Abstract
Context: Soybean and its fermented products are the most common source of isoflavones in human food.
Objective: The present study quantifies the major glycosides and aglycones in soybean and its fermented product tempeh isoflavone extracts. The comparision of antioxidant effects and BACE1 inhibitory activity between the isoflavones of soybean and tempeh were also established.
Materials and methods: The major isoflavones such as daidzein and genistein (aglycones), and their sugar conjugates (glycosides) daidzin and genistin in soybean and tempeh isoflavones were quantified using HPLC analysis. Comparative studies on BACE 1 (β-site amyloid precursor protein cleaving enzyme 1 or β-secretase 1) inhibition and free-radical scavenging activities (diphenyl-1-picrylhydrazyl (DPPH) and ferrous ion chelating ability) were conducted.
Results: The amount of actives (mg/100 g) in soybean isoflavone compared with tempeh isoflavone is as follows: daidzein 16.72 mg/100 g versus 38.91 mg/100 g, genistein 11.10 mg/100 g versus 24.03 mg/100 g, daidzin 6.16 mg/100 g versus 0.69 mg/100 g, and genistin 24.61 mg/100 g versus 6.57 mg/100 g. The IC50 values of soybean and tempeh isoflavones against BACE1 were 10.87 and 5.47 mg/ml, respectively. The tempeh isoflavone had a more potent DPPH free-radical scavenging activity (IC50 = 2.67 mg/ml) than the soybean isoflavone (IC50 = 10 mg/ml). The ferrous ion chelating ability of the isoflavones was practically similar (IC50 = 10.40 mg/ml, soybean and 11.13 mg/ml, tempeh).
Discussion and conclusion: The present study indicates that tempeh is a healthy supplement to alleviate oxidative stress through the enrichment of aglycones.
Introduction
There is great interest in free radicals or reactive oxygen species (ROS) due to their role in the progression of diseases such as cancer, diabetes, atherosclerosis, arthritis, and neurodegenerative disorders (Shinde et al., Citation2010). Free radicals are released during normal metabolic and oxidation processes. An excessive amount of free radicals in the body leads to oxidative damage in proteins, lipids, DNA, and RNA. Perpetual oxidative damage may disrupt the activity of a biological system and weaken the body defense mechanism (Droge, Citation2002). The central nervous system (CNS) is more vulnerable to free radical damage as a result of the brain’s high oxygen consumption rate and the relative paucity of antioxidant enzymes when compared with other tissues. Alzheimer’s disease (AD) is the most common form of adult onset dementia. In AD, free radicals may be induced by the accumulation of β-amyloid and neurofibrillary tangles, the well-pronounced hallmarks of AD resulting in neuronal death (Perry et al., 2002).
The insoluble amyloid plaques are predominantly aggregates of Aβ peptides of 39–43 amino acids formed from the sequential cleavage of amyloid precursor protein (APP). It is cleaved by β-site APP cleaving enzyme-1 (BACE-1) followed by subsequent cleavage by the γ-secretase complex to form Aβ peptides. Since the main culprit of Aβ aggregation is the β-secretase (BACE1), the inhibition of this enzyme is believed to play an important role in the prevention of AD (Tresadern et al., Citation2011). Oxidative stress has been found to elevate BACE1 levels in cultured neurons and animal models (Guglielmotto et al., Citation2009).
Isoflavones are weak phytoestrogen antioxidants as they have the ability to trap singlet oxygen. Soybean is known to possess a high amount of isoflavones which are weak phytoestrogen antioxidants. The major isoflavones present in soybean are glycosides followed by aglycones. Aglycones, however, are much more active and easily absorbed in the small intestine compared with glycosides. The less complex structure and lower molecular weight of the former are the reasons for the better bioavailability. Therefore, there is much interest in fermented soybean food product worldwide, as this process often results in higher amount of aglycones.
Tempeh is soybean that is fermented with Rhizopus oligosporus Saito (Mucoraceae) (Haron et al., Citation2009). In Asia, fermented soybean food products differ in their name, ways of preparation as well as the type of strain used. In Japan, the fermented soybean products are known as natto and miso (Yanaka et al., Citation2012), in Korea, cheonggukjang (Cho et al., Citation2011), and in China, douchi (Wang et al., Citation2008). The health benefits of tempeh especially on antioxidant effects have been documented in previous studies (Lin et al., Citation2006; Chang et al., Citation2009) but this is the first report on BACE1 activity. Therefore, this study is aimed to compare the antioxidant and BACE1 inhibititory activities of total isoflavones from soybean and tempeh isoflavones.
Materials and methods
Materials
Materials used in this study were soybean [Glycine max (L.) Merr. (Fabaceae)] and Rhizhopus sp., the mould used for tempeh preparation. Daidzein, genistein, daidzin, genistin, flourescein, 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, iron (II) sulfate (FeSO4), and ferrozine were procured from Sigma-Aldrich (St. Louis, MO) Acetonitrile, methanol, dimethyl sulfoxide (DMSO), and n-hexane were purchased from Fisher Scientific (Waltham, MA). BACE1 (β-secretase) FRET Assay kit produced by Pan Vera Co. (Madison, WI) was bought from Invitrogen (Selangor, Malaysia). The fluorescence was read using a spectrofluorometer (Infinite M200, Tecan, San Jose, CA). The HPLC system used was from Agilent (1200 Series) (Palo Alto, CA) consisting of an auto sampler, diode array detector (DAD), and fraction collector, with a Phenomenex Luna C18 (2) 100 A (250 × 4.6 mm i.d., 5 μm) column (Dikma Technologies Inc, Lake Forest, CA).
Sample preparation
Soybeans (1 kg) were soaked overnight in tap water and the beans were dehulled by soaking for another 24 h. The dehulled beans were boiled for 30 min, cooled and divided into two parts. Part of the beans (500 g) was directly dried by placing at −80 °C (soybean) and this acted as the control. The other part (500 g) was fermented with Rhizhopus sp. and incubated in air tight plastic at 28 °C for 3 d (tempeh). Both soybean (control without fermentation) and tempeh were lyophilized. The samples were grinded before being stored in air tight condition.
HPLC analysis of isoflavones
The isoflavone was extracted according to Wei et al. (Citation2004), but with slight modification. A solution of 10 ml of 80% methanol and 1 ml of internal standard (0.15 mg/ml) was added into the sample (1 g) and shaken at 60 °C for an hour. The sample was then centrifuged at 3800g for 10 min. The upper layer solution was rotary evaporated (R215, BUCHI, Flawil, Switzerland) to dryness and dissolved in 5 ml of 50% methanol followed by extraction (three times) in 20 ml n-hexane. The methanol phase was concentrated. Then 80% of methanol was added to the residue to a final volume of 10 ml. The solution was filtered through 0.2 μm filter membrane prior to HPLC analysis. The standards used were daidzein, genistein, daidzin, and genistin. Each standard (1 mg) was dissolved in 20 μl dimethyl sulfoxide and 980 μl 80% methanol in water as a stock solution. The standards were prepared at different concentrations (0.5, 1, 5, 10, 20, 40, and 80 μg/ml) in 80% methanol containing flourescein as the internal standard (0.15 mg/ml).
The method published by Hutabarat et al. (Citation1998) was adopted. A Phenomenex Luna C18 (2) 100 A (250 × 4.6 mm i.d., 5 μm) column (Dikma Technologies Inc, Lake Forest, CA) was used. The mobile phase consisted of (A) water containing 0.05% formic acid (v/v) and (B) acetonitrile. The gradient solvent system started with: 10% (A) and 90% (B) from 10 min, linear gradient from 10% to 55% (A) for 5 min, and then from 55% to 10% in another 5 min. The flow rate was 1.0 ml/min and the injection volume was 20 μl. The isoflavones were detected at 265 nm. The quantitation of isoflavones from soybean and tempeh was calculated using the following formula (Wei et al., Citation2004):
where,
Determination of DPPH free-radical scavenging activity
The free-radical scavenging activity of total isoflavone extracts of soybean and tempeh was evaluated as described by Niciforovic et al. (Citation2010). DPPH (8 mg) was dissolved in 100 ml methanol to obtain a concentration of 80 μg/ml. Serial dilution (20, 2, 0.2, 0.02, and 0.002 mg/ml) of the sample was carried out. Each sample of 2 ml was then mixed with DPPH (2 ml) and incubated 30 min for reaction to occur, and the absorbance was measured at 517 nm. Gallic acid was used as a reference standard. A control sample was prepared containing the same volume without test compounds or standard. Methanol was used as a blank. The DPPH free-radical scavenging activity (%) was calculated using the following equation:
where Ac is the absorbance of control and As is the absorbance of the sample.
The IC50 value, which is the concentration of the test material that reduces 50% of the free-radical concentration, was calculated as mg/ml through a sigmoidal dose–response curve.
Determination of ferrous ion chelating ability
The ferrous ion chelating activity of the total isoflavones of soybean and tempeh was measured by the decrease in the absorbance at 562 nm of the iron(II)–ferrozine complex (Niciforovic et al., Citation2010). About 1 ml of 0.125 mM FeSO4 was added to 1.0 ml sample (at a concentration of 20, 2, 0.2, 0.02, and 0.002 mg/ml) followed by 1.0 ml of 0.3125 mM ferrozine. The mixture was allowed to equilibrate for 10 min before measuring the absorbance. The ability of the sample to chelate ferrous ion was calculated relative to the control (consisting of iron and ferrozine only) using the following formula:
where Ac is the absorbance of control and As is the absorbance of the sample.
The IC50 value, which is the concentration of the test material that reduces 50% of the ferrous ion concentration, was calculated as mg/ml through a sigmoidal dose–response curve.
Determination of β-secretase inhibition
The β-secretase inhibition of soybean and tempeh isoflavones was determined using BACE1 (β-secretase) FRET assay kit (BUCHI, Flawil, Switzerland) that uses baculovirus-expressed BACE1 and a specific substrate (Rh-EVNLDAEFK-quencher BUCHI, Flawil, Switzerland) based on the Swedish mutation of the amyloid precursor protein (APP). This peptidic substrate becomes highly fluorescent upon enzymatic cleavage. Serial dilutions were carried out with the stock solution (20 mg/ml) of the isoflavones. The concentrations of the isoflavones used were 20, 2, 0.2, 0.02, and 0.002 mg/ml. A mixture of 10 µL of BACE-1 substrate (Rh-EVNLDAEFK-quencher, BUCHI, Flawil, Switzerland, in 50 nM ammonium bicarbonate), 10 µL of soybean or tempeh isoflavones, and 10 µl of BACE1 (β-secretase) enzyme [(50 mM Tris (pH 7.5), 10% glycerol) (1.0 U/ml)] was incubated for 60 min at room temperature in the dark. Then 10 µl of BACE1 stop buffer (2.5 M sodium acetate) was added to the mixture. The fluorescence was read using a multiwell spectrofluorometer (InfiniteM200, Tecan, San Jose, CA) under excitation and emission wavelengths of 545 nm and 585 nm, respectively (Mani et al., Citation2012). The percentage of inhibition was obtained by the equation below:
where E is the relative fluorescence unit (RFU) of the tested samples and PC is the RFU of the positive control. IC50 is defined as the concentration of BACE1 inhibitor that is required to inhibit 50% of BACE1 activity.
Statistical analysis
All data were presented as mean values ± SD of three parallel measurements. Statistical analysis of IC50 values of DPPH free-radical scavenging activity and ferrous ion chelating ability was analyzed by one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparisons’ test while quantification of isoflavones and β-secretase inhibition was analysed by the Student unpaired t-test. A probability value of 0.05 was considered significant.
Results
Fermentation process improved bioavailable aglycone levels
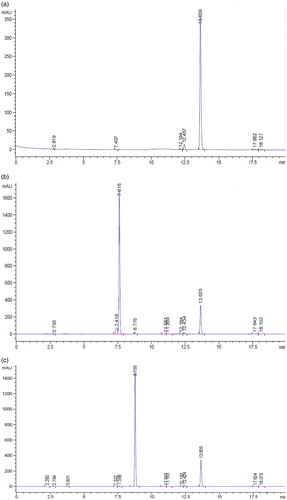
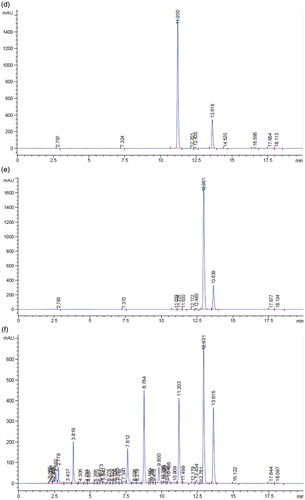
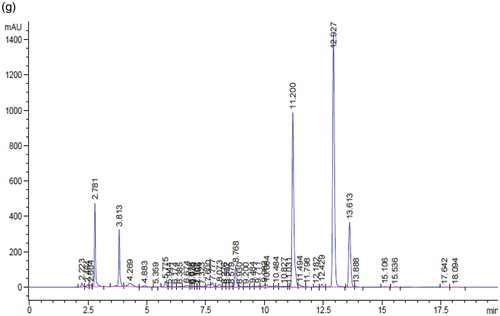
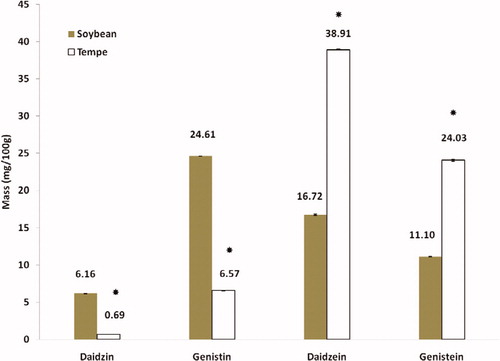
Following the extraction method, the total yield of soybean and tempeh isoflavones was recorded as 560 mg/500 g (0.112% w/w) and 670 mg/500 g (0.134% w/w), respectively. The HPLC chromatograms of glycosides daidzin and genistin, and aglycones daidzein and genistein are as shown in . The amount of isoflavones in soybean and tempeh was determined by comparing the area under the peak of its standard linear curve. The amount of glycosides was higher than aglycones in soybean (). The amounts of daidzin and genistin in soybean were 6.16 mg/100 g and 24.61 mg/100 g, while aglycones daidzein and genistein were 16.72 mg/100 g and 11.10 mg/100 g, respectively. However, after fermentation of the soybean and formation of tempeh, the amounts of daidzein, genistein, daidzin, and genistin were 38.91 mg/100 g, 24.03 mg/100 g, 0.69 mg/100 g, and 6.57 mg/100 g, respectively.
Figure 1. HPLC profile for flourescein (a), daidzin (b), genistin (c), daidzein (d), genistein (e), soybean (f), and tempeh (g). The peaks were flourescein (13.639 min), daidzin (7.615 min), genistin (8.756 min), daidzein (11.200 min), and genistein (12.691 min).
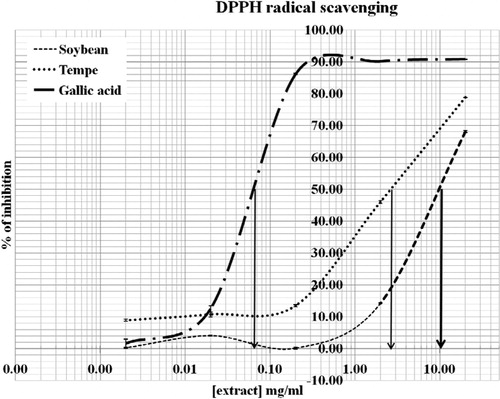
DPPH free-radical scavenging activity of soybean and tempeh
The results from DPPH radical scavenging activity of soybean and tempeh isoflavones are shown in . The IC50 value of gallic acid was 0.065 mg/ml while the tempeh extract was 2.67 mg/ml and the soybean extract was 10 mg/ml. The present study showed that tempeh possessed greater (p < 0.001) antioxidant activity than soybean. However, both isoflavones were not as potent (p < 0.001) as gallic acid that was used as a reference standard.
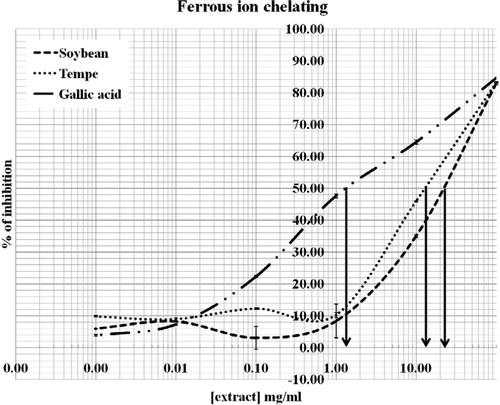
Ferrous ion chelating ability of soybean and tempeh
The ferrous ion chelating ability of the isoflavones was determined by measuring the decrease in absorbance at 562 nm of the iron(II)–ferrozine complex (). Both the extracts increased the ability of ferrous ion chelating in a dose-dependent manner. Tempeh possessed a higher ability (p < 0.001) to chelate than soybean. Moreover, both soybean and tempeh extracts showed significant (p < 0.001) difference as compared with gallic acid which is used as a standard drug. The IC50 values were 1.50, 11.13, and 10.40 mg/ml for gallic acid, soybean, and tempeh, respectively.
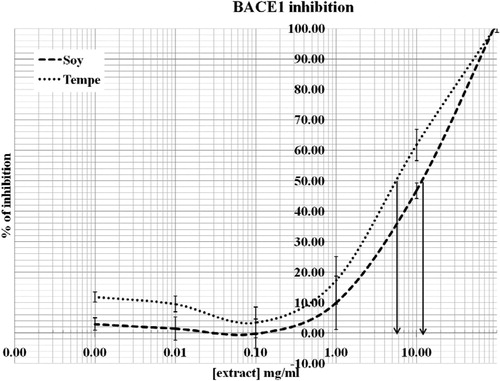
β-Secretase (BACE1) inhibition of soybean and tempeh
Soybean and tempeh isoflavones were tested on the ability to act as a BACE1 inhibitor. The plots were established between various concentrations of isoflavones (20, 2, 0.2, 0.02, and 0.002 mg/ml) and percentage of BACE1 inhibition. Based on , the IC50 values for soybean and tempeh were 10.87 and 5.47 mg/ml, respectively. The results showed that tempeh possessed a greater ability (p < 0.001) to inhibit BACE1 than soybean.
Discussion
Soybean isoflavones are diphenolic compounds that are similar to estrogen and bind to estrogen receptors. These compounds appear to compete with endogenous estrogen for receptor binding and act as an agonist and antagonist to estrogen receptors. Soybean isoflavones can be categorized into several groups based on their chemical structures. The glycosides are daidzin and genistin while aglycones are daidzein and genistein. The isoflavones of interest were selected due to their abundance in soybean. Aglycones are the conjugated form of glycosides, where the sugar moiety is broken down during hydrolysis, most of the time during fermentation from soybean to other soybean food products.
Results showed ( and ) that after the fermentation process, the amount of glycosides decreased while the amount of aglycones increased. Hong et al. (Citation2012) reported a similar pattern where isoflavone glycosides in black soybean before fermentation were 2.84 mg/100 g (daidzin) and 2.05 mg/100 g (genistin) but after a 24 h fermentation with Bacillus subtilis, the amount decreased to 0.13 mg/100 g for daidzin and 0.57 mg/100 g for genistin. In the meantime, isoflavone aglycones were found to increase with daidzein from 0.26 to 0.95 mg/100 g and genistein from 0.50 to 3.5 mg/100 g. Additionally, a quantitative study on cheonggukjang, soybean fermented with Bacillus subtilis CS90, also showed a decrease in daidzin content, from 487.45 to 213.45 mg/kg. Daidzen increased from undetected level to 287.32 mg/kg. However, after 60 h of fermentation, the value rose to 330.09 mg/kg. A similar trend was observed for genistein, rising from an undetected level to 25.62 mg/kg after 60 h of fermentation (Cho et al., Citation2011).
The bioavailability of glycosides and aglycones is different. In the small intestines, aglycones are easily absorbed by passive diffusion due to its less complex structure and lower molecular weight (Haron et al., Citation2009; Wei et al., Citation2004). Glycosides, however, must be hydrolyzed to aglycones by β-glucosidases in the gut. This indicates that aglycone-rich products may be more effective than glucoside-rich products in preventing diseases such as coronary heart disease, cancer, and also neurodegeneration diseases (Hsieh et al., Citation2009). Through the inhibitory effect of tyrosine kinase, topoisomerase, and angiogenesis, soybean isoflavones are believed to reduce the risk of cancer (Adlercreutz & Mazur, Citation1997). Data from various epidemiological and clinical studies have highlighted that soybean consumption may be associated with a lower risk of atherosclerosis and coronary heart diseases. The mechanisms and specific pathways of the effect of soybean extracts on cardiovascular health using omics technologies were also documented recently (Gil-Izquierdo et al., Citation2012).
The DPPH free-radical scavenging assay is widely used to evaluate antioxidant activity. It is considered that substances which are able to donate an electron or a hydrogen to DPPH, nitrogen-centered free radical, can be considered as radical scavengers. The hydrogen-donating ability confers the antioxidant radical scavenging action. This free-radical scavenging potential of antioxidant is designated by the reduction in the magnitude of discoloration of DPPH (Prior et al., Citation2005). The present study showed that tempeh possessed greater antioxidant activity than soybean.
A previous study by Hu et al. (Citation2010) reported that 5 mg/ml methanol extract of the soaked black bean soybean, steamed black soybean, and fermented black soybean showed a DPPH scavenging effect at 30.38, 28.78, and 78.22%, respectively. These results indicated that the fermentation process enhanced the antioxidant activity by an increase in DPPH scavenging effects. The higher percentage of inhibition of fermented black soybean might be due to the changes of polyphenolic compounds during the fermentation process, where at least partial alteration in the glycosides took place, releasing the potent antioxidant substances by the transformation of flavanoids. A recent research highlighted that the total phenolics increased from 253 to 9414 mg/kg during cheonggukjang after 60 h of fermentation which correlated with the elevation of DPPH radical scavenging activity from 53.6 to 93.3% by cheonggukjang (Cho et al., Citation2011).
The substances that act as ion chelators are crucial to attenuate the harmful effects of oxidation. Ferrous ions are the most effective pro-oxidants and they are commonly found in the food system. In biological systems, the presence of ferrous ion can initiate lipid peroxidation as these ions react with hydrogen peroxide to promote the formation of free hydroxyl radical (Gutteridge & Halliwell, Citation1988). Isoflavone extracts of soybean and tempeh showed the ability of ferrous ion chelating but tempeh extract showed higher ability of ferrous ion chelating as compared with the soybean extract.
Results from previous studies also suggested that fermentation process in general increases the ability to chelate iron. According to a study by Huang et al. (Citation2011), sufu a fermented soybean with Aspergillus oryzae showed EC50 with a range of 3.7–5.3 mg/ml, which was significantly lesser than that of non-fermented soybean (19.9 mg/ml). Another fermented product soybean koji using Aspergillus sojae, Aspergillus oryzae, Aspergillus awamori, Actinomucor taiwanesis, and Rhizopus sp. registered an increase of Fe+2 ion chelating ability by 2.1–6.7-fold (Lin et al., Citation2006). A number of previous reports suggested that plants containing phenolic compounds are most effective in sequestering ferrous ion by intercepting all coordination sites of metal ions. Glycoside and aglycone forms of isoflavones present in soybean exact such type of phenolics with multiple hydroxyl and carbonyl groups in ring C that are considered as available sites for metal complexation (Leopoldini et al., Citation2006). In particular, the iron chelating ability of isoflavones from both the extracts (this study) might control the elevation of lipid peroxidation and this can be associated with the reduction of antioxidant glutathione concentration in various neuronal dysfunctions.
It is well known that natural antioxidants demonstrated beneficial effects in the maintenance of health, management of age-related diseases, and the amelioration of harmful effects of both chemical and physical toxic agents. Sesamol, curcumin, and ferulic acid are believed to be potential antioxidant-based therapeutic agents. Antioxidants like gallic acid and butylated hydroxytoluene (BHT) are used as food preservatives in industries and as reference standards in the evaluation of antiradical properties of pure antioxidant compounds or plant extracts of different nature (Erkan et al., Citation2008). It is believed that the generation of ROS and its oxidative damage are involved in the pathogenesis of neurodegenerative disorders including AD. Studies with different animal models revealed that oxidative stress played a critical role leading to neuronal as well as vascular abnormalities in AD (Massaad, Citation2011). DNA oxidation products, such as 8-oxoguanosine, and protein oxidation products, such as dityrosine, are markers of oxidative stress that are found to be increased in the AD brain. In addition, senile plaques and neurofibrillary tangles are altered in ways characteristic of oxidative damage including advanced glycation end products (AGE) modification, protein cross-linking, and carbonyl- and acyl-modification (Srikanth et al., Citation2011). Fermentation of soybean resulted in great improvement of isoflavones and free-radical scavenging ability of tempeh further promote it as a health food. Tempeh can be considered as a natural antioxidant to alleviate oxidative-induced diseases including AD.
On one hand, the ability of β-secretase (BACE1) to cleave amyloid precursor protein (APP) is deleterious, as the release and accumulation of β-amyloid will lead to AD. Evidences suggested that progressive cerebral aggregation of Aβ in the brain plays a central role in the pathogenesis and development of AD. Therefore, lowering the concentration of this peptide through inhibition of β-secretase (BACE-1) or γ-secretase in the brain appears to be a rational therapeutic approach for treating AD. Because of the great interest of BACE-1, several BACE-1 inhibitors are currently under preclinical and clinical trials. On the other hand, the accumulation of Aβ also causes intracellular reactive free radicals and ROS that can induce neuronal toxicity. An increase in the level of ROS and free radicals due to the Aβ peptide leads to an accumulation of lipid peroxidation, protein oxidation, and oxidation of mitochondrial DNA. These abnormalities lead to oxidative neuronal death and cognitive decline in patients with AD (Kim et al., Citation2011). A previous study showed that soybean isoflavones decreased the expression of BACE1 (Hsieh et al., Citation2009). The soybean isoflavones decreased the Aβ formation by inhibiting the regulation of Aβ-biosynthetic enzymes. This would suggest that soybean isoflavones not only act as an antioxidant but also as a BACE1 inhibitor. This study showed tempeh possessed a higher ability to inhibit the BACE1 than soybean.
The oxidative markers also positively correlated with BACE 1 activity in AD brain. A study using transgenic mouse models of AD (Tg2576) found age-related changes in brain antioxidant enzyme activities and its cortical levels of reactive nitrogen species were correlated with an alteration of BACE activity and Aβ levels (Apelt et al., Citation2004). Moreover, in vitro studies revealed that oxidative stress in neuronal cells increased BACE 1 transcription, expression, and activity (Tamagno et al., Citation2002; Tong et al., Citation2005). Also blockade of c-Jan N-terminal kinases (JKN) pathway contributed in the prevention of oxidative stress-induced upregulation of BACE 1 in mouse fibroblasts as well as in brain (Tamagno et al., Citation2008). From our results, both tempeh and soybean isoflavones possess antioxidant activity and the ability to inhibit Aβ accumulation through BACE-1 inhibition. Both the isoflavones appeared to be useful in the prevention of AD through BACE-1 inhibition; however, the tempeh isoflavone showed higher inhibition ability than unfermented soybean isoflavone.
Conclusion
Recently, the development of bioactive compounds from natural compounds with BACE1 inhibitory properties received great attention in the quest for therapeutic targets for AD. From our results, both soybean and tempeh isoflavones have a promising role as an antioxidant with the potential to counter Aβ accumulation through BACE-1 inhibition. The fermented product tempeh showed higher BACE-1 inhibition, and free-radical scavenging and ferrous ion chelating ability as compared with the unfermented soybean. Furthermore, the phytochemical analysis indicated that tempeh possessed a higher amount of daidzein and genistein as compared with soybean. Both daidzein and genistein are more bioavailable as compared with the glycosidic daidzin and genistin; therefore, tempeh isoflavone may have a higher preventive therapeutic potential, and thus needs to be investigated through in vivo studies.
Declaration of interest
The authors report they have no declarations of interest. This study was supported by the Research Excellence Fund (600-RMI/ST/DANA 5/3/DST (465/2011), Research Management Institute, Universiti Teknologi MARA, Malaysia and Institute of Graduate Studies, Universiti Teknologi MARA, Malaysia.
References
- Adlercreutz H, Mazur W. (1997). Phyto-oestrogens and Western diseases. Ann Med 29:95–120
- Apelt J, Bigl M, Wunderlich P, Schliebs R. (2004). Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci 22:475–84
- Chang CT, Hsu CK, Chou ST, et al. (2009). Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int J Food Sci Tech 44:799–806
- Cho KM, Lee JH, Yun HD, et al. (2011). Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J Food Compos Anal 24:402–10
- Droge W. (2002). Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
- Erkan N, Ayranci G, Ayranci E. (2008). Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, black seed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110:76–82
- Gil-Izquierdo A, Penalvo JL, Gil JI, et al. (2012). Soy isoflavones and cardiovascular disease epidemiological, clinical and -omics perspectives. Curr Pharm Biotechnol 13:624–31
- Guglielmotto M, Aragno M, Autelli R, et al. (2009). The up-regulation of BACE1 mediated by hypoxia and ischemic injury: Role of oxidative stress and HIF1alpha. J Neurochem 108:1045–56
- Gutteridge JM, Halliwell B. (1988). The deoxyribose assay: An assay both for ‘free' hydroxyl radical and for site-specific hydroxyl radical production. Biochem J 253:932–3
- Haron H, Ismail A, Azlan A, et al. (2009). Daidzein and genestein contents in tempeh and selected soy products. Food Chem 115:1350–6
- Hong GE, Prabhat KM, Lim KW, Lee CH. (2012). Fermentation increases isoflavone aglycone contents in black soybean pulp. Asian J Anim Vet Adv 7:502–11
- Hsieh HM, Wu WM, Hu ML. (2009). Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol 47:625–32
- Hu Y, Ge C, Yuan W, et al. (2010). Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J Sci Food Agric 90:1194–202
- Huang YH, Lai YJ, Chou CC. (2011). Fermentation temperature affects the antioxidant activity of the enzyme-ripened sufu, an oriental traditional fermented product of soybean. J Biosci Bioeng 112:49–53
- Hutabarat LS, Mulholland M, Greenfield H. (1998). Development and validation of an isocratic high-performance liquid chromatographic method for quantitative determination of phytoestrogens in soybean. J Chromatogr A 795:377–82
- Kim MJ, Lee J, Seong AR, et al. (2011). Neuroprotective effects of Eriobotrya japonica against β-amyloid-induced oxidative stress and memory impairment. Food Chem Toxicol 49:780–4
- Leopoldini M, Russo N, Chiodo S, Toscano M. (2006). Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 54:6343–51
- Lin CH, Wei YT, Chou CC. (2006). Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol 23:628–33
- Mani V, Ramasamy K, Ahmad A, et al. (2012). Protective effects of total alkaloidal extract from Murraya koenigii leaves on experimentally induced dementia. Food Chem Toxicol 50:1036–44
- Massaad CA. (2011). Neuronal and vascular oxidative stress in Alzheimer’s disease. Curr Neuropharmacol 9:662–73
- Niciforovic N, Mihailovic V, Maskovic P, et al. (2010). Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem Toxicol 48:3125–30
- Perry G, Cash AD, Smith MA. (2002). Alzheimer disease and oxidative stress. J Biomed Biotechnol 2:120–3
- Prior RL, Wu X, Schaich K. (2005). Standardized methods for the determination antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–302
- Shinde AN, Malpathak N, Fulzele DP. (2010). Determination of isoflavone content and antioxidant activity in Psoralea corylifolia L. callus cultures. Food Chem 118:128–32
- Srikanth V, Maczurek A, Phan T, et al. (2011). Advanced glycation end products and their receptor RAGE in Alzheimer's disease. Neurobiol Aging 32:763–77
- Tamagno E, Bardini P, Obbili A, et al. (2002). Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 10:279–88
- Tamagno E, Guglielmotto M, Aragno M, et al. (2008). Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 104:683–95
- Tong Y, Zhou W, Fung V, et al. (2005). Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm 112:455–69
- Tresadern G, Delgado F, Delgado O, et al. (2011). Rational design and synthesis of aminopiperazinones as β-secretase (BACE) inhibitors. Bioorg Med Chem Lett 21:7255–60
- Wang D, Wang L, Zhu F, et al. (2008). In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food). Food Chem 107:1421–8
- Wei QK, Jone WW, Fang TJ. (2004). Study on isoflavones isomers contents in Taiwan's soybean and GM soybean. J Food Drug Anal 12:324–31
- Yanaka K, Takebayashi J, Matsumoto T, Ishimi Y. (2012). Determination of 15 isoflavone isomers in soy foods and supplements by high-performance liquid chromatography. J Agric Food Chem 60:4012–16