Abstract
Context: Carica papaya L. (Caricaceae) fruit was shown to exhibit wound healing properties.
Objectives: We investigated anti-inflammatory and antioxidant potential of papaya fruit phosphate-buffered saline extract (PE) during wound healing and enhancement of the potentials due to trace ions addition.
Materials and methods: Rat excision wounds were topically treated twice/day with 20 µL of PE (5 mg extract/mL), 0.5 µg Se2+ added PE (PES), or 100 µM Zn2+ added PE (PEZ). Control groups were treated with deionized water (negative) and deproteinized calf blood extract ointment (Solcoseryl®, positive). Lipid peroxidation (LPX), antioxidant, proinflammatory, and arginine metabolic enzymes were estimated in the wound excised on days 4 and 10 post wounding.
Results: PE (5 mg/mL; 9.80 ± 0.33 d) and PES (PE + 0.5 µg Se2+; 8.90 ± 0.23 d) significantly (p < 0.05) reduced the average time for complete wound closure compared with the negative (13.00 ± 0.37 d) and positive (9.80 ± 0.33 d) controls, respectively. Biochemical evaluations of LPX product (malondialdehyde), antioxidant (catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx)), and pro-inflammatory (cyclooxygenase-2 and myeloperoxidase (MPO)) enzyme activities and metabolites (nitrite and urea), on days 4 and 10 post wounding, confirmed the anti-inflammatory and antioxidant properties of PE and PES in this study.
Discussion and conclusion: Treatment of excision wounds with papaya extract, especially with the addition of selenium for 10 d, reduced inflammation associated oxidative damage apparently via cyclooxygenase specific inhibition, arginine metabolism, and up-regulation of antioxidant enzymes.
Introduction
Many fruits are known to have important pharmacological properties such as pineapple for allergic airway diseases and asthma (Secor et al., Citation2005), chinaberry for leprosy (Marimuthu et al., Citation2013), and feijoa fruit for goiter in traditional Turkish medicine (Keles et al., Citation2012). Carica papaya L. (Caricaceae) fruit is consumed often as a fruit, sometimes as a vegetable, especially unripe papaya which is used as a meat tenderizer (Maiti et al., Citation2008). It is also well known for its various pharmacological properties such as antioxidant (Oboh et al., Citation2013), antimicrobial (Osato et al., Citation1993) anti-inflammatory (Owoyele et al., Citation2008), and wound healing (Anuar et al., Citation2008).
Pharmacological properties of natural products are mostly evaluated using in vitro models such as antioxidant activity by DPPH and β-carotene-linoleate bleaching methods (Oboh et al., Citation2013), antimicrobial activity by disk diffusion and minimum inhibitory concentration (MIC) determination methods (Klancnik et al., Citation2010), and wound healing using a scratch assay (Liang et al., Citation2007). However, it is important to evaluate how these in vitro pharmacological assays are translated to in vivo conditions. A number of studies have reported the pharmacological potential of natural products using in vivo models, such as antioxidant properties of Pyrostegia venusta extract using wound tissue (Roy et al., Citation2012), and anti-inflammatory properties of papaya using carrageenan-induced rat paw edema (Owoyele et al., Citation2008).
Open full thickness excision wound was used as in vivo model in the present study to investigate both the anti-inflammatory and antioxidant properties of unripe papaya. Open full thickness excision wound is prone to microbial invasion which increases inflammatory responses at the wound site. The increased inflammatory responses aggravate respiratory burst to increase the release of reactive oxygen species (ROS), reactive nitrogen species (RNS), and peroxides from the inflammatory cells beyond physiological need (Süntar et al., Citation2010). These oxidants, at physiological doses, could stimulate angiogenesis by up-regulating the vascular endothelial growth factors (VEGF) expression thereby improving nutrients and oxygen supply to the wound (Kanta, Citation2011). These beneficial effects of oxidants generated in the wound are achieved due to the homeostatic state of ROS and peroxides which are maintained by the endogenous antioxidant enzymes (Süntar et al., Citation2010). Thus, open full thickness excision wound provides a complete in vivo model to study both the anti-inflammatory and antioxidant properties of any drug including natural products to be tested in terms of wound healing properties.
Rats orally administered with higher concentrations of papaya extract showed reduced inflammatory paw-swelling (Owoyele et al., Citation2008). Consumption of papaya fruit also reduced oxidative stress and altered lipid profiles comparable with the standard drug α-tocopherol (Krishna et al., Citation2008). High concentrations of antioxidant substances such as phenolic, flavonoid, β-carotene, lycopene, and ascorbic acid in papaya exhibited a good correlation with the antioxidant activity of papaya fruit extract analyzed using β-carotene-linoleate bleaching assay and DPPH free radical scavenging assay (Oboh et al., Citation2013). Topical agents which have antioxidant properties improve wound healing significantly and protect tissues from oxidative damage (Roy et al., Citation2012). Furthermore, wound healing process was associated with the presence of antioxidants and phenolic compounds (Chidambara et al., Citation2004; Süntar et al., Citation2010).
Thus, the rationale of using an excision wound model to study anti-inflammatory and antioxidant properties lies on the requirement of protection against inflammation associated oxidants (free radical) production during healing process. Here we are reporting the anti-inflammatory and antioxidant properties of unripe papaya extract, with or without additional trace element, using excision wound as a model. Unripe papaya with additional selenium (Se2+) was earlier reported to increase wound healing efficiency (Abdulrazaq et al., Citation2013) and provides protection against excessive inflammation and oxidative tissue damage which are major requirements for efficient healing (Roy et al., Citation2012).
Materials and methods
Unripe papaya extract preparation
The unripe C. papaya L. var. exotica fruits were collected during July 2011, from a farm at Jabatan Pertanian Tanjung Malim, Perak, Malaysia. The fruit was identified and authenticated by Dr. Nurziana of Herbal Laboratory, Faculty of Pharmacy, International Islamic University Malaysia (IIUM). The voucher specimen (Eiium 34) was deposited in the herbarium at the Faculty of Pharmacy, IIUM, for future reference. The fruits were washed, drained at room temperature, and peeled. The pulps were cut into small pieces and homogenized (1:3 w/w) in sterile phosphate-buffered saline (PBS). The resulting homogenate was transferred to an incubator shaker at 37 °C for 8 h. The mixture was filtered and then centrifuged at 200 g for 30 min at 4 °C. The supernatant was collected and freeze-dried as previously reported (Abdulrazaq et al., 2013; Anuar et al., Citation2008). Standard solutions of either Na2SeO3 (54.75 µg/mL) or ZnSO4 (100 µM) were added to PE for topical applications.
Experimental animals
Adult female Sprague–Dawley rats weighing 200 ± 20 g body weight were purchased from the university breeding center (UKM, Kuala Lumpur, Malaysia). Rats (5–10) were used for each treatment group (specified in the respective sections). Each rat, housed separately, was maintained on standard pellet diet and tap water. Throughout the experiments, all animals received humane care and the study procedure was approved by the ethics committee (no. IIUM/305/20/4/10) for animal experimentation.
Experimental design
Ten rats (n = 10) per group were used for the evaluation of wound size while wound samples taken from five rats (n = 5) were used for the evaluation of each of the biochemical parameters analyzed in the current study. Rat excision wounds were topically treated twice/day with either PBS extract only (PE) or PE with the addition of either selenium (PES) or zinc (PEZ). In a final volume of 20 µL of the solution prepared in PBS, the concentration of the PBS extract, selenium (Se2+), and zinc (Zn2+) were 5 mg extract/mL, 0.5 µg/20 µL, and 100 µM, respectively. Control groups were treated with either deionized water as a negative control (NC) or a thin layer of deproteinized calf blood extract ointment (10% Solcoseryl®, Solco Basle, Birsfelden, Switzerland) as a positive control (PC).
Wound induction and size measurement
The dorsal region of the animals’ neck (specifically, the nape of the neck) was shaved using hair clippers. A uniform circular full thickness open excision wound was created using a 6 mm biopsy punch under light ethyl ether anesthesia. Wounds were left undressed and exposed to the open environment. This model was used to monitor the wound size over time. Rats showing any sign of skin infection or abnormal skin appearance were excluded from the study.
Pictures of wounds were taken using a digital camera (Canon Powershot 5.0 MP, Canon, Tokyo, Japan) on alternative days to measure the wound size from the first day of wound induction until the day of complete wound closure (CWC). Photos were taken with the same settings of distance and aperture and were analyzed for wound surface area (mm2) using Adobe® CS3 Photoshop software (Extended version 12.0.4 × 32 Adobe Inc., Adobe Systems Incorporated, San Jose, CA) as previously described (Abdulrazaq et al., Citation2013).
Sample collection for the biochemical assays
Wound tissue samples from rats were carefully excised using scissors to remove a sufficient and constant amount of the surrounding wound margin from the healing skin of rats from all groups on days 4 and 10 post wounding. Cuts were made deep into the underlying tissue to completely remove the granulation tissue from the healing wound. The wound samples were frozen and stored in −80 °C. These were used for the biochemical estimations of endogenous antioxidant enzyme activities, lipid peroxidation (LPX), inflammation, and arginine metabolic enzyme activities.
Sample preparation for the biochemical assays
Wound samples were prepared for the protein estimation following the method of Dieterich et al. (Citation2000). The wound samples were homogenized in an ice-cold 0.01 mol/L sodium phosphate buffer (pH 7.4) and centrifuged for 5 min at 12 000 g at 4 °C and the supernatant was collected and stored at −80 °C prior to analysis. Protein content was quantified following the standard method of Bradford (Citation1976). Briefly, the Bradford reagent (5.0 mL) was mixed with 100 µL of prepared wound sample supernatant which was previously diluted in 0.15 M NaCl solution. Absorbance value was read at 595 nm. Bovine serum albumin was used as the standard.
Catalase enzyme assay
Catalase activity was measured in the sample supernatant using the rapid spectrophotometric method described by Cohen et al. (Citation1970). The rate of H2O2 decomposition by addition of sample was determined by allowing the reaction to continue for 3 min with a standard excess of KMnO4 and by subsequent measurement of the residual KMnO4 at 480 nm. Measurements were performed in triplicate. In this assay, 1 unit of enzyme activity equals k/(0.00693), where k = log (S0/S2) × (2.3/t), S0 = absorbance of standard − absorbance of blank, S2 = absorbance of standard − absorbance of sample, and t = time interval (Aebi, Citation1974). The measured activities were normalized with the protein content of each sample. Catalase activity was calculated as units per milligram of protein.
Superoxide dismutase (SOD) enzyme assay
SOD activity was measured in the sample supernatant according to the method described by Marklund and Marklund (Citation1974). This assay is based on the ability of SOD to scavenge superoxide anion radical (), which inhibits the auto-oxidation of pyrogallol. In brief, 1 mL of 0.05 mol/L Tris-HCL buffer (pH 8.2) containing 1 mmol/L diethylenetriamine penta acetic acid (DTPA) was added to 50 µL of sample supernatant. The reaction was initiated by the addition of pyrogallol (a final concentration of 0.2 mM), and the absorbance measured kinetically at 420 nm, 25 °C for 3 min. SOD activity was calculated as units per milligram of protein, with 1 U of SOD defined as the amount that inhibited the rate of pyrogallol autoxidation by 50%.
Glutathione peroxidase (GPx) enzyme activity
GPx activity was measured using a GPx cellular activity assay kit. It is based on the oxidation of reduced glutathione by GPx coupled to the disappearance of NADPH by glutathione reductase. Sample supernatant (40 µL) was added to 950 µL NADPH assay reagent containing 5 mM NADPH, 42 mM reduced glutathione, 10 units/mL of glutathione reductase, 0.5 mM EDTA, and 50 mM Tris HCl (pH 8.0). The reaction was initiated by the addition of 10 µL of 30 mM tert-butyl hydroperoxide solution. The decrease in the absorbance at 340 nm was recorded for 3 min. GPx activity was calculated as units per gram of protein, with 1 U GPx causing the formation of 1.0 µmol of NADP+ from NADPH per minute.
LPX assay
Sample used for this assay was taken after homogenization without centrifuge. The analysis of LPX was carried out based on the thiobarbituric acid reactive substance TBARS method as described by Buege and Aust (Citation1978) with little modification. The reaction mixture was prepared by adding 1 mL homogenate into 4 mL reaction solution containing 15% trichloroacetic acid, 0.375% thiobarbituric acid, and 0.25 N NaOH. The mixture was heated at 100 °C for 10 min, cooled to room temperature, and centrifuged at 10 000 g for 10 min. The supernatant was removed and the absorbance was recorded at 532 nm. LPX in the form of malondialdehyde (MDA) equivalent was expressed as nmol/mg protein in the supernatant.
Cyclooxygenase-2 (COX-2) enzyme activity
The COX-2 enzyme activity was measured using the COX Colorimetric-based Assay Kit (Cayman, Ann Arbor, MI) according to the manufacturer’s protocol. In this method, the peroxidase activity of COX was measured based on the oxidation of N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) during the reduction of hydroperoxy endoperoxide (prostaglandin G2 or PGG2) to corresponding alcohol (prostaglandin H2 or PGH2), the precursor of prostaglandins and prostacyclins (Copeland et al., Citation1994) at 590 nm.
Myeloperoxidase (MPO) enzyme activity
Wound tissue sample was homogenized in 10 volumes of ice-cold 50 mM sodium phosphate buffer, pH 6.0, containing 0.5% hexadecyl trimethyl ammonium bromide (Sigma Chemical, St. Louis, MO) and 5 mM EDTA. The homogenate was sonicated on ice for 15 s, freeze-thawed three times, and centrifuged for 30 min at 14 000 rpm at 4 °C. Protein concentration was determined using the standard method of Bradford. MPO in the supernatant was estimated by o-dianisidine oxidation previously described (Andrews & Krinsky, Citation1985) and activity calculated based on the earlier study (Feng et al., Citation2007). MPO activity (units/mg protein) = (ΔA460 × 13.5)/amount of protein in sample (mg). ΔA460: change in absorbance at 460 nm in 3 min. The coefficient 13.5 was empirically determined so that one unit of MPO activity was defined as the quantity able to convert 1 mmol of H2O2 to water in 1 min at 25 °C.
Nitrite content determination
Wound tissue sample was homogenized in lysis buffer (1% Triton X-100, 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 15 µg leupepetin per mL). Tissue lysates (1 mL) was cleared at 100 000 g for 30 min, diluted with deionized water, and stored at −20 °C. The supernatant (200 µL) was maintained at 4 °C, mixed with 20 µL of 1% sulfanilamide (dissolved in 1.2 M HCl), 20 µL of 0.1% N-naphtylethylenediamine dihydrochloride, and incubated for 5 min at room temperature. The absorbance was measured at 540 nm using a microplate reader. Nitrite concentration was determined using NaNO2 as a standard.
Arginase enzyme activity
Wound tissue sample was homogenized in 25 mM Tris-HCl, (pH 7.5) containing 5 mM MnCl2 and protease inhibitors cocktail (10 mg/mL pepstatin A, 10 mg/mL leupeptin, 100 mg/mL phenylmethylsulfonyl fluoride). Homogenate was cleared at 5000g for 20 min, protein content was determined using the standard method of Bradford, and the arginase enzyme activity was measured as previously described (Corraliza et al., Citation1994).
Statistical analysis
The statistical analysis was carried out using the software SPSS for Windows version 11.01 (SPSS Inc., Chicago, IL). Data were expressed as mean with standard error. One way-Anova was used to analyze the data and Tukey’s post hoc test was used to find the mean differences within the groups at different time interval. Differences were considered statistically significant at p < 0.05.
Results
Se2+ addition to papaya extract enhanced wound closure
On days 1, 3, and 5, sizes of the wounds treated with PC and PES were significantly reduced (p < 0.05) compared with that of NC. However, on days 1 and 7, the size of the wound treated with PES was also significantly reduced (p < 0.05) compared with that of PC. Notably, PES also reduced significantly (p < 0.05) the wound size as compared with PE () on days 1, 3, and 5. On days 1, 7, and 9, size of the wound treated with PE was significantly reduced (p < 0.05) compared with that of NC (). On the day 11, the wound closure was ∼100% with PE and PES, while it was ∼98%, ∼93%, and ∼85% for PC, PEZ and NC, respectively. NC produced CWC after 2 weeks of treatment ().
Figure 1. (A) Representative photographs of full-thickness excision wounds at different time intervals following treatment with 5 mg/mL papaya PBS extract (PE) with or without trace elements. (B) Wounds were more rapidly healed in PE + 0.5 µg Se2+ (PES) treated group than the positive control (PC), negative control (NC), PE + 100 µM Zn2+ (PEZ). Bars indicate mean ± SEM (n = 10). *, #, and † indicate significantly (p < 0.05) reduced wound size as compared with NC, PC, and PE respectively.
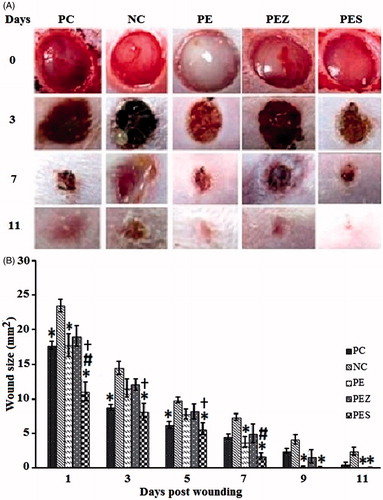
Table 1. Grouping of animals into respective treatment groups.
Average time for CWC was significantly reduced (p < 0.05) in the PES group (9.80 ± 0.33 d) compared with the PC group (8.90 ± 0.23 d) (). Average time for CWC was also significantly reduced (p < 0.05) in all the experimental groups, i.e., PC, PE (9.80 ± 0.33 d), PEZ (10.80 ± 0.25 d), and PES compared with the NC group (13.00 ± 0.37 d) ().
Table 2. Time for complete wound closure in response to Se2+ and Zn2+ added papaya extract.
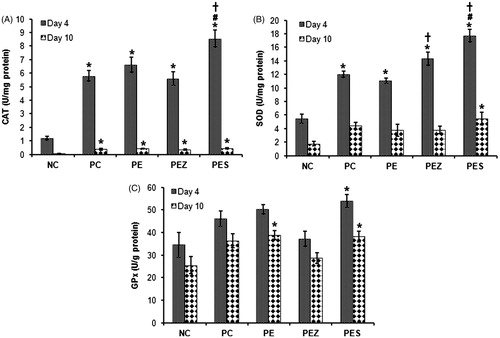
Se2+ added papaya extract increased catalase, SOD, and GPx activities on days 4 and 10
PC, PE, and PEZ significantly increased (p < 0.05) wound catalase activity on days 4 and 10 () and SOD activity on day 4 only () when compared with NC. These treatments did not show any significant effect (p < 0.05) on the GPx activity on days 4 and 10 post wounding (). However, PES significantly increased (p < 0.05) the activity of wound catalase (), SOD (), and GPx () on days 4 and 10 post wounding when compared with NC and significantly increased (p < 0.05) further the wound catalase () and SOD () activities on day 4 when compared with PC. In addition, PES significantly (p < 0.05) increased catalase and SOD activities on day 4 when compared with PE.
Figure 2. Catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities in wound tissues following treatment with papaya extract added with Se2+ and Zn2+. NC, negative control; PC, positive control; PE, PBS extract; PES, PE + 0.5 µg Se2+; PEZ, PE + 100 µM Zn2+. Bar indicate mean ± SEM (n = 5); *, #, and † indicate significantly increased (p < 0.05) enzymes’ activities as compared with NC, PC, and PE, respectively.
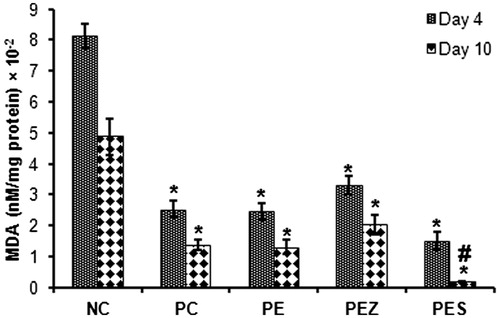
All treatment reduced LPX (MDA concentration) in wound
MDA concentration was significantly reduced (p < 0.05) in all the treated groups versus the NC rats on days 4 and 10 (). However, PES only significantly reduced (p < 0.05) MDA concentration compared with PC on day 10 ().
Figure 3. Malondialdehyde (MDA) levels in wound tissues following treatment with papaya extract added with Se2+ and Zn2+. NC, negative control; PC, positive control; PE, PBS extract; PES, PE + 0.5 µg Se2+; PEZ, PE + 100 mM Zn2+. Bars indicate mean ± SEM (n = 5); * and # indicate significantly (p < 0.05) decreased tissue MDA content as compared with NC and PC, respectively.
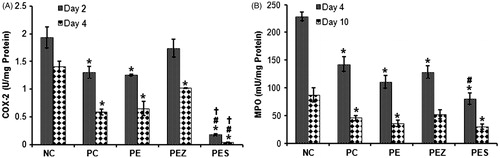
Se2+ added papaya extract decreased COX-2 and MPO activities in wound on days 4 and 10
Treatment with PE, PEZ, and PES significantly decreased (p < 0.05) the COX-2 enzyme activity as compared with NC on days 2 and 4 post wounding corresponding to peak and declining phase of inflammation except PEZ which produced no significant effect on day 2 (). PES only decreased COX-2 enzyme activity significantly (p < 0.05) versus PC and PE on days 2 and 4 post wounding (). PE, PEZ, and PES significantly decreased (p < 0.05) the MPO enzyme activity as compared with NC on days 4 and 10 post wounding () except the PEZ which produced no significant effect on day 10. Moreover, PES only decreased MPO enzyme activity significantly (p < 0.05) versus PC on day 4 post wounding ().
Figure 4. Cyclooxygenase-2 (COX-2) and myeloperoxidase (MPO) activities in wound tissues following treatment with papaya extract added with Se2+ and Zn2+. NC, negative control; PC, positive control; PE, PBS extract; PES, PE + 0.5 µg Se2+; PEZ, PE + 100 mM Zn2+. Bars indicate mean ± SEM (n = 5); *, #, and † indicate significantly (p < 0.05) decreased enzymes’ activities as compared with NC, PC, and PE, respectively.
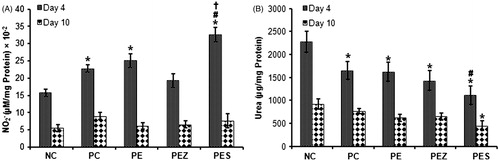
Se2+ added papaya extract increased wound nitrite and decreased urea content on day 4
Treatment with PE and PES significantly increased (p < 0.05) wound nitrite content versus NC on day 4 (). However, PES only significantly increased (p < 0.05) wound nitrite content versus PC and PE on day 4 (). The wound nitrite content reduced in all the experimental groups on day 10 post wounding, however, control and treated groups showed no significant difference. PE, PEZ, and PES significantly decreased (p < 0.05) wound urea content as compared with NC while PES only decreased wound urea content significantly (p < 0.05) when compared with PC on day 4. Treatment with PES only significantly decreased (p < 0.05) wound urea content versus NC on day 10 ().
Figure 5. Nitrite () and urea content of wound tissues following treatment with papaya extract added with Se2+ and Zn2+. NC, negative control; PC, positive control; PE, PBS extract; PES, PE + 0.5 µg Se2+; PEZ, PE + 100 mM Zn2+. Bars indicate mean ± SEM (n = 5); *, # and † indicate significantly (p < 0.05) increased nitrite content and decreased urea content as compared with NC, PC, and PE, respectively.
Discussion
Papaya is known for its antimicrobial, anti-inflammatory, immunomodulatory (Otsuki et al., Citation2010), and antioxidant properties (Oboh et al., Citation2013; Osato et al., Citation1993; Owoyele et al., Citation2008). Anti-inflammatory and antioxidant properties of papaya are attributed to primary and secondary bioactive components such as papain, chymopapain, papaya lipase, and carotenoids. Lycopene, β-criptoxhantin, and β-carotene were reportedly most abundant carotenoids in papaya fruit with proven antioxidant property in the biological system (Rivera-Pastrana et al., Citation2010). Addition of Se2+, a well-known antioxidant, to papaya PBS extract was shown to improve healing efficiency of the extract (Abdulrazaq et al., Citation2013). This improved healing efficiency was hypothesized to be linked with anti-inflammatory and antioxidant activities during healing. As expected, the current study has shown that application of papaya extract alone or in combination with additional antioxidants such as Se2+ and Zn2+ on wound suppressed excessive inflammation and enhanced activities of endogenous antioxidant enzymes such as catalase and superoxide dismutase in vivo conditions.
Injury to the skin induces intricate reactions, including early inflammation (inflammatory cell influx), during which free radicals and oxidants are excessively released in to the wound area. Free radicals (ROS and RNS), released during inflammation, can cause severe oxidative damage to the healing wound (Korkina et al., Citation2007). Endogenous antioxidants like SOD, catalase, and glutathione (GSH), in granulation tissues, accelerate the process of wound healing by neutralizing the free radicals (Gupta et al., Citation2002). Therefore, cutaneous wound must be properly protected against excessive inflammatory cell influx and free radical-mediated processes in cutaneous wound.
Healing takes place in stages which involve inflammation, repair, and remodeling phases in order. Depending on the type and etiology of wound, duration of those phases varies (Sherman & Barkley, Citation2011) and could be overlapping. In the excision wound model that was used, the duration of the phases generally proceed by the following pattern: inflammation (days 1–4), repair phase (days 5–10 or 11), and remodelling (days 11–21 or more) (Li et al., Citation2007). Therefore, biochemical markers of inflammation and oxidative damage were assessed in the wound tissues, excised on days 4 and 10 post wounding, except COX-2 which was assessed on days 2 and 4 post wounding as it was not detectable beyond day 4.
Endogenous antioxidants, such as SOD, GPx, and catalase, constitute the first line of defense against oxidants (Guo & DiPietro, Citation2010). Activities of these enzymes in response to papaya extracts application were analyzed using wound tissue. The extract preparations enhanced activities of catalase and SOD and papaya extract alone increased activity of GPx later on day 10. However, addition of Se2+ enhanced exclusively activity of SOD at day 10 and increased activity of both catalase and SOD on day 4 better than Solcoseryl®. Furthermore, Se2+ addition to papaya PBS extract increased activity of GPx both at the early time (day 4) and the later time (day 10). The findings of the present study thus suggest that PBS extract of unripe papaya pulp increased the rate of wound healing by increasing the activity of wound endogenous antioxidant enzymes which suppressed oxidant-induced tissue oxidative damage and reduced accumulation of break down products of LPX.
Addition of Se2+ to papaya PBS extract can be linked with increased activity of endogenous antioxidant enzymes. As Se2+ is an essential component of various selenoproteins like GPxs (Sunde, Citation2001), thioredoxin reductase, iodothyronine deiodinases, and selenoprotein P which are also responsible for controlling oxidative stress (Mukherjee et al., Citation1998; Papp et al., Citation2007).
Results in the present study did not reflect the role of Zn2+ on the induction and up-regulation of antioxidants notably catalase. Topical zinc, in the form of divalent zinc ions, has been reported to provide antioxidant photoprotection for skin (Aricioglu et al., Citation2001; Rostan et al., Citation2002). Again, elevated level of GSH, GPx, and SOD activities was observed in the blood of wrestlers given zinc supplement at physiological doses (Kara et al., Citation2010). The apparent contradiction in the present study can be explained in terms of the effect of Zn2+ on inflammatory responses during cutaneous wound healing. Zinc supplementation, in mice inflicted with excision wound, caused altered inflammatory responses, and delayed the rate of wound closure at a very low or high dose (Lim et al., Citation2004)
Antioxidant property of the extract in excision wound model was further analyzed by measuring the accumulated product of oxidative damage (MDA equivalent). Papaya extract decreased MDA both at days 4 and 10 (). Compared with the positive control group, the level of MDA in wound tissues was observed to be significantly lower only in Se2+ added papaya PBS extract group on day 10. Oxidative stress at the site of injury is known to impair the healing process by causing damage to cellular membranes, nucleotides, proteins, and lipids (Clark, Citation2008; Moseley et al., Citation2004). Decreased level of MDA found in all wounds treated with papaya PBS extract alone or papaya PBS extract with additional trace element strongly suggests antioxidant property of the preparations could be due to increased quenching of free radicals by the elevated levels of enzymatic antioxidants or suppressed level of free radical production.
COX-2 enzyme and its enzymatic product prostaglandin E2 (PGE2) are known critical mediators of inflammation (Wilgus et al., Citation2004). Papaya PBS extract suppressed the activity of COX-2 at days 2 and 4 post wounding (). However, COX-2 activity was more markedly suppressed in the wound tissues with additional Se2+ in the extract. Addition of Zn2+, however, did not cause further reduction in COX-2 enzyme activity compared with that obtained using papaya PBS extract alone. The enzymatic product of COX-2, i.e., PGE2, increases vascular permeability, infiltration, and activation of inflammatory cells in the early inflammation (Wilgus et al., Citation2000). Thus reduced activity of COX-2 enzyme diminishes PGE2-mediated inflammatory cell infiltration of cutaneous wound. Thus, it can be concluded that the papaya PBS extract might mimic COX inhibitors. Notably, specific COX-2 inhibitor was shown to dampen the inflammatory responses and reduces scarring in cutaneous wound (Wilgus et al., Citation2003).
The present study also quantified the level of MPO, which converts hydrogen peroxide to hypochlorous acid, as a measure of PMNL influx/activation in the excised wound tissue (Debats et al., Citation2009). We have found that the MPO activity was significantly decreased by papaya PBS extract (). Again, Se2+ addition enhanced the effect of papaya PBS extract on inflammatory cell activation by further reducing MPO activity when compared with Solcoseryl®.
We have further estimated urea content () in the wound tissue as a function of total arginase activity. Enzymes of arginine metabolism have been identified as essential in the course of wound healing especially during acute inflammation. Arginase enzyme activity in wound was considerably reduced by topical application of Se2+ added papaya PBS extract both on days 4 and 10, as shown through reduced wound urea content compared with both negative and positive control. Arginine supplementation in animals and humans improved skin repair (Debats et al., Citation2009). l-Ornithine and urea are the main enzymatic products of arginase. l-Ornithine is further converted to the polyamines (involved in cell growth and differentiation) and proline, the building block for collagen synthesis (Kapoor et al., Citation2004). Reduced collagen synthesis and deposition are required to maintain the hemostatic state of extracellular matrix for remodeling phase of wound healing (Kapoor et al., Citation2004). Regression of many of the newly formed capillaries is also essential as this would produce scar more closely resemble the surrounding normal dermal architecture (Guo & DiPietro, Citation2010). Hence, reduced arginase activity can be attributed to less scar formation which was observed in the current study after the treatment with the PBS extract or Se2+ added PBS extract compared with negative control (, Day 11).
Nitrite is the stable breakdown product of nitric oxide (NO). NO synthase (NOS) metabolized l-arginine to produce NO and l-citrulline. Treatment with papaya PBS extract resulted in a significant increase in wound nitrite content on day 4 but Se2+ added papaya PBS extract produced the highest wound tissue nitrite content on day 4. The wound nitrite content falls to almost control level in all groups by day 10 (). These suggest that decreased arginase activity from the control level with increased NOS activity at the early phase (day 4) favors NOS catalyzed production of NO which is an angiogenic factor. Attenuation of NOS activity to barely control level in all treated group indicated a shift in metabolic direction towards formation of urea, proline, polyamines, protein, and collagen deposition which might be sufficient for normal wound healing but not enough to produce excess collagen for abnormal scar formation. A study has shown that elevated NO associated with cutaneous wound healing in the early phase of wound healing was exclusively due to the inducible isoform of NOS. NO derived from the inducible NOS (iNOS) reportedly increased VEGF expression, angiogenesis, collagen synthesis, and has cytostatic activity during cutaneous wound healing (Kapoor et al., Citation2004). Thus, it can be hypothesized that the increase in iNOS activity in the early phase due to papaya PBS extract treatment of wound may induce transient up-regulation of VEGFA and angiogenesis. Again, decrease of NO production in the later phase might halt or suppress excessive angiogenic activity and collagen deposition that otherwise would cause abnormal scar formation. This might be one of the mechanisms through which papaya PBS extract enhanced wound healing. Additional Se2+ in the extract increased the rate at which these activities are amplified. The results of this study have demonstrated that papaya PBS extract possesses anti-inflammatory property in cutaneous wound by regulating activity of inflammatory mediators (COX-2 and MPO) and metabolic enzymes (arginase and iNOS) and effect can be improved with additional selenium in the extract.
Based on the current findings and earlier studies, papaya PBS extract induced mechanism of antioxidant and anti-inflammatory pathways in wound healing is proposed (). One of the early responses to an inflammatory stimulus such as wounding is the induction of the enzyme COX-2. The enzyme COX-1 in conjunction with its inducible isoform COX-2 catalyzes the conversion of arachidonic acid to prostaglandins. However, COX-2 specifically mediates wound-induced elevation of prosglanding E2 (PGE2) which induces vascular permeability, infiltration, and activation of inflammatory cells (Wilgus et al., Citation2000). Therefore, it can be proposed that papaya PBS extracts significantly inhibited COX-2 enzyme activity () thereby reduced the PGE2-mediated PMNL infiltration and activation ( and ) at the wound area. These actions of COX-2 as modulated by papaya PBS extracts in this study may be more complex than these since it involves the activity of proinflammtory cytokines that also regulate the inflammatory responses. However, this is one of the biological pathways through which papaya PBS might work as a topical anti-inflammatory agent during wound healing.
Figure 6. Role of anti-inflammatory and antioxidative bioactive components of papaya PBS extract during wound healing. Bioactive components of papaya PBS extract are involved in regulating the functions of superoxide dismutase (SOD), catalase (CAT), myeloperoxidase (MPO), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and arginase. Activity of these enzymes eventually contributes to the improved wound healing. PGE2, prostaglandin E2; OAT, ornithine amino transferase; ODC, ornithine decarboxylase; NO, nitric oxide; PMNL, polymorphonuclear leukocytes. [symbols: - -|, inhibition; →, synthesis or activation].
![Figure 6. Role of anti-inflammatory and antioxidative bioactive components of papaya PBS extract during wound healing. Bioactive components of papaya PBS extract are involved in regulating the functions of superoxide dismutase (SOD), catalase (CAT), myeloperoxidase (MPO), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and arginase. Activity of these enzymes eventually contributes to the improved wound healing. PGE2, prostaglandin E2; OAT, ornithine amino transferase; ODC, ornithine decarboxylase; NO, nitric oxide; PMNL, polymorphonuclear leukocytes. [symbols: - -|, inhibition; →, synthesis or activation].](/cms/asset/2d398549-9225-4a65-a051-161902e0f839/iphb_a_936470_f0006_c.jpg)
However, while reduced inflammation benefit wound healing, minimal or moderate inflammation is essential to wound healing. Endogenous or microbial activation of inflammatory cells release ROS and RNS which at physiological doses protect wound against infection and participates in the activation of angiogenesis (Broughton et al., Citation2006). In contrast, a slight increased level of ROS or RNS could be debilitating to wound healing (Süntar et al., Citation2010). In this study, papaya PBS extract markedly increased endogenous antioxidant enzyme activities () both in the early and later time thereby suppressing the ROS and RNS mediated oxidative tissue damage, which is reflected in the reduced amount of accumulated tissue break down products such as MDA ().
As the healing process continues, inflammatory macrophages replace PMNL as the main cells of inflammation. At this point, cytokines play significant role in the alternative activation and differentiation of macrophages which results in the release of growth factors and MMPs necessary for cellular proliferation, differentiation, and migration at the wound area. These mark the beginning of proliferation phase of wound healing. In the current study, papaya PBS extract treatment exhibited considerable increase in the wound nitrite content which indicates increased activity of iNOS. Studies have reported essential role of iNOS in wound healing especially in the early phase of wound healing (Pacher et al., Citation2007). NO and its breakdown products such as RNS are involved in the signaling pathways that stimulate increase angiogenic response.
The arginase-mediated arginine metabolism might have contributed to collagen and extracellular matrix synthesis at the early and later phases (days 4 and 10). Previous studies have shown the activation of iNOS and arginase1 enzymes after wounding with increased expression that diminish as the wound heals. Arginase-mediated arginine metabolic pathway produces urea as the end product and l-ornithine which yield poly-amines and proline, a cellular proliferation factor and the precursor of collagen synthesis, respectively (Debats et al., Citation2009). On day 10, we observed a significant decrease in the wound tissue urea content and no significant change in the nitrite content, as compared with the negative control groups, in the papaya PBS extract-treated group. These implied decreased activities of iNOS and arginase1 enzymes. These might serve as “switch off” stimulus for the VEGF protein expression thereby stopped excessive vascularization, ECM, and collagen synthesis. Excessive vascularization, ECM, and collagen synthesis are the cornerstone of abnormal scar formation (Gauglitz et al., Citation2011). Since wound healing is a homeostatic mechanism for restoration of disrupted tissue integrity, decreased activities of arginase1, and iNOS enzyme to control level served to establish a homeostatic state for normal skin structure and function.
Conclusions
Treatment of excision wound with papaya PBS extract (5 mg/mL) with the addition of selenium (0.5 µg/20 µL) attenuates inflammation associated oxidative damage and improved cutaneous wound healing. The anti-inflammatory and antioxidative effects are apparently mediated through cyclooxygenase specific inhibition, changes in the arginine metabolic pathways, and up-regulation of endogenous antioxidant enzymes activities.
Acknowledgements
We thank Izzati Abd Malik for contributing to the preparation of plant extracts.
Declaration of interest
The authors report no declarations of interest. This research is supported by the grant under the research matching grant scheme (RMGS-09-01) from IIUM in collaboration with Sultan Qaboos University.
References
- Abdulrazaq NB, Akram HB, Bero DN, et al. (2013). Addition of selenium to Carica papaya Linn pulp extract enhances dermal wound healing activity. Trop J Pharm Res 12:77–84
- Aebi H. (1974). Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. New York and London: Academic Press, 673–7
- Andrews PC, Krinsky NI. (1985). Myeloperoxidase activity. In: Greenwald RA, ed. Handbook of Methods for Oxygen Radical Research. Florida: CRC Press, 297–302
- Anuar NS, Zahari SS, Taib IA, Rahman MT. (2008). Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chem Toxicol 46:2384–9
- Aricioglu A, Bozkurt M, Balabanli B, et al. (2001). Changes in zinc levels and superoxide dismutase activities in the skin of acute ultraviolet-B-irradiated mice after treatment with Ginkgo biloba extract. Biol Trace Elem Res 80:175–9
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–54
- Broughton G, Janis JE, Attinger CE. (2006). The basic science of wound healing. Plast Reconstr Surg 117:12–34S
- Buege JA, Aust SD. (1978). Microsomal lipid peroxidation. In: Fleischer S, Packer L, eds. Methods in Enzymology. New York: Academic Press, 302–10
- Chidambara Murthy KN, Reddy VK, Veigas JM, Murthy UD. (2004). Study on wound healing activity of Punica granatum peel. J Med Food 7:256–9
- Clark RA. (2008). Oxidative stress and “senescent” fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol 128:2361–4
- Cohen G, Dembiec D, Marcus J. (1970). Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–8
- Copeland RA, Williams JM, Giannaras J, et al. (1994). Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci USA 91:11202–6
- Corraliza IM, Campo ML, Soler G, Modolell M. (1994). Determination of arginase activity in macrophages: A micromethod. J Immunol Methods 174:231–5
- Debats IBJG, Wolfs TGAM, Gotoh T, et al. (2009). Role of arginine in superficial wound healing in man. Nitric Oxide 21:175–83
- Dieterich S, Bieligk U, Beulich K, et al. (2000). Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation 101:33–9
- Feng X, Hu Y, Ding J, et al. (2007). Early treatment with hydroxyethyl starch 130/0.4 causes greater inhibition of pulmonary capillary leakage and inflammatory response than treatment instituted later in sepsis induced by cecal ligation and puncture in rats. Ann Clin Lab Sci 37:49–56
- Gauglitz GG, Korting HC, Pavicic T, et al. (2011). Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med 17:113–25
- Guo S, DiPietro LA. (2010). Factors affecting wound healing. J Dent Res 89:219–29
- Gupta A, Singh RL, Raghubir R. (2002). Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem 241:1–7
- Kanta J. (2011). The role of hydrogen peroxide and other reactive oxygen species in wound healing. ACTA MEDICA (Hradec Králové) 54:97–101
- Kapoor M, Howard R, Hall I, Appleton I. (2004). Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am J Pathol 165:299–307
- Kara E, Gunay M, Cicioglu I, et al. (2010). Effect of zinc supplementation on antioxidant activity in young wrestlers. Biol Trace Elem Res 134:55–63
- Keles H, Ince S, Küçükkurt I, et al. (2012). The effects of Feijoa sellowiana fruits on the antioxidant defense system, lipid peroxidation, and tissue morphology in rats. Pharm Biol 50:318–25
- Klancnik A, Piskernik S, Jersek B, Mozina SS. (2010). Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods 81:121–6
- Korkina LG, Mikhal’chik E, Suprun MV, et al. (2007). Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol Biol (Noisy-le-grand) 53:84–91
- Krishna KL, Paridhavi M, Jagruti AP. (2008). Review on nutritional, medicinal and pharmacological properties of Papaya (Carica papaya Linn.). Nat Product Radiance 7:364–73
- Li J, Chen J, Kirsner R. (2007). Pathophysiology of acute wound healing. Clinics Dermatol 25:9–18
- Liang CC, Park AY, Guan JL. (2007). In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–33
- Lim Y, Levy M, Bray TM. (2004). Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD-1 mice. J Nutr 134:811–6
- Maiti AK, Ahlawat SS, Sharma DP, Khanna N. (2008). Application of natural tenderizers in meat – A review. Agric Rev 29:226–30
- Marimuthu S, Balakrishnan P, Nair S. (2013). Phytochemical investigation and radical scavenging activities of Melia azedarach and its DNA protective effect in cultured lymphocytes. Pharm Biol 51:1331–40
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–74
- Moseley R, Hilton JR, Waddington RJ, et al. (2004). Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 12:419–29
- Mukherjee B, Anbazhagan S, Roy A, et al. (1998). Novel implications of the potential role of selenium on antioxidant status in streptozotocin-induced diabetic mice. Biomed Pharmacother 52:89–95
- Oboh G, Olabiyi AA, Akinyemi AJ. (2013). Inhibitory effect of aqueous extract of different parts of unripe pawpaw (Carica papaya) fruit on Fe2+-induced oxidative stress in rat pancreas in vitro. Pharm Biol 51:1165–74
- Osato JA, Santiago LA, Remo GM, et al. (1993). Antimicrobial and antioxidant activities of unripe papaya. Life Sci 53:1383–9
- Otsuki N, Dang NH, Kumagai E, et al. (2010). Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol 127:760–7
- Owoyele BV, Adebukola OM, Funmilayo AA, Soladoye AO. (2008). Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 16:168–73
- Pacher P, Beckman JS, Liaudet L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
- Papp LV, Lu J, Holmgren A, Khanna KK. (2007). From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
- Rivera-Pastrana DM, Yahia EM, González-Aguilar GA. (2010). Identification of phenolic and carotenoid compounds in Carica papaya L. ‘Maradol’ using liquid chromatography – Mass spectrometry. Acta Hort (ISHS) 877:1197–204
- Rostan EF, DeBuys HV, Madey DL, Pinnell SR. (2002). Evidence supporting zinc as an important antioxidant for skin. Int J Dermatol 41:606–11
- Roy P, Amdekar S, Kumar A, et al. (2012). In vivo antioxidative property, antimicrobial and wound healing activity of flower extracts of Pyrostegia venusta (Ker Gawl) Miers. J Ethnopharmacol 140:186–92
- Secor Jr ER, Carson IV WF, Cloutier MM, et al. (2005). Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol 237:68–75
- Sherman AR, Barkley M. (2011). Nutrition and wound healing. J Wound Care 20:357–67
- Sunde RA. (2001). Selenium. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. Washington, DC: ILSI Press, 480–97
- Süntar I, Akkol EK, Nahar LD, Satyajit S. (2010). Wound healing and antioxidant properties: Do they coexist in plants? Free Radic Antiox 2:2–7
- Wilgus TA, Bergdall VK, Tober KL, et al. (2004). The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol 165:753–61
- Wilgus TA, Ross MS, Parrett ML, Oberyszyn TM. (2000). Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat 62:367–84
- Wilgus TA, Vodovotz Y, Vittadini E, et al. (2003). Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen 11:25–34

