Abstract
Context: Amaranthus spinosus Linn. (Amaranthaceae), commonly known as “spiny pigweed”, is used both in the Indian traditional system and in folk medicine to treat diabetes.
Objective: The present study evaluates the scientific basis of antidiabetic activity of chloroform fraction of methanol extract of A. spinosus and of an isolated constituent of A. spinosus.
Materials and methods: HPLC analysis was performed to determine the purity and the amount of the constituent present in the plant extract. The yeast α-glucosidase inhibition technique was used to determine the antidiabetic activity of A. spinosus. Acarbose was used as a standard. An appropriate therapeutic approach for preventing diabetes mellitus and obesity is to retard the absorption of glucose by inhibition of α-glucosidase.
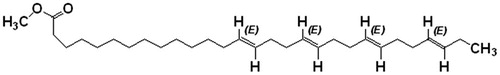
Results: One novel fatty acid with strong α-glucosidase inhibitory activity – (14E, 18E, 22E, 26E) – methyl nonacosa-14, 18, 22, 26 tetraenoate [1] (IC50 value 6.52 µM/mL) and β-sitosterol [2] were purified. Compound 1 was found to be more potent than the methanol extract. HPLC quantative analysis revealed that 0.15% of compound 1 and 0.06% of compound 2 were present in the plant extract.
Conclusion: This novel fatty acid can potentially be developed as a novel natural nutraceutical for the management of diabetes.
Introduction
Amaranthus spinosus Linn. (Amaranthaceae), commonly known as “spiny pigweed”, is used as a vegetable and cultivated throughout India, Sri Lanka, and many tropical countries (Kirtikar & Basu, Citation2001). It is an important plant used traditionally in diabetic complications, and also as a diuretic, analgesic, antipyretic, antileprotic agent, and in the treatment of bronchitis and piles (Girija et al., Citation2011). There are many scientific contributions to the antidiabetic activities shown by the plant extract (Sangameswaran & Jayakar, Citation2008; Sangameswaran & Pandhare, Citation2010) and isolated active metabolites.
Some previous studies revealed that the plant possesses antidiabetic activity (Balakrishnan & Pandhara, Citation2010; Girija et al., Citation2011) but only a few have been investigated in detail, especially the active constituents responsible for showing antidiabetic activity. The present investigation aimed to identify the active constituent responsible for α-glucosidase inhibition, in order to justify the use of this plant as an antidiabetic agent in the folk medicine. The yeast α-glucosidase inhibition technique was employed for the determination of α-glucosidase inhibitory activity of the isolated constituent.
Material and methods
Apparatus
IR spectra were measured on a FT-IR Shimadzu spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1H Nuclear magnetic resonance spectra were recorded in CDCl3 using either a FT-NMR or Bruker Avance 300 MHz spectrometer. The EI-MS mass spectra were obtained using a MAT 95 XL mass spectrometer (Thermofinigan, Thermo Fisher Scientific, Inc., Waltham, MA).
Chemicals
HPLC grade methanol and water used in HPLC analysis were procured from Merck (Darmstadt, Germany). All other chemicals were of analytical grade purchased locally.
Plant material
The whole plant was collected in the month of June 2012 and was identified by Dr. V. P. Prasad, Scientist F, Botanical Survey of India, Howrah, India. A voucher specimen (no. CNH/18/2011/Tech.II/419) was deposited in the Department of Pharmaceutical Technology, Jadavpur University. The collected plant material was washed, shade-dried, and then milled to coarse powder with a mechanical grinder for further studies.
Extraction and isolation of secondary metabolites
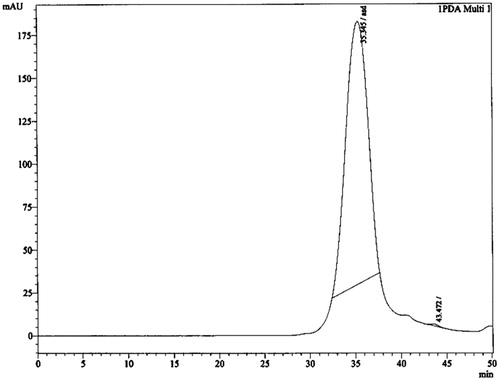
The plant material (2 kg) was extracted with methanol at room temperature for 48 h. The extract was filtered and dried under reduced pressure at 45 °C, to afford a crude methanol extract (59.46 g). A part of the methanol extract (50 g) was suspended in water and fractioned successively with chloroform, ethyl acetate, and n-hexane. Then the extracts were evaporated under reduced pressure in a rotatory evaporator and lyophilized to give residues of the chloroform (7.76 g), ethyl acetate (7.06 g), and n-hexane (20.12 g) fractions. All the fractions were evaluated for α-glucosidase inhibitory activity. The chloroform fraction was found to be more potent than the ethyl acetate and n-hexane fraction. Hence, the chloroform fraction was further exploited for isolation of secondary active metabolites. The chloroform fraction (5 g) was subjected to CC over a bed of silica gel (100–200 mesh size, Merck, Mumbai, India) and separated with a benzene:chloroform gradient into 10 fractions (fractions 1–10). Fraction 9 was further subjected to silica gel CC and eluted with petroleum ether:benzene (40:60) to give 63 fractions (9.1–9.63). The fraction (9.44–9.63) obtained from recolumn contains a mixture of two components revealed by HPLC analysis (). These fractions were subjected to preparative TLC to yield compound 1 (13 mg) and compound 2 (8 mg).
Quantitative analysis by HPLC-DAD
HPLC analyses were performed at 30 °C using sample solutions filtered through 0.45 μm membrane (Whatman syringe filter, Shimadzu Corporation, Kyoto, Japan) and analyzed (2.5 μL injected volume) using a Shimadzu (Shimadzu Corporation, Kyoto, Japan) system fitted with SCL-10A VP Shimadzu system controller, SPD-M10A VP Shimadzu diode array detector (Shimadzu Corporation, Kyoto, Japan), LC-10AT VP Shimadzu liquid chromatography pump, Class VP software (VP Software Engineering, Okinawa, Japan), and a Rheodyne injector (Sigma-Aldrich, Tokyo, Japan) having 20 µL loop. Separation was achieved using a COSMOSIL C18-MS-II column, 4.6 × 150 mm, 5 μm particle size; isocratic elution was achieved using the mobile phase methanol: 20 mmol/L phosphate buffer (pH 2.5) (90:10 v/v) at a flow rate of 1 mL/min. The eluate was monitored at 210 nm. Quantitative estimation of the constituents present in the methanolic extract and fractions was determined. The standard solutions of extract fractions (10 mg/mL) and compounds (1 mg/mL) were prepared in methanol.
α-Glucosidase inhibitory activity and kinetic studies
α-Glucosidase inhibitory assay was performed as described by Kumar et al. (Citation2012). The extract (25 μL) and constituent solution at different concentrations were prepared separately, in 0.1 M phosphate buffer having pH 6–8, was mixed with 25 μL of enzyme solution containing 0.5 U/mL in 0.1 M phosphate buffer. The mixed solution was incubated at 37 °C for 10 min to allow the inhibition of the enzyme by the extract and isolated compound under test. After incubation, 25 μL of the substrate, p-nitrophenyl α-d-glucopyranoside, 0.5 mM solution in 0.1 M phosphate buffer was added to the above prepared mixture and incubated at 37 °C for 30 min. Then the reaction was terminated by the subsequent addition of 100 μL of 0.2 M sodium carbonate solution. The absorbance of the solution containing p-nitrophenol was measured at 405 nm. The uninhibited enzyme was taken as a control and an appropriate blank was used for all the samples. DMSO control was used whenever required and the final concentration of DMSO was maintained below 2% v/v, which was found that it is not affecting the enzyme activity. Acarbose was used as a standard. The assay was performed in triplicate and in three independent experiments.
For kinetic studies, various concentrations of substrate p-nitrophenyl α-d-glucopyranoside (10, 15, 20, and 25 mM) was incubated with α-glucosidase in the absence of inhibitor, in the presence of methanol extract of A. spinosus (20 and 40 µg/mL), and isolated fatty acid (5 and 10 µg/mL), in phosphate buffer pH 7.2 (100 mM) at 37 °C and the amount of glucose formed was determined. Double reciprocal Lineweaver Burk plots were used to determine the nature of inhibition in the enzyme kinetics.
Results and discussion
The present study was conducted to isolate and evaluate the inhibitory activity of the bioactive compound present in A. spinosus. The whole plants of A. spinosus were extracted with methanol and fractioned by column chromatography. The column fractions were pooled according to their TLC and HPLC profiles. The combined fractions were concentrated and allowed to crystallize two compounds. The purity of compounds was confirmed by HPLC. The isolated constituents were characterized using mass spectrometry, FT-IR, and 1H and 13C NMR spectroscopic analysis, and by comparison of literature data (Jayaprakasha et al., Citation2007; Zhang et al., Citation2005). Based on the spectral analysis, the two structures of the compounds 1 and 2 were identified as (14E, 18E, 22E, 26E)-methyl nonacosa-14, 18, 22, 26 tetraenoate () and β-sitosterol. This is the first report on isolation and identification of (14E, 18E, 22E, 26E)-methyl nonacosa-14, 18, 22, 26 tetraenoate and β-sitosterol from A. spinosus. The isolated fatty acid was purified in an octadecyl-bonded silica packed column using the methanol/isopropanol (95:5, v/v) solvent system as a brownish colored compound.
Compound 1
Brownish color crystal, m.p. 174–176 °C. 1H NMR (300 MHz, CDCl3) δ 3.66 (methyl ester), δ 1.29–1.25 (alkanes), and δ 5.34–4.947 (–C=C–). IR (KBr) 1735.62 cm−1 indicates the carbonyl (C=O) group in ester, 2923.46 cm−1 asymmetric and 2854.13 cm−1 symmetric C–H stretching in alkanes and 1564.95–1460.81 cm−1 indicates the C–H stretching of alkenes. MS m/z 444.106 (calculated value C30H52O2, 444.73), m/z (%): 444.106 (100), 445.092 (33.1), 446.091 (5.5). A quantitative estimation of the constituents present in the chloroform fraction of the methanol extract was performed using RP-HPLC. The chromatogram for the chloroform fraction revealed two peaks (). The amount of constituents present in the fraction was 0.15% (compound 1) and 0.06% (compound 2). The retention time for compounds 1 and 2 is 35.487 and 42.321 min, respectively. The compound 1 was further purified by HPLC as shown in . According to Shim et al. (Citation2003), postprandial hyperglycemia could induce the non-enzymatic glycosylation of various proteins, resulting in the development of chronic diseases. Therefore, the control of postprandial plasma glucose levels is the essential factor in the early treatment of diabetes and in reducing the chronic vascular complications. By preventing the absorption of carbohydrates after food uptake, postprandial hyperglycemia in patients with diabetes mellitus can be reduced. Monosaccharides can only be transported out of the intestinal lumen into the bloodstream, so oligosaccharides and disaccharides should be broken down into individual monosaccharides before it can be absorbed in the duodenum and upper jejunum. This digestion is assisted by α-glucosidases which is attached to the brush border of the intestinal cells. Acarbose is the competitive inhibitors of intestinal α-glucosidases and reduces the postpandrial digestion and absorption of starch and disaccharides (Ortiz-Andrade et al., Citation2007). The isolated fatty acid along with the methanol extract demonstrated concentration-dependent inhibition of the enzyme (). The ester of fatty acid exhibited a potent inhibition of enzyme α-glucosidase (IC50 6.52 µM/mL) than the methanol extract (IC50 8.49 µM/mL) and acarbose. Acarbose is a standard drug, which provides inhibition at IC50 values of 15.25 µM/mL.
Figure 2. HPLC chromatograms of two isolated compounds from chloroform fraction of methanol extract of Amaranthus spinosus.
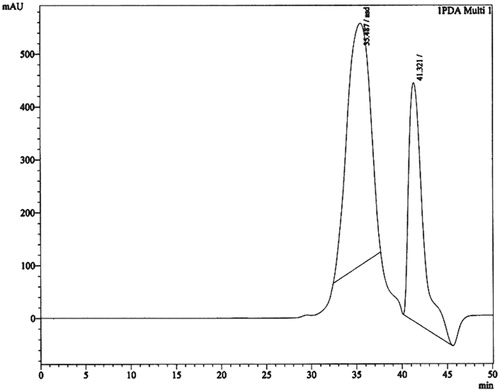
Figure 4. Inhibitory effect of methanol extract and fatty acid on α-glucosidase (0.5 U/mL, using substrate p-nitrophenyl α-d-glucopyranoside 0.5 mm) determined in the concentration range from 1 to 10 µM/mL.
The Lineweaver–Burk plot showed a decrease in Vmax with an increased concentration of methanol extract and the isolated compound. The data suggested an uncompetitive type of inhibition for isolated compound and methanol extract of A. spinosus ().
Figure 5. Kinetic analysis of α-glucosidase inhibition by novel fatty acid and methanol extract of A. spinosus. The Lineweaver–Burk plot of hydrolysis of p-nitrophenyl α-d-glucopyranoside (10, 15, 20, and 25 mM) by α-glucosidase (0.5 U/mL) in the presence and absence of isolated fatty acid and methanol extract.
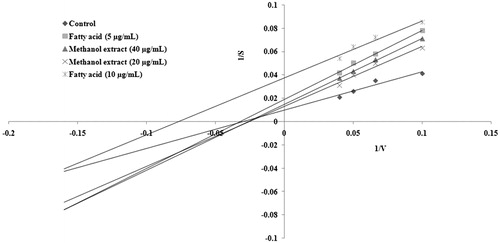
The α-glucosidase inhibitory activity of the isolated fatty acid has not been reported in the literature previously. The reports of this investigation establish that the isolated and purified (14E, 18E, 22E, 26E)-methyl nonacosa-14, 18, 22, 26 tetraenoate could be an important antidiabetic agent.
Conclusion
The present study showed that the purified fatty acid showed significant inhibition of α-glucosidase and it might be the active constituent at least in part, responsible for such inhibitory activity.
Acknowledgements
The authors would like to thank Jadavpur University, Kolkata, India, for providing research facilities and CSIR-Indian Institute of Chemical Biology, Kolkata, India, for instrumental analysis facilities.
Declaration of interest
The authors have no conflicts of interests.
References
- Balakrishnan S, Pandhara R. (2010). Antihyperglycemic and antihyperlipidaemic activities of Amaranthus spinosus Linn extract on alloxan induced diabetic rats. Malays J Pharm Sci 8:13–22
- Girija K, Lakshman K, Udaya C, et al. (2011). Anti-diabetic and anti-cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac J Trop Biomed 1:133–8
- Jayaprakasha GK, Mandadi KK, Poulose SM, et al. (2007). Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg Med Chem 15:4923–32
- Kirtikar KR, Basu BD. (2001). Indian Medicinal Plants, 2nd ed., Vol 1. New Connaught Place, Dehradun, Uttranchal, India: Oriental Enterprises, 2832–6
- Kumar D, Gaonkar RH, Ghosh R, Pal BC. (2012). Bioassay guided isolation of α-glucosidase inhibitory constituents from Eclipta alba. Nat Prod Commun 7:989–90
- Ortiz-Andrade RR, Garcia-Jimenez S, Castillo-Espana P, et al. (2007). α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J Ethnopharmacol 109:48–53
- Sangameswaran B, Jayakar B. (2008). Anti-diabetic, antihyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn. on streptozotocin-induced diabetic rats. J Nat Med 62:79–82
- Sangameswaran B, Pandhare R. (2010). Antihyperglycemic and antihyperlipidaemic activities of Amaranthus spinosus Linn extract on alloxan induced diabetic rats. Malays J Pharm Sci 8:13–22
- Shim YJ, Doo HK, Ahn SY, et al. (2003). Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85:283–7
- Zhang X, Geoffroy P, Miesch M, et al. (2005). Gram-scale chromatographic purification of β-sitosterol. Synthesis and characterization of β-sitosterol oxides. Steroids 70:886–95