than 5 years old and accounts for nearly 1.4 million child deaths in developing countries every year (Black et al., 2010). A child under 5 years of age is estimated to have approximately 3.2 episodes of diarrhea each year. Diarrhea is also an important cause of malnutrition, particularly when it is prolonged. Treatment of diarrhea with oral rehydration therapy (ORT) reduces mortality due to dehydration. Other treatment options include antibiotics and gut motility suppressing agents which could help in reducing the duration and severity of diarrhea (Martini & Eloff, Citation1998; Rates, Citation2001). Nowadays, enteropathogens are developing a high level of resistance to commonly used antibiotics; hence, resistance to new drugs is emerging very fast. Diarrheal diseases are far more common in children and the search for new drugs with activity against enteropathogens is a public health priority. The ethno-botanical studies and the previously reported antimicrobial activity of many medicinal plants suggest these naturally occurring compounds as an alternative therapy in treatment of infectious diarrhea (Martini & Eloff, Citation1998; Rates, Citation2001). Essential oils are very potent bioactive compounds extracted from plants using steam distillation and/or hydro-distillation. They are complex mixtures with a number of constituents that may influence the biological activities either alone or in combination with other agents. Terpenes occur in the essential oils of plants and are a family of chemical structures that are derivatives of isoprene.
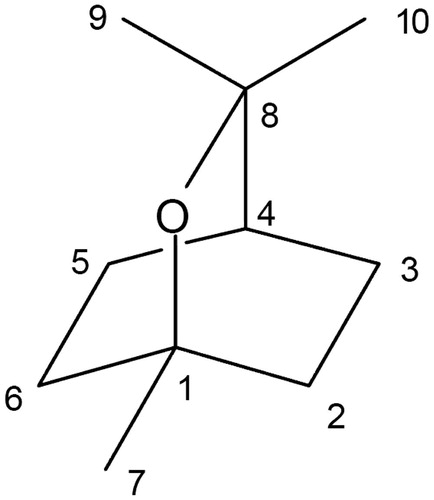
1,8-Cineole () belongs to the terpenes, characterized predominantly as a major or minor constituent occurring in a number of popular biologically active aromatic plants and spice oils, such as Mentha longifolia L. (Labiatae), Artemisia dracunculus L. (Compositae), Coriandrum sativum L. (Umbelliferae), Origanum vulgare L. (Labiatae), Rosmarinus officinalis L. (Labiatae), Thymus vulgaris L. (Labiatae), and Zingiber officinale Rosc. (Zingiberaceae) (Vuuren & Viljoen, Citation2007). It is often used as a flavoring agent for food products and has been used traditionally for treatment of infectious respiratory diseases. It is also frequently employed by the pharmaceutical industry in drug formulations as a percutaneous enhancer and for its decongestant and antitussive effects (Santos & Rao, Citation2001).
Figure 1. Chemical structure of 1,8-cineole which is the principal constituent of the many medicinal plant essential oils.
Recent studies have confirmed antimicrobial (Krist et al., Citation2008; Pattnaick et al., Citation1997) antifungal (Pattnaick et al., Citation1997), antimalarial (Su et al., 2008), insecticidal (Prates et al., Citation1998), anthelmintic (Shah et al., Citation2011), antiexudant, cytotoxic, antitumor (Asanova et al., Citation2003), antispasmodic (Magalhaes et al., Citation1998), antiinflammatory (Santos & Rao, Citation2000), analgesic (Asanova et al., Citation2003; Santos & Rao; Citation2000), and gastroprotectant (Santos & Rao, Citation2001) properties of 1,8-cineole. It has been reported that 1,8-cineole alters neural firing in certain areas of the olfactory lobe. It also possesses hypotensive and smooth muscle relaxant activities (Lahlou et al., Citation2002). To the best of our knowledge, there have been no studies conducted so far concerning in vivo antidiarrheal properties of this substance. The present study was carried out to evaluate the possible antidiarrheal activity of 1,8-cineole.
Materials and methods
Drugs/chemicals
1,8-Cineole, loperamide (the standard reference antidiarrheic drug), and atropine sulfate were purchased from Sigma Chemical Co., St. Louis, MO. Castor oil (a laxative agent), Tween 80, and charcoal meal (10% activated charcoal in 5% tragacanth powder) were obtained from Merck Co. (Darmstadt, Germany). To perform the experiments, 1,8-cineole was dissolved in 2% Tween. Charcoal meal, atropine sulfate, and loperamide were prepared in normal saline.
Animals
Male Wistar rats (200–230 g) were obtained from the Central Animal House of Urmia University. The animals were housed in polypropylene cages at room temperature (23 ± 2 °C) 2 weeks before the experiment under standard environmental conditions (humidity 55–60% and 12 h light/dark cycle) with free access to rodent pellet diet (Dane-Pars Co., Tehran, Iran) and tap water. Food was withheld 18–24 h prior to experimentation but they were allowed free access to water, as acclimatization. All the experiments reported in this study were carried out in accordance with current guidelines for the care and use of research animals (NIH guidelines).
Acute oral toxicity
The acute oral toxicity of 1,8-cineole in rat was measured in accordance with Lork’s method (Lorke, Citation1983). Animals were randomly divided into seven groups. Groups 1–6 received the following doses of 1,8-cineole separately: 10, 100, 600, 1000, 1600, and 2900 mg/kg diluted in Tween 80 (2%) to the final volume of 2 mL, only once through an oral intubation cannula. The animals in the last group were considered as the control and were administered 2 mL of Tween 80 (2%). Signs of toxicity and mortality within 24 h were noted. The LD50 was then calculated based on the pattern of death observed using the Probit-log analysis. Animals were kept under observation for a period of 14 consecutive days.
Effect on gastrointestinal transit time
The rats were randomly divided into seven groups of six and were fasted for 18 h before the commencement of the experiment. Animals in the first four groups orally received 120, 80, 60, and 20 mg/kg of 1,8-cineole. The animals in group 5 received 10 mL/kg of Tween 80 (2%) prepared in normal saline, while those in group 6 were given a standard antispasmodic agent (atropine) at a dose of 0.1 mg/kg. The seventh group received atropine sulfate 30 min before 1,8-cineole (80 mg/kg) administration. Thirty minutes later each animal was treated orally with charcoal meal as a marker diet (Aye-Than et al., Citation1989). Half an hour after the charcoal meal was given, all the animals were sacrificed by euthanasia, the abdomens were cut open and the intestines were carefully removed from cardia to the anus. The intestines were immediately immersed in formalin to arrest peristalsis.
Thereafter, the distance the meal traveled through the intestine, as represented by the charcoal meal progress, was measured in every individual rat. The inhibition of gastrointestinal transit of the charcoal meal by atropine and 1,8-cineole was calculated as a function of the negative control using the following formula:
In this formula, “N” represents the length traveled by charcoal meal in the non-treated rats and “n” represents the length traveled by the charcoal meal in the treated animals.
Castor oil-induced diarrhea
The method of Atta and Mouneir (Citation2005) was followed; overnight-fasted rats were divided into four groups each in which six animals were placed and diarrhea was induced by oral administration of 2 mL of castor oil to the rats. Group 1 served as a negative control (10 mL/kg of 2% Tween 80 solution, p.o.); groups 2 and 3 received 120 and 20 mg/kg (p.o.) of 1,8-cineole; while group 4 received loperamide (3 mg/kg, p.o.) and was considered as the positive control. One hour after treatment, each animal was challenged orally with 2 mL of castor oil and placed separately over clean filter papers inside cages. The filter papers were inspected for the presence of diarrheal droppings at hourly intervals for a period of 4 h. The following parameters were observed: the time delayed between the administration of castor oil and the excretion of the first diarrheic feces, the total number of fecal outputs, and the number of diarrheic stools excreted by the animals in 4 h, as well as the total weight of the diarrheic feces in the mentioned period of time. The severity of the castor oil-induced diarrhea was noted and recorded as a score. A numerical score based on stool consistency was assigned as follows: normal stool (or lack of diarrhea), 1; semi-solid stool, 2; watery stool/feces, 3.
Castor oil-induced intestinal transit
The animals in all groups were treated orally with 1,8-cineole (120, 80, 60, and 20 mg/kg), loperamide (3 mg/kg) or with the vehicle (10 mL/kg) 30 min before the administration of castor oil (2 mL/rat). Thirty minutes after castor oil administration, the animals were given the standard charcoal meal (10% suspension in 5% tragacanth powder). All the animals in each treatment group were sacrificed 20 min after administration of the charcoal meal, and the small intestines were immediately isolated. Peristaltic index (as percentages) for each rat was then determined as described earlier (Aye-Than et al., Citation1989).
Castor oil-induced enteropooling
Following the method of Robert et al. (Citation1976), the rats were allotted into six animals per group and were pretreated orally with 2% of Tween 80 (10 mL/kg), loperamide (3 mL/kg), or 1,8-cineole (120 and 20 mg/kg). One hour later, the rats received castor oil (2 mL/rat) intragastrically. The animals were sacrificed 1 h later and the small intestines were removed after ligation at the pyloric end and the ileocaecal junction. The contents of the intestines were then expelled into a graduated tube and the volume was measured.
Statistical analysis
Data are presented as means ± SE mean. The statistical analysis for the animal experiments was carried out using one-way ANOVA followed by Dunnet’s multiple comparisons or Chi-square test as appropriate. The obtained results were compared with the control group. p Values less than 0.05 were considered significant.
Results
Acute toxicity study
The LD50 of 1,8-cineole was estimated to be 1280 mg/kg for the oral route in an adult rat. There were no visible signs of toxicity and treatment-related mortality in animals treated with 1,8-cineole at doses of 10 and 100 mg/kg for 14 d. Even high-dose males and females did not exhibit a significant decrease in food and water consumption 14 d following the treatment (data not shown).
1,8-Cineole, when administered orally, produced visible signs of toxicity in the animals at doses of greater than 100 mg/kg. The signs include tremor, convulsion, abnormal gait and ataxia, increased respiration, decreased activity, unresponsiveness to writhing test, and flaccid paralysis that lead to recumbencey (). There was no significant post-treatment drop in body weight in both males and females of all groups. Visual inspection on necropsy did not reveal any signs of damage to organs.
Table 1. Acute oral toxicity of 1,8-cineole in rats.
Normal intestinal transit
In this study, administration of atropine sulfate at 0.1 mg/kg and 1,8-cineole at 80 mg/kg accompanied with atropine significantly inhibited the gastrointestinal transit of charcoal in rats by 38.89% and 16.69%, respectively, compared with the control group ().
Table 2. Effect of 1,8-cineole on normal gastrointestinal transit in rat.
As shown in , pure 1,8-cineole (20–120 mg/kg) produced a slight and non-significant decrease in charcoal meal traversing in the small intestine compared with the control group which showed a value of 64.00 ± 2.08%.
Effect on castor oil-induced intestinal transit
In the control group, the charcoal meal moved farther (85.22 ± 4.31) in this model compared with the normal intestinal transit test (64 ± 2.08). 1,8-Cineole produced dose-dependent reduction in intestinal transit. Significant inhibition at the most effective dose (120 mg/kg) was 68 ± 0.36. An additional application of 1,8-cineole at lower doses (20–80 mg/kg) produced non-significant inhibitions of castor oil-induced small intestinal transit (data not shown).
Castor oil-induced diarrhea in rats
About 90 min after castor oil administration, all the rats in the control group produced copious and watery diarrhea. The induced diarrhea was significantly inhibited with loperamide (3 mg/kg, p.o.) for 4 h. In general, the anti-diarrheal activity of 1,8-cineole was not significant (). Pretreatment of the rats (in the test groups) with 1,8-cineole (20 and 120 mg/kg, p.o.) dose dependently delayed the onset of diarrhea (p < 0.05). 1,8-Cineole caused a non-significant reduction in the frequency of defecation (the number of total stools), the weight of stools, and the general diarrheal score, including the hard, mild, and copious stools. Loperamide (3 mg/kg, p.o.) produced a more significant (p < 0.001) inhibitory effect on all the diarrheal parameters compared with the highest dose of 1,8-cineole (120 mg/kg, p.o.) administered ().
Table 3. Effect of the 1,8-cineole on diarrhea onset and the time course of diarrhea induced by castor oil in rats.
Table 4. Antidiarrheal activity of 1,8-cineole on the diarrhea parameters that induced by castor oil in rats.
Castor oil-induced enteropooling in rats
Oral administration of castor oil produced a significant (p < 0.05) increase in the intestinal fluid volume of castor oil-treated groups compared with the control group treated with sole distilled water (10 mL/kg, p.o.). Compared with the control group, pretreatment of the animals with 1,8-cineole (20 and 120 mg/kg, p.o.) significantly (p < 0.05–0.01) inhibited castor oil-induced fluid accumulation in rats (). Loperamide (3 mg/kg) produced a greater (p < 0.001) inhibitory effect on castor oil-induced fluid accumulation than the highest dose of 1,8-cineole (120 mg/kg) used (). The intestinal fluid of the animals pretreated with 1,8-cineole and loperamide were found to be more viscous than those of the distilled water-treated rats.
Table 5. Effect of 1,8-cineole on castor oil-induced enteropooling in rat.
Discussion
The LD50 value for oral administration of 1,8-cineole was found to be 1280 mg/kg. Oral administration of 1,8-cineole at 100 mg/kg did not cause any visible toxic symptoms, respiratory distress, ataxia, convulsion, or mortality in the animals. Cineole acute toxicity has been studied previously and the oral LD50 in rats was reported as 2500 mg/kg (Jenner et al., Citation1964). LD50 values in other species have been reported to be 2300 mg/kg (IM) in Guinea pig, 1500 mg/kg (SC) in dog, and 50 mg/kg in mouse (McLean et al., Citation2007). Various LD50 values have been reported for mouse: 1070 and 100 mg/kg (McLean et al., Citation2007) and more than 3500 mg/kg (p.o.) (Santos & Rao, Citation2001), indicating that these values should be interpreted with caution. The numerical value of the LD50 is influenced by many factors such as animal species and strain, age and sex, diet, food deprivation prior to dosing, temperature, season, and experimental procedures. Thus, the LD50 value cannot be regarded as a biological constant (Zbinden & Flury-Roversi, Citation1981). Finally, an oral LD50 of 1280 mg/kg was observed in our experimental conditions and procedures. The doses utilized in the present study were safe and demonstrated no untoward behavioral effects in the rats.
The results of the present study indicate that 1,8-cineole does not possess full antidiarrheal activity in rodents. To the best of our knowledge, this is the first report on the antidiarrheal activity of 1,8-cineole in biomedical literature. 1,8-Cineole (120 and 20 mg/kg, p.o.) slightly (non-significant) inhibited all the diarrheal parameters measured including frequency and severity of diarrhea, total number of stools, and weight of wet stools. Furthermore, 1,8-cineole decreased normal intestinal propulsive movement and transit in the rats. The inhibition exerted by the highest dose of 1,8-cineole (120 mg/kg) was lower than those of loperamide (3 mg/kg) and atropine (0.1 mg/kg) which are used as standard antidiarheal drugs. In the present study, loperamide and atropine inhibited the gastrointestinal motility (propulsion), delayed the onset of diarrhea and reduced the intestinal fluid secretion and accumulation, thus ameliorating diarrhea.
Castor oil is reported to induce diarrhea via increasing the volume of intestinal content by prevention of reabsorption of water. The release of ricinoleic acid results in irritation and inflammation of the intestinal mucosa, leading to release of prostaglandins and nitric oxide, which stimulate gastrointestinal secretion (Franca et al., Citation2008), motility, epithelial permeability, and edema of the intestinal mucosa (Zavala et al., Citation1998), thereby preventing the re-absorption of sodium, chloride, and water. Studies on enteropooling showed that pretreatment with 1,8-cineole reduces the volume of intraluminal contents. These effects, which are direct consequences of reduced secretion into the intestine, suggest that 1,8-cineole may enhance electrolyte reabsorption consistent with the inhibition of hyper-secretion or may encourage the absorption of other intestinal contents. The precise mechanism of hyper-secretion affected by 1,8-cineole is not clear, hence, the reduction in fecal wetness strongly indicates that it may inhibit gastrointestinal hyper-secretion. It has been shown that cineole may produce various effects based on the route of its application. It locally results in inflammatory edema and systemically provokes an anti-inflammatory effect (Santos & Rao, Citation1997). In ex vivo studies, cineole-treated monocytes subjected upon to stimulation by LPS or calcium ionophore demonstrated a significant inhibition in the production of cytokines (TNFa and IL1-b), leukotriene B4, thromboxane B2, and prostaglandin E2, compared with non-treated cells (Santos & Rao, Citation2000). This may explain anti-secretory effect of cineole observed in the present study through diminution in the formation of prostaglandin and other inflammatory mediators in ricinoleic acid-induced irritation and inflammation.
Hypermotility is a causative type of diarrhea where the secretory component is not the contributing aspect. Pre-treatment with the cineole suppressed the transit of charcoal meal through the gastrointestinal tract, which clearly indicates that the cineole may be capable of reducing the frequency of stooling in diarrheal conditions. Beside, delay in the intestinal motility causes further absorption of water from feces and may additionally contribute to reduce its watery texture. Our experimental data show that it is likely that cineole inhibit gastrointestinal hypermotility through its anticholinergic effect. Castor oil-induced gastrointestinal hypermotility has been suggested to be indirectly mediated by the cholinergic system since it is inhibited by atropine (Brown & Taylor, Citation1996). An in vitro study has shown that cineole provides a minimal and weak relaxant an antispasmodic activity on the isolated ileum basal tonus (Magalhaes et al., Citation1998). A similar finding regarding the effect of cineol on intestinal motility was obtained in the present study that may contribute to the delaying of diarrheal onset, stooling, and intraluminal fluid reduction. This also implies that the antidiarrheal activity afforded by 1,8-cineole may be associated with its anti-inflammatory action, its prevention of castor oil-induced hypermotility and hypersecretion, and also lipoxygenase inhibition, blocking of leukotrienes, and prostaglandin formation.
Conclusion
These data demonstrated that 1,8-cineole offers some protection against onset of diarrhea in rat at non-toxic doses. However, dose–response studies should be conducted for 1,8-cineole in order to determine the most effective dose that this substance may exerts its maximal efficacy in experimentally induced diarrhea models. Further studies are required to clarify the precise mechanisms responsible for the antidiarrheal activity of 1,8-cineole.
Acknowledgements
The authors wish to thank Milad Moloudizargari and Shahin Aghajanshakeri for revising the manuscript. We also thank Laya Hatefi-Afshar, Alireza Yusefi, and Mehdi Abdollahi for their technical assistance.
Declaration of interest
The authors declare no conflicts of interest associated the present work. Financial support for this work was provided by the Research Council of the Urmia University, Iran.
References
- Asanova ZhK, Suleimenov EM, Atazhanova GA, et al. (2003). Biological activity of 1,8-cineole from levant wormwood. Pharm Chem J 37:28–30
- Atta AH, Mouneir SM. (2005). Evaluation of some medicinal plant extracts for antidiarrhoeal activity. Phytother Res 19:481–5
- Aye-Than HJ, Kukarni W, Tha SJ. (1989). Antidiarrhoeal efficacy of some Burmese indigenous drug formulations in experimental diarrhoeal test models. J Crude Drug Res 27:195–200
- Black RE, Cousens S, Johnson HL, et al. (2010). Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 375:1969–87
- Brown JH, Taylor P. (1996). Muscarinic receptor agonists and antagonist. In: Hardman JG, Limbird LE, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 9th ed. New York: MacGraw, Hill, 141–60
- Franca CS, Menezes FS, Costa LCB, et al. (2008). Analgesic and antidiarrheal properties of Ocimum selloi essential oil in mice. Fitoterapia 79:569–73
- Jenner PM, Hagan EC, Taylor JM, et al. (1964). Food flavourings and compounds of related structure. Acute oral toxicity. Food Cosmetic Toxico 2:327–43
- Krist S, Sato K, Glasl S, et al. (2008). Antimicrobial effect of vapours of terpineol, (R)-(–)-linalool, carvacrol, (S)-(–)-perillaldehyde and 1,8-cineole on airborne microbes using a room diffuser. Flavour Fragr J 23:353–6
- Lahlou S, Figuereido AF, Magalhães PJC, Leal-Cardoso JH. (2002). Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol 80:1125–30
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Magalhaes PJC, Criddle DN, Tavares RA, Melo EM. (1998). Intestinal myorelaxant and anti-spasmodic effects of the essential oil of Croton nepetaefolius, and its constituents cineole, methyl-eugenol and terpineol. Phytother Res 12:172–7
- Martini N, Eloff JN. (1998). The preliminary isolation of several antibacterial compounds from Combretum erytrophyllum (Combretaceae). J Ethnopharmacol 62:255–63
- McLean S, Boyle RR, Brandon S, et al. (2007). Pharmacokinetics of 1,8-cineole, a dietary toxin, in the brushtail possum (Trichosurus vulpecula): Significance for feeding. Xenobiotica 37:903–22
- Pattnaick S, Subramanyam VR, Bapaji M, Kole CR. (1997). Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 89:39–46
- Prates HT, Santos JP, Waquil JM, et al. (1998). Insecticidal activity of monoterpenes against Rhyzopertha dominica (F.) and Tribolium castaneum (Herbst). J Stored Prod Res 34:243–9
- Rates SMK. (2001). Plants as source of drugs. Toxicon 39:603–13
- Robert A, Nezamis JE, Lancaster C, et al. (1976). Enteropooling assay: A test for diarrhoea produced by prostaglandins. Prostaglandins 11:809–14
- Santos FA, Rao VSN. (1997). Mast cell involvement in the rat paw oedema response to 1,8-cineole, the main constituent of eucalyptus and rosemary oils. Eur J Pharmacol 331:253–8
- Santos FA, Rao VSN. (2000). Anti-inflammatory and antinociceptive effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils. Phytother Res 13:1–5
- Santos FA, Rao VSN. (2001). 1,8-Cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci 46:331–7
- Shah AJ, Gilani AH, Abbas K, et al. (2011). Studies on the chemical composition and possible mechanisms underlying the antispasmodic and bronchodilatory activities of the essential oil of Artemisia maritima L. Arch Pharm Res 34:1227–38
- Su V, King D, Woodrow I, et al. (2008). Plasmodium falciparum growth is arrested by monoterpenes from eucalyptus oil inhibition of malaria by eucalyptus oil. Flavour Fragr J 23:315–18
- Vuuren SF, Viljoen AM. (2007). Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr J 22:540–4
- Zavala MA, Perez S, Perez C, et al. (1998). Antidiarrheal activity of Walfheria americana, Commelina coelestis and Alternanthera repens. J Ethnopharmacol 61:41–7
- Zbinden G, Flury-Roversi M. (1981). Significance of the LD50 test for the toxicological evaluation of chemical substances. Arch Toxicol 47:77–99

