Abstract
Context: Polyphenol-rich marine macroalgae are gaining dietary importance due to their influence over diabetes mellitus and the role as a vital source of high-value nutraceuticals. Their assorted beneficial effects on human health include competitive inhibition of digestive enzymes, varying the activity of hepatic glucose-metabolizing enzymes, lowering the plasma glucose levels, and lipid peroxidation, delaying the aging process.
Objective: In this paper, we review the health beneficial effects of polyphenols and phlorotannins from brown seaweeds with special emphasis on their inhibitory effects on carbohydrate-metabolizing enzymes.
Methods: A survey of literature from databases such as Sciencedirect, Scopus, Pubmed, Springerlink, and Google Scholar from the year 1973 to 2013 was done to bring together the information relating to drug discovery from brown seaweeds as a source for diabetes treatment.
Results: Over the past two decades, 20 different bioactive polyphenols/phlorotannins have been isolated and studied from 10 different brown algae. Discussion of the positive effect on the inhibition of enzymes metabolizing carbohydrates in both in vitro and in vivo experiments are included.
Conclusion: Despite the recent advancements in isolating bioactive compounds from seaweeds with potential health benefit or pharmaceutical behavior, studies on the polyphenol effectiveness on glucose homeostasis in human beings are very few in response to their functional characterization. Added research in this area is required to confirm the close connection of polyphenol rich seaweed-based diet consumption with glucose homeostasis and the exciting possibility of prescribing polyphenols to treat the diabetes pandemic.
Introduction
Diabetes mellitus (DM) is one of the most common chronic non-communicable diseases globally; it involves multiple organs and several pathways that weaken the human body’s capability to sustain glucose equilibrium. Such impaired glucose metabolism results in hyperglycemia. The long-lasting blood glucose level elevation is a principal reason for a number of complications such as cardiovascular disorders, nephropathy, retinopathy, neuropathy, foot and leg ulcers, and limb amputation that lead to the mortality and morbidity of a diabetic person. King et al. (Citation1998) assumed that the estimated total of individuals (aged ≥20) with diabetes in the world would be increased by 122%, from 135 million in 1995 to 300 million in 2025. But, according to the 2009 report by World Health Organization (WHO, Citation2010), more than 346 million people worldwide have diabetes and the condition will worsen by doubling the number by 2030. Unnikrishnan et al. (Citation2007) suggest that the incidence of nephropathy and microalbuminuria amid the urban rural population of Chennai, South India was 2.2 and 26.9%, respectively. According to Chakrabarti and Rajagopalan (Citation2002), the management of diabetic condition with no side effects is a challenge to medical research. Medical scientists consider diabetes mellitus as a chronic disease caused by hereditary or acquired insufficiency in insulin secretion, and via increased insensitivity of other organs to the secreted insulin. The ethnical variations of the genes leading to the type-2 diabetes have been studied in the offspring of the conjugal parents in India (Viswanathan et al., Citation1985). On one hand, the consequence of this inefficiency leads to elevation in blood glucose level; while on the other hand, it imparts damage to blood vessels and nerves in the human body (Matsui et al., Citation2007). The most important diabetes associated risk factors is hypertension. An epidemiological investigation by Shanthirani et al. (Citation2003) found that the pervasiveness of hypertension among the South Indian population has a positive correlation among diabetic individuals. The findings of Boulton et al. (Citation1985) explain the significance of metabolic factors of diabetic patients and their interrelationship with postprandial glycemia and impaired nerve function. Diabetes mellitus is of two basic types: type I and type II. Type 1 diabetes (also known as insulin dependent, juvenile, or childhood onset) is categorized by deficient secretion of insulin and can be efficiently kept in check by administration of insulin. Whereas, in type 2 diabetes (also called as non-insulin-dependent or adult onset), the body is incompetent to insulin usage, and it is still difficult to find a completely effective controlling measure. Prevalence of type 2 diabetic people is around 90% (Apostolidis & Lee, Citation2010) among the total diabetics around the world.
α-Amylase and α-glucosidase are the two key enzymes in carbohydrate digestion, located on the pancreas and the brush-border surface membranes of intestinal cells, respectively (Lebovitz, Citation1997). Elevation in blood glucose level takes place due to the glucose released by the combined action of these two enzymes on carbohydrate foods. α-Amylase catalyzes the hydrolysis of α-1,4-glucosidic linkages of starch, glycogen, and a variety of oligosaccharides and further, α-glucosidase degrades disaccharides into simple sugars which are readily absorbed by the intestine. Profound inhibition of intestinal α-glucosidase and mild inhibition of pancreatic α-amylase is an effective approach which can be applied in the management of type 2 diabetes through reducing the release of glucose from starch by these enzymes (Krentz & Bailey, Citation2005). Evaluation of several natural resources for their ability to reduce the conversion of carbohydrates into glucose in the gut or absorption of glucose by the intestine was done (Matsui et al., Citation2007).
Potent synthetic glucosidase inhibitor drugs like acarbose, miglitol, and voglibose are used so far to restore the ameliorated postprandial hyperglycemia, which function directly in lowering the elevated blood glucose levels that occur immediately after having a meal (van de Laar et al., Citation2005). However, the long-term use of such man-made agents may provoke side effects such as vomiting, flatulence, diarrhea, and abdominal cramps (Hanefeld, Citation1998). Furthermore, reports on the incidence of renal tumors, acute hepatitis, and serious hepatic injury are greater than before (Charpentier et al., Citation2000) that might have some relation with the long-term use of these synthetic drugs. Therefore, non-toxic, competent inhibitors of α-glucosidase and α-amylase have widely been sought after from marine natural resources. The global nutraceutical market for diabetes was estimated to be worth $117.3 billion in 2007 (Ahmad et al., Citation2011). This review attempts to enumerate the favorable health actions of seaweed polyphenols and phlorotannins and their promising function in glucose homeostasis in human beings.
Glucose homeostasis and its mechanism
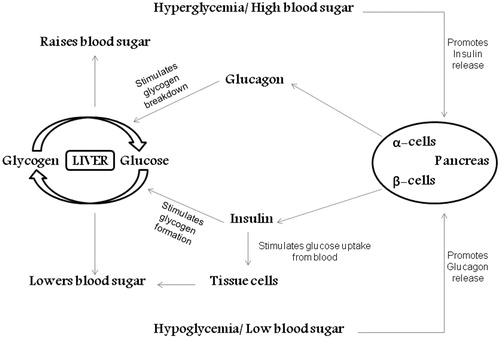
Each and every cell, tissue, and organ in a human body constantly needs glucose as a vital source of cellular energy to sustain. Lower levels of blood glucose could lead to seizures, loss of perception, and even fatality. Besides, long-standing elevated blood glucose levels could result in the loss of eye sight, renal failure, cardiovascular diseases, etc; therefore, blood glucose level should to be controlled. The mechanism to uphold blood glycemic index is called “glucose homeostasis” (DeFronzo, Citation1988) ().
A number of harmonizing physiological processes, including glucose absorption, glucose reabsorption, glycogenolysis, gluconeogenesis, and glucose excretion, are required to maintain glucose homeostasis (Szablewski, Citation2011). In order to circumvent fasting hypoglycemia and postprandial hyperglycemia, the human body could adjust blood glucose levels by secreting two alternatively functional hormones, namely insulin and glucagon. For the period of postprandial hyperglycemia, the β-cell of the pancreatic Islets of Langerhans produces extra insulin in response to the rise in blood sugar and amino acid past a meal. Insulin’s key purpose is to counteract the hyperglycemia-inducing hormones to retain low-glucose levels. It regulates glucose homeostasis through various activities like lowering the hepatic glucose production when the blood glucose level exceeds 5 mmol/L via glycogenolysis and gluconeogenesis, thus facilitating the transportation of glucose into striated muscle, adipose tissue as well as inhibiting glucagon secretion (Aronoff et al., Citation2004). It is noted that the postprandial secretion of insulin takes place in two stages: an early discharge of pre-synthesized insulin, pursued by raised insulin secretion based on the blood glucose level. In contrast, during the hypoglycemia, additional volumes of glucagon are secreted by the α-cells of Islets of Langerhans of the pancreas. This hormone is responsible for maintaining the plasma glucose in respective levels when there is additional prerequisite (Cryer, Citation2002). Glucagon will repress low-blood glucose level by inducing hepatic glucose production and preventing the actions of insulin. The glycogenolysis and gluconeogenesis pathways are activated by this hormone, resulting in glucose release, thus raising the blood glycemic index.
Polyphenols and their health benefits
Until now, clinical and epidemiological studies have specified that the use of plant-derived foods like soya bean products and drinks like tea and red wine could lessen the jeopardy of oxidative damage related diseases like ageing and other lifestyle diseases (Shibata et al., Citation2008). The health aspects of natural foods are considered to be credited for the antioxidant activity of polyphenols and many active substances, e.g. D-catechins, isoflavones, and resveratrol present in them (Aggarwal & Shishodia, Citation2006).
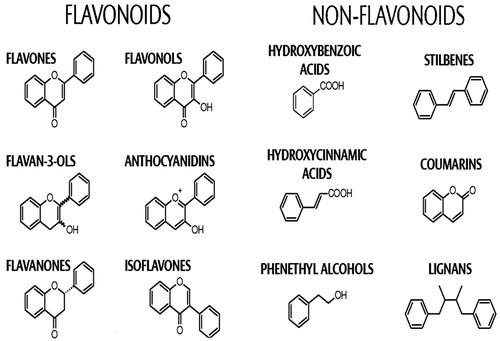
Amelioration of type 2 diabetes risk usually targets the lifestyle and diet, primarily with the aim of reducing obesity. Dietary polyphenols may possibly assist in type 2 diabetes prevention through weight control (Martin de Bock et al., Citation2012). Dietetic polyphenols are chemicals of plant origin that are abundant in fruit, vegetables, chocolates, and nuts as well as in tea, coffee, wine, and soy milk (Manach et al., Citation2004; Torabian et al., Citation2009). Thousands of natural polyphenols are present in the plant kingdom, every single one of which shares the basic structure of an aromatic ring with attached hydroxyl groups; variation in their configuration leads to individual classifications of polyphenols, with at least 10 separate classes identified (Bravo, Citation1998), four among them are significant in the human diet: phenolic acids, flavonoids, stilbenes, and lignans (Perez-Jimenez et al., Citation2010). Although terrestrial polyphenols, flavonoids, and gallic acids are recognized to have a number of bioactive functions, the literature on marine macroalgae polyphenols is very sparse from a human physiological point of view (Shibata et al., Citation2008). Currently, researchers are focusing on the prevention of significant diseases including cancer, coronary heart diseases, and allergies with nutritional factors such as phenolic compounds or polyphenols (Shibata et al., Citation2003).
Polyphenols on cellular and molecular metabolism of carbohydrates
Epidemiological evidence on the consumption of polyphenolic-rich diet and the risk of habitual human diseases show an inverse relationship. Altered metabolism of carbohydrates and resistance to insulin leads to the metabolic syndrome with the onset of high blood glucose level and diabetes mellitus type-2. Deformity in digestion and absorption of culinary carbohydrates, depreciation of stored glycogen, inflated gluconeogenesis and excessive production of hepatic glucose, β-cells dysfunction, peripheral tissue insulin resistance, and flaw in insulin signal transduction pathways are more important root for hyperglycemia (Dinneen et al., Citation1992). While, the use of synthetic antidiabetic medications like inhibitors of α-glucosidase, sulfonylureas, meglitinides, thiazolidindiones, and biguanides, or administration of exogenous insulin are widespread clinical practices in the treatment of diabetes mellitus type-2 and hyperglycemia, however, indigenous or folk medicines that use natural products have been under consideration from ancient era. Amidst the very well-acknowledged naturally occurring bioactive products, polyphenols are prominent for their antidiabetic functions with no after-effects (Hanhineva et al., Citation2010).
Polyphenols like catechins, epicatechins, ferulic acids, chlorogenic acids, quercetin, naringenin, tannic acid, and caffic acid could relate with the small intestinal glucose absorption via inhibition of sodium glucose co-transporter (SGLT1 and SGLT2) (Johnston et al., Citation2005; Kobayashi et al., Citation2000). Investigations from Johnston et al. (Citation2003) and Dao et al. (Citation2011) have shown that the polyphenols are able to mediate hyperglycemia and impaired glucose tolerance by an aided insulin response and constricted synthesis of glucagon-like polypeptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Flavanols like epicatechins and catechins moderate hyperglycemia and hepatic glucose production through regulating the expression of hepatic glucokinase and phosphoenolpyruvate carboxykinase (PEPCK) (Waltner-Law et al., Citation2002). In vitro study of epigallocatechin gallate (EGCG) revealed that it could trigger AMP-activated protein kinase pathway (AMPK) for inhibiting the expression of enzymes of gluconeogenesis (Collins et al., Citation2007). AMP-activated protein kinase, an energy sensory and regulatory protein, plays a critical role in the metabolism, the efficacy of the new treatment for metabolic syndrome, obesity, postprandial hyperglycemia, and a foremost objective for anti-diabetic drugs like metformin lies in the activation of this pathway (Towler & Hardie, Citation2007; Zhou et al., Citation2001). Ferulic acid, a derivative of hydroxycinnamic acid, efficiently contains glycemic index of blood by increased glucokinase activity, glycogen production by liver, and increased plasma insulin level (Jung et al., Citation2007). Hesperidin and naringin, two citrus bioflavonoids, when supplemented to diabetic rats with increased liver glucokinase activity and glycogen concentration, weaken hepatic gluconeogenesis through decreasing G6Pase and PEPCK action and result in the improvement of blood glucose level (Jung et al., Citation2004, Citation2006). Prabhakar and Doble (Citation2009) found that improvement of glucose uptake by PI3K-dependent pathway was due to ferulic acid, whereas glucose uptake by augmenting GLUT4 expression through PI3K-independent pathway by chlorogenic acid was comparable with common oral postprandial glycemia drugs like metformin and thiazolodinedione. In vitro studies of Zhang et al. (Citation2011) and Park et al. (Citation2007) exhibit that epigallocatechin gallate, quercetin, and resveratrol regulate the AMPK pathway and facilitate the insulin-dependent glucose uptake in muscle cells and translocation of glucose transporter (GLUT4) by adipocytes to plasma membrane. Similarly, Zang et al. (Citation2006) found that resveratrol and apigenin increased the acetyl-CoA carboxylase (ACC) and phosphorylation of AMPK hereby increasing the functions of AMPK 200 times more than the synthetic drug metformin. Likewise, polyphenols have the potential to trigger phosphatidylinositide 3-kinase (PI3K), an intracellular signal transducer for the upregulation of blood glucose uptake (Kumar et al., Citation2009).
The antihyperglycemic effect of genistein did not correlate with induction of insulin biosynthesis, glucose transporter-2 expression, or glycolytic pathway, rather than it acts as a novel agonist in cAMP-dependent protein kinase signaling, which is an important physiological inducer of pancreatic β-cells for glucose-induced insulin secretion (Liu et al., Citation2006). Genistein, an isoflavone, demonstrated antidiabetic effect by positively harmonizing the pancreatic β-cell functions through activation of cyclic AMP/PKA-dependent ERK1/2 signaling pathway (Fu et al., Citation2010). Resveratrol (3,4,4′-trihydroxystilbene), a stilbenoid, a type of phenol, enhances glucose intolerance, constrict β-cell loss, and lessen oxidative stress damage in pancreatic islet (Szkudelski & Szkudelska, Citation2011). Efficacy of polyphenols on β-cells is associated with their capability to regulate key cell-signaling pathways; anthocyanins exhibit such positive effects on β-cells of pancreas against the oxidative stress-induced damage via upregulation of hemeoxygenase-1, modulation of PI3K/Akt, and ERK1/2 signaling pathway as well as even moderate inhibition of β-cells apoptosis (Zhang et al., Citation2011). Polyphenols from brown seaweed Ecklonia cava showed significant improvements in diabetic condition by activating together AMPK/ACC and PI3K/Akt signal transduction pathways in STZ-induced hyperglycemia (Kang et al., Citation2010). Similarly, polyphenol-rich extract from brown alga Ishige okamurae significantly reduced hyperinsulinemia among diabetes mellitus type-2 mice by enhancing the glucokinase activity, whereas G6Pase and PEPCK activities were reduced. Also the hepatic glycogen level was elevated (Min et al., Citation2011). Only very few investigations have been done at the cellular level to study the functions of polyphenols isolated from marine algae. The functions of polyphenols in human healthiness are still a prospective area to explore. Added research in marine algal polyphenols is needed at the cellular and human clinical trial levels to authenticate the positivity of dietary and supplementary polyphenols is the treatment of diabetic patients.
Classes of seaweeds and their bioactivity and health benefits
So far, around 10 000 different marine algae or seaweeds are known in the world. They are classified into three divisions, Chlorophyta (green algae), Phaeophyta (brown algae), and Rhodophyta (red algae) based on their pigment composition. On one hand, the green algae are fully green with no pigments to mask the chlorophyll. They are highly diverse in size and behavior, ranging from microscopic free-swimming single cells to large membranous, tubular, and bushy plants. On the other hand, brown algae are solely multi-cellular but differ in their physical forms: some are crusts and others are filaments. However, like other photosynthetic organisms, brown algae possess the green pigment chlorophyll. In addition, they contain other golden and brown pigments to mask the green color of chlorophyll. The dominant pigment found in brown algae is called fucoxanthin. The red algae, in addition to chlorophyll, contain the pigments phycocyanin and phycoerythrin which give the red colorations. Red algae are found in a variety of physical forms, including simple and branched filaments.
Seaweeds are a rich resource of structurally diversified bioactive compounds exhibiting a range of biological behavior and their value as a source of novel bioactive substance is increasing swiftly. With marine organisms comprising around half of the total global biodiversity, the ocean offers a vast resource for novel compounds (Aneiros & Garateix, Citation2004; Barrow & Shahidi, Citation2007). The benefits of macroalgae as a source of novel bioactive products are being revealed by the scientific community to mankind with the increasing interest on their different biological activities and the consumers are getting more attracted to marine algae-derived foods (Kim & Wijesekara, Citation2010; Wijesekara et al., Citation2010). In Asian countries such as China, Japan, and Korea, seaweeds are considered as sea vegetables in their diet as well as an alternative medicine since primordial (Ali et al., Citation2000). Due to their low lipid content, high polysaccharides, natural richness in minerals, polyunsaturated fatty acids, vitamins, and other bioactive molecules, marine algae are known to be a source of high-quality healthy food (Gupta & Abu-Ghannam, Citation2011).
Seaweed-derived polyphenols
Polyphenols are a heterogeneous group of compounds and are structurally classified into phenolic acids, flavonoids, stilbenes, and lignans () (Corona, Citation2011). Marine macroalgae are a wealthy source of polyphenolic antioxidants such as catechins, flavonols, and phlorotannins in particular (Heo et al., 2005a). Polyphenols are well-characterized antioxidants and seaweed-derived polyphenols have been explored as a component of functional foods (Cofrades et al., Citation2010). Dietary intake of seafood-derived polyphenols have been ascribed to several potential health benefits; however, epidemiological and interventional studies are not well documented. Edible seaweed polyphenols have been suggested to influence responses related to diabetes through modulation of glucose-induced oxidative stress (Lee et al., Citation2010) as well as the inhibition of starch-digesting enzymes.
Figure 2. Structures of major polyphenols found in plant and seaweeds (Corona, Citation2011).
Phlorotannins derived from seaweeds
Phlorotannins are polyphenols found in several brown macroalgae. They are biosynthesized by the polymerization of phloroglucinol (1,3,5-trihydroxybenzene) monomer units through the acetate-malonate pathway or the polyketide pathway (Arnold & Targett, Citation2002). They are highly hydrophilic components with high molecular sizes ranging from 126 Da to 650 kDa (Rangan & Glombitza, Citation1986). Brown alga accumulates an array of phloroglucinol-derived polyphenols, containing both phenyl and phenoxy units. The different types of phlorotannins isolated are listed (). Based on the linkages, phlorotannins can be classified into four subclasses such as fucols (with a phenyl linkage), fuhalols and phlorethols (phlorotannins with an ether linkage), fucophlorethols (a mixture of an ether and phenyl linkage), and eckols (with a dibenzodioxin linkage). The isolated and structurally defined phlorotannins from brown seaweeds are shown in (Singh & Bharate, Citation2006; Thomas & Kim, Citation2011). The members of Laminariaceae are rich resources of phlorotannins among other marine macroalgae (Okada et al., Citation2004). The well-studied phlorotannins from Eisenia bicyclis and E. cava are phloroglucinol, phloroglucinol tetramer, eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol, dioxinodehydroeckol (Jung et al., Citation2010; Li et al., Citation2009; Shibata et al., Citation2004).
Figure 3. Chemical structures of different phlorotannins from marine algae (Singh & Bharate, Citation2006; Thomas & Kim, Citation2011).
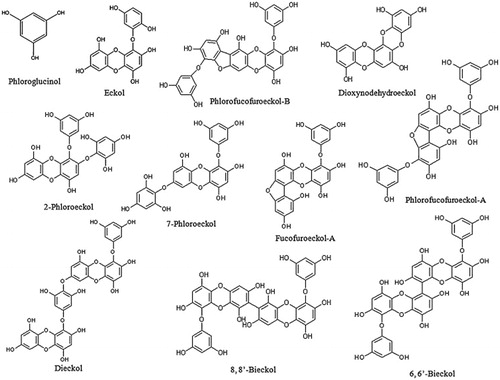
Table 1. Summary of bioactive phlorotannins (polyphenols) isolated from brown marine algae.
Seaweed antioxidants
It is observed in many research reports that phytochemicals having antioxidant capacity also demonstrate hypoglycemic properties. Marine brown algae are being studied widely now days as a source of antioxidants and, therefore, reports on the antioxidant properties of seaweed extracts that have been reviewed. The failing of cells and tissues to detoxify free radicals that are generated during the metabolic activities contributes to the oxidative stress. Diabetes mellitus caused by the overloading of glucose metabolic pathway leads to the excessive production of free radicals and contribute to oxidative stress. Both acute and chronic high blood glucose level induce oxidative stress in the peripheral nervous system which can support the onset of the diabetic neuropathy (Vincent et al, Citation2004). Hence, increased oxidative stress and impaired antioxidative defense mechanisms play a vital role in the pathogenesis and progression of diabetes mellitus. Thus, to reduce the jeopardy of pathological damages caused by diabetes, lessening of oxidative stress induced by hyperglycemia is essential. Currently, there is a growing curiosity in identifying antioxidant compounds that do not exhibit side effects or toxicity. Natural polyphenols are secondary metabolites which are abundantly found in plant and seaweed origin. Epidemiological studies indicate the potential health benefits of foods and beverages containing polyphenols due to their protective effects on cellular constituents against oxidative stress and also reduce tissue injuries either by direct action on free radicals or by inducing the endogenous antioxidant defense mechanisms (Scalbert et al., Citation2005).
Marine brown algae have been documented as an important source of naturally bioactive polyphenols with unique linkages and are widely known as phlorotannins. Screening of potential radical scavengers from 25 Japanese seaweeds was done (Nakai et al., Citation2006). Among them, Sargassum ringgoldianum exhibited the strongest scavenging activity. The bioactive fraction as analyzed by MALDI-TOF-MS was found to contain high molecular weight polymerized bifuhalol, a phlorotannin which was found to be five times stronger that tea catechins. The study of E. cava extract revealed that it contains phlorotannin derivatives (eckol, dieckol, 6,6′-bieckol, phloroglucinol, fucodiphloroethol G, phlorofucofuroeckol A, and 7-phloroeckol). Among these, dieckol showed the strongest antioxidant activity (Li et al., Citation2009). In a comparative study of the bioprotective properties of seaweeds (Devi et al., Citation2008), methanolic extracts of Gelidiella acerosa showed high hydrogen donating ability and also exhibited scavenging effect of OH free radicals which can be supportive in preventing or minimizing the progression of oxidative stress-associated disorders. The free radical scavenging ability of 2,7″-phloroglucinol-6,6′-bieckol (PHB) isolated from E. cava were evaluated (Kang et al., 2012b) using ESR spectrometry. PHB’s efficiency was evidenced on DPPH, alkyl, hydroxyl, and superoxide radical scavenging activities and was found to be higher than ascorbic acid.
Phloroglucinol derivatives (eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol) isolated from the brown marine algae E. bicyclis, E. cava, and E. kurome, exhibited potent inhibitory activity against phospholipids peroxidation and significant free radical scavenging against superoxide anion and DPPH. The EC50 values of eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol were 26, 12, 13, and 15 µM, respectively, and their effects were twice that of the catechins ascorbic acid and α-tocopherol (Shibata et al., Citation2008). Antioxidant properties of 10 species of Chlorophyta and 25 species of Phaeophyta in Jeju Island were measured by Heo et al. (Citation2005b). The methanolic and aqueous extracts showed positive effects against reactive oxygen species. The methanolic extract of S. thunbergii exhibited highest radical-scavenging activities (97.44%) against singlet oxygen, hydroxyl anion, H2O2, and DPPH-free radicals. Turbinaria conoides, Padina tetrastomatica, and Sargassum marginatum are three Indian brown seaweeds that are reported to demonstrate the antioxidant activity (Kumar & Ganesan, Citation2008). Among them ethyl acetate fraction of Sargassum marginatum demonstrated the highest antioxidant activity (39.62 mg ascorbic acid equivalent/g extract). In contrast, petroleum ether fraction of Turbinaria conoides exhibited lower deoxyribose activity (47.81%). Epidemiological study on rodents revealed the protective effect of green and red algae against intestinal, breast, and skin cancer. Indian brown and red seaweed extracts were evaluated for their different radicals scavenging and singlet oxygen quenching effects (Sachindra et al., Citation2010). Higher 2,2′-azinobis (3-ethyl benzothizoline-6-sulfonic acid) radical scavenging activity was exhibited by the methanol extract from brown seaweed and the activity was correlated to the high polyphenol content.
Radical scavenging and metal chelation properties of 10 species of Icelandic seaweeds were studied by Wang et al. (Citation2009). Acetone (70%) extract of three fucoid species exhibited high correlation between their total phenolic content and strong radical scavenging ability, indicating the profound role of algal phlorotannins as good antioxidants. The multi-functional antioxidant properties of polyphenols are mainly correlated to the number of phenol rings present in a compound. The phenol rings act as electron traps to scavenge hydroxyl, peroxy, and superoxide-anions. Phlorotannins isolated so far from brown algae have up to eight phenol rings interconnected; therefore, they are more effective free radical scavengers than other terrestrial plant polyphenols. The antioxidant capacities of different solvent extracts and different molecular weight phlorotannins extracted from F. vesiculosus were studied by Wang et al. (Citation2012). Among the crude extracts of different polarities of solvent, the ethyl acetate fraction enriched with phlorotannins showed the highest DPPH free radical scavenging activity and reducing power.
The phlorotannins isolated from F. vesiculosus consisted mainly of high molecular weight phloroglucinol polymers and no clear correlation was found among the degree of polymerization, molecular size, and its antioxidant capability. Increased protective effects along with increasing concentration of E. cava phlorotannins were observed against H2O2-mediated DNA damage in mouse T-cell lymphoma cell line (L5178Y-R) (Ahn et al., Citation2007). During the assessment of the free radical scavenging potentiality of the five different enzymatic extracts of I. okamurae, all of them showed strong dose-dependent scavenging abilities. In particular, the Kojizyme extract reduced the H2O2-induced DNA damage significantly with increasing dosages (Heo & Jeon, Citation2008). Analysis of protective effects of dieckol, a phlorotannin isolated from E. cava against high glucose induced oxidative stress in human umbilical vein endothelial cells (HUVECs), has been studied (Lee et al., Citation2010). Dieckol effectively inhibited TBARS formation in HUVECs, and also the high glucose-induced increased intracellular ROS levels were also inhibited by dieckol. The overproduction of NO and superoxide anion due to the high glucose treatment was found to be scavenged by the dieckol. In an another study by Lee et al. (Citation2012), the elevated levels of lipid peroxidation in the C57BL/KsJ-db/db diabetic mice model was found to be reduced both in the erythrocyte and in the liver by supplementation of AG-dieckol isolated from E. cava. This could be possibly related to upregulation of antioxidant enzyme activities by AG-dieckol. Kang et al. (Citation2013) reported that increased activities of antioxidant enzymes SOD, CAT, and GSH-px were observed in the treatment with dieckol isolated from E. cava on db/db mice models. The antioxidant property of three edible seaweeds from South East Asia was evaluated by Chew et al. (Citation2008). A strong correlation between the total phenolic content and the antioxidant activity was found in all the three seaweeds with P. antillarum showing the highest antioxidant capacities.
Dieckol, a phlorotannin extracted E. cava, possesses more stable scavenging effect than ascorbic acid. The degree of thermostability in addition to the scavenging ability was tested by Kang et al. (2012a). The compound efficiently scavenged the H2O2-induced intracellular ROS generated in Vero cells at 60 °C up to 7 d. Furthermore, dieckol also protected the Vero cells from oxidative stress-induced apoptosis. The purified phenolic fraction of A. nodosum was assessed for its radical scavenging abilities (Audibert et al., Citation2010). It is found that phenolic compound with a MW ≥50 kDa showed high antioxidant activity and also correlated with total phenolic content with an correlation coefficient R2 = 0.93. Connan et al. (Citation2004) hypothesized a similar relationship between the molecular weight of phlorotannin and its activity in Fucales and Laminariales. In recent years, the consumption of seaweeds has increased many folds because of their beneficial effects and no adversity. The in vitro and in vivo antioxidant activities of edible F. vesiculosus extract were studied Zaragoza et al. (Citation2008). The extracts showed no toxicity effects in the acute toxicity studies in mice and rats. The extracts also exhibited antioxidant activity in non-cellular systems and in active RAW 264.7 macrophages, as well as in plasma and erythrocytes in vivo assays. The findings support the daily consumption of seaweeds that would have potent effects in human health.
α-Glucosidase and α-amylase inhibitory activity
An overview of the antidiabetic activity of brown marine algae is presented in and has been discussed in detail in this section. α-Glucosidase and α-amylase are two vital enzymes in carbohydrate metabolism, the inhibition of which are considered to be a key tool in controlling hyperglycemia. Lots of in vitro studies are being carried out with new plant extracts using this tool to screen new antidiabetic principles. The effect of phloroglucinol oligomers eckol, phlorofucofuroeckol A, dieckol, 8,8′-bieckol, and an unidentified tetramer, from E. bicyclis, a brown seaweed, on the activity of different glucosidases present in the viscera of the turban shell (Turbo cornutus) were studied (Shibata et al., Citation2002). Among the phloroglucinol oligomers, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol strongly inhibited α-fucosidase, β-galactosidase, and β-mannosidase, while others were weakly active. The competitive inhibition of dieckol against α-fucosidase with an inhibition constant (Ki) of 0.12 mM was recorded. This fact seems to indicate the deterrent activity of phloroglucinol and its oligomers against the glucosidase enzymes. The anti-diabetic activity of the new phloroglucinol derivative from E. bicyclis, 2-phloroeckol and two known eckol and dieckol were determined (Okada et al., Citation2004). The percentage inhibition of glycation by these three compounds as tested by ELISA were 91.1, 96.2, and 86.7% for 2-phloroeckol, eckol, and dieckol, respectively, as well as the inhibition of α-amylase was 89.5% for 2-phloroeckol, 87.5% for eckol, and 97.5% for dieckol, respectively.
Table 2. An overview of the antidiabetic properties of the brown marine algae.
Chlorella and Petalonia exhibited antidiabetic effects via both insulin-like and insulin-sensitizing behavior on in vitro tests (Cherng & Shih, Citation2006; Kang et al., Citation2008). The ethanolic extract of Ascophyllum nodosum inhibited rat-intestinal α-glucosidase, with an IC50 value of 77 µg/ml and stimulated the reduction in liver glycogen levels in diabetic mice (Zhang et al., Citation2007). The polyphenol-rich methanol extracts of Ecklonia stolonifera (MEE) were tested for their antidiabetic activity in vitro in a genetically non-insulin-dependent diabetic mice model KK-Ay (Iwai, Citation2008). The male KK-Ay mice showed decreasing levels of plasma glucose and lipid peroxidation. Potent inhibitory effects of phlorofucofuroeckol A from Ecklonia stolonifera on diabetic complications such as angiotensin converting enzyme (ACE), advanced glycation end products (AGE), rat lens aldose reductase (RLAR), reactive oxygen species (ROS), and peroxynitrite in vitro (Jung et al., Citation2008). The results suggest that phlorotannins derived from E. stolonifera as a hopeful natural product for the treatment of the diabetic complications.
Five phloroglucinol derivatives (fucodiphloroethol G, dieckol, 6,6′-bieckol, 7-phloroeckol, and phlorofucofuroeckol) from E. cava were studied for their antidiabetic functions (Lee et al., 2009). Of the five compounds, dieckol displayed a significant activity with an IC50 value of 10.8 µmol/l for α-glucosidase and 124.9 µmol/l for α-amylase, respectively. The compounds also exhibited non-competitive inhibitory activity, which is evidenced from the Lineweaver–Burk plot results. A new phlorotannin, diphlorethohydroxycarmalol (DPHC) isolated from Ishige okamurae significantly suppressed the postprandial blood glucose level as well as inhibited α-glucosidase and α-amylase functions in streptozotocin-induced diabetic and normal mice (Heo et al., Citation2009). The IC50 values were 0.16 and 0.53 mM against α-glucosidase and α-amylase, respectively, which appeared to be as effective as acarbose, a commercial drug. DPHC also did not exert any cytotoxicity effects when tested in HUVEC’s. Therefore, these results suggested DPHC as a potent inhibitor for α-amylase and α-glucosidase in type 2 diabetic patients.
The highest content of polyphenols (4.2 mg/g wet wt.) extracted from Ascophyllum nodosum showed significant α-amylase and α-glucosidase inhibitory activity equivalent to acarbose, a commercially potent inhibitor drug (Apostolidis & Lee, Citation2010). The IC50 value was 0.24 µg and 1.3 µg phenolics for the α-glucosidase and α-amylase inhibition, respectively, compared with the IC50 value of acarbose being 0.37 µg (α-glucosidase) and 0.68 µg (α-amylase). This study suggested the pharmaceutical potentiality of A. nodosum against hyperglycemia in type 2 diabetes. On consumption of seaweeds polyphenol mix, there was no adverse effect. But, it was associated with a non-significant reduction (3.6%) in the area under cover (AUC) of plasma glucose levels (p = 0.16), a 5.9% reduction in insulin AUC (p = 0.031) and an increase in insulin sensitivity index by 6.9% (p = 0.047). These results suggested that polyphenols from brown seaweeds may help in improving the acute glycemic condition and insulin response in type 2 diabetic patients.
Accordingly, hyperglycemic condition in streptozotocin-induced type 1 diabetic rats was improved when the rats were given polyphenol-rich extracts of E. cava (Kang et al., Citation2010). In vitro studies demonstrated that therapeutic values of E. cava extract significantly lowered the plasma glucose level (66.4%) in diabetic rats. This result shows that E. cava extract could assist in improving the hyperglycemic status of type 1 diabetes. In another report, dieckol isolated from E. cava exhibited α-glucosidase and α-amylase inhibitory activities and also alleviated postprandial hyperglycemia in streptozotocin-induced diabetic male ICR mice (Lee et al., Citation2010). The IC50 value of dieckol against α-glucosidase and α-amylase was 0.24 and 0.66 mM, respectively. No cytotoxicity was evidenced in HUVEC’s, as well as area under cover (AUC) was significantly reduced due to the administration of dieckol (259 versus 483 mmol min−l) in the diabetic mice and thereby also delayed the assimilation of dietary carbohydrates, ensuing the restraint of an increase in postprandial blood glucose levels.
The antidiabetic effect of Ishige okamurae methanolic extract (IOE) on postprandial glucose load and insulin resistance in male C57BL/KsJ-db/db mice was studied (Min et al., Citation2011). The insulin resistance and fasting blood glucose levels were enhanced and HbA1c levels were lowered in IOE-treated group mice as compared with DMC (diabetic mellitus control) group mice. While, IOE supplement enhanced the glucokinase activity, there was a significant decrease in the glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activity in the IOE group mice. In addition, the enhanced rate of glycogenesis was also observed in the IOE group mice as compared with that of DMC group mice. As a result of enhancement of IOE in diets, blood glucose levels were lowered by varying the hepatic glucose-metabolizing enzyme functions. Phenolic-rich extract of Palmaria, Ascophyllum, Alaria, and Ulva were documented for their potential antidiabetic activity (Nwosu et al., Citation2011). Alaria, Ascophyllum, and Palmaria yielded phenolics, but not Ulva. Ascophyllum extracts were very potent inhibitor of α-glucosidase, with an IC50 value of ∼20 µg/ml GAE. Similarly, α-amylase inhibitory activity was noted in Palmaria, Ascophyllum, and Alaria, with Ascophyllum very effective, with an IC50 value of ∼0.1 µg/ml GAE, which is 500-fold more potent than similar raspberry extracts as reported (McDougall et al., Citation2005) and lower than the IC50 value of ∼0.8 µg/ml GAE for acarbose, determined in the same assay condition. The LC-MS results of the extracts strongly suggested the presence of MS signals assignable to phlorotannins structures.
Conclusion
With the advances in biotechnological tools for extraction and identification of drug compounds from marine macroalgae resources, a positive trend in the available food ingredients with potential functional activities in human health and nutrition is observed. Despite promising data from in vitro and animal studies, the effects of polyphenols on glucose homeostasis in human beings have not been consistently shown. Research on the interaction of polyphenols and its derivatives with human cellular systems could provide beneficial understanding on parameters like bioavailability, molecular mechanisms of actions of polyphenols in glucose homeostasis in humans, which may translate into long-term health benefits. Further research would widen the likelihood of screening more biologically proficient polyphenols and its derivatives and provide with resourceful drug candidates for pharmaceutical purpose to sustain reduction or regulation of the diet-linked chronic malfunctions.
Acknowledgements
The authors gratefully acknowledge the facilities provided by the Faculty of Industrial Sciences and Technology, Universiti Malaysia Pahang for carrying out this study.
Declaration of interest
The authors alone are responsible for the content and writing of the paper. The authors report that they have no conflicts of interest. The authors wish to thank the Ministry of Higher Education, Malaysia, for providing the financial assistance to the project under the Exploratory Research Grant Scheme (ERGS) (2012-RDU 120 603) and Universiti Malaysia Pahang for providing the Doctoral Student Scholarship.
References
- Aggarwal B, Shishodia S. (2006). Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–421
- Ahmad MF, Ashraf SA, Ahmad FA, et al. (2011). Nutraceutical market and its regulation. Am J Food Technol 6:342–7
- Ahn GN, Kim KN, Cha SH, et al. (2007). Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol 226:71–9
- Ali M, Jahangir M, Saleem M, et al. (2000). Metabolites of marine algae collected from Karachi-coasts of Arabian sea. Nat Prod Sci 6:61–5
- Aneiros A, Garateix A. (2004). Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J Chromatogr B 803:41–53
- Apostolidis E, Lee C. (2010). In vitro potential of Ascophyllum nodosum phenolic antioxidant mediated α-amylase inhibition. J Food Sci 75:97–102
- Arnold TM, Targett NM. (2002). Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J Chem Ecol 28:1919–34
- Aronoff SL, Berhowitz L, Shreiner B, Want L. (2004). Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectr 17:183–90
- Artan M, Li Y, Karadeniz F, et al. (2008). Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem Lett 16:7921–6
- Audibert L, Fauchon M, Blanc N, et al. (2010). Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical scavenging activities. Phytochem Anal 21:399–405
- Barrow C, Shahidi F. (Eds.). (2007). Marine Nutraceuticals and Functional Foods. Boca Raton (FL): Taylor and Francis, CRC Press, 517
- Boulton AJM, Knight G, Drury J, Ward JD. (1985). The prevalence of symptomatic, diabetic neuropathy in an insulin-treated population. Diabetes Care 8:125–8
- Bravo L. (1998). Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev 56:317–33
- Chakrabarti R, Rajagopalan R. (2002). Diabetes and insulin resistance associated disorders: Diseases and the therapy. Curr Sci 83:1533–8
- Charpentier G, Riveline JP, Varroud-Vial M. (2000). Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 26:73–85
- Cherng JY, Shih MF. (2006). Improving glycogenesis in streptozotocin (STZ) diabetic mice after administration of green algae Chlorella. Life Sci 78:1181–6
- Chew YL, Lim YY, Omar M, Khoo KS. (2008). Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT 41:1067–72
- Cofrades S, Lopez-Lopez I, Bravo L, et al. (2010). Nutritional and antioxidant properties of different brown and red Spanish edible seaweeds. Food Sci Technol Int 6:361–70
- Collins QF, Liu HY, Pi J, et al. (2007). Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282:30143–9
- Connan S, Goulard F, Stiger V, et al. (2004). Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot Mar 47:410–6
- Corona G. (2011). Seaweed polyphenols: Bioavailability and healthy benefits. Available from: http://www.seaweedsforhealth.org/swafax/ [last accessed Aug 2012]
- Cryer PE. (2002). Hypoglycemia: The limiting factor of type I and type II diabetes. Diabetologia 45:937–48
- Dao TM, Waget A, Klopp P, et al. (2011). Resveratrol increases glucose induced GLP-1 secretion in mice: A mechanism which contributes to the glycemic control. PLoS One 6:e20700
- DeFronzo RA. (1988). The triumvirate: Beta cell, muscle, liver – A conclusion responsible for NIDDM. Diabetes 37:667–84
- Devi KP, Suganthy N, Kesika P, Pandian SK. (2008). Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern Med 8:38
- Dinneen S, Gerich J, Rizza R. (1992). Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med 327:707–13
- Fu Z, Zhang W, Zhen W, et al. (2010). Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 151:3026–37
- Fukuyama Y, Kodama M, Miura I, et al. (1989). Structure of an anti-plasmin inhibitor, eckol, isolated from the brown algae Ecklonia kurome OKAMURA and inhibitory activities of its derivatives on plasma–plasmin inhibitor. Chem Pharm Bull 37:349–53
- Fukuyama Y, Kodama M, Miura I. (1990). Anti-plasmin inhibitor VI. Structure of phlorofuco-furoeckol A, a novel phlorotannins with both dibenzo-1,4-dioxin and dibenzofuran elements, from Ecklonia kurome OKAMURA. Chem Pharm Bull 38:133–5
- Glombitza KW, Keusgen M. (1995). Fuhalols and deshydroxyfuhalols from the brown alga Sargassum spinuligerum. Phytochemistry 38:987–95
- Glombitza KW, Keusgen M, Hauperich S. (1997). Fucophlorethols from the brown algae Sargassum spinuligerum and Cystophora torulosa. Phytochemistry 46:1417–22
- Glombitza KW, Rosener HU, Vilter H, Rauwald W. (1973). Antibiotics from algae. 8. Phloroglucinol from Phaeophyceae. Planta Med 24:301–3
- Gupta S, Abu-Ghannam N. (2011). Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol 22:315–26
- Ham YM, Baik JS, Hyun JW, Lee NH. (2007). Isolation of a new phlorotannins, fucodiphlorethol G, from a brown alga Ecklonia cava. Bull Korean Chem Soc 28:1595–7
- Hanefeld M. (1998). The role of acarbose in the treatment of non-insulin dependent diabetes mellitus. J Diabetes Complications 12:228–37
- Hanhineva K, Torronen R, Bondia-Pons I, et al. (2010). Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 11:1365–402
- Heo SJ, Cha SH, Lee KW, et al. (2005a). Antioxidant activities of chlorophyta and phaeophyta from Jeju Island. Algae 20:251–60
- Heo SJ, Hwang JY, Choi JI, et al. (2009). Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 615:252–6
- Heo SJ, Jeon YJ. (2008). Radical scavenging capacity and cytoprotective effect of enzymatic digest of Ishige okamurae. J Appl Phycol 20:1087–95
- Heo SJ, Park EJ, Lee KW, Jeon YJ. (2005b). Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol 96:1613–23
- Iwai K. (2008). Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Food Hum Nutr 63:163–9
- Johnston K, Sharp P, Clifford M, Morgan L. (2005). Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett 579:1653–7
- Johnston KL, Clifford MN, Morgan LM. (2003). Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 78:728–33
- Jung EH, Kim SR, Hwang IK, Ha TY. (2007). Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem 55:9800–4
- Jung HA, Oh SH, Choi JS. (2010). Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg Med Chem Lett 20:3211–5
- Jung HA, Yoon NY, Woo MH, Choi JS. (2008). Inhibitory activities of extract from several kinds of seaweeds and phlorotannins from brown alga Ecklonia stolonifera on glucose-mediated protein damage and rat lens aldose reductase. Fish Sci 74:1363–5
- Jung UJ, Lee MK, Jeong KS, Choi MS. (2004). The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr 134:2499–503
- Jung UJ, Lee MK, Park YB, et al. (2006). Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 38:1134–45
- Kang C, Jin YB, Lee H, et al. (2010). Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem Toxicol 48:509–16
- Kang KA, Lee KH, Chae SW, et al. (2005). Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Rad Res 39:883–92
- Kang MC, Kim EA, Kang SM, et al. (2012a). Thermostability of a marine polyphenolic antioxidant dieckol, derived from the brown seaweed Ecklonia cava. Algae 27:205–13
- Kang MC, Wijesinghe WAJP, Lee SH, et al. (2013). Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem Toxicol 53:294–8
- Kang SI, Jin YJ, Ko HC, et al. (2008). Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem Biophys Res Commun 373:264–9
- Kang SM, Heo SJ, Kim KN, et al. (2012b). Isolation and identification of new compound, 2,7″-phloroglucinol-6,6′-bieckol from brown algae, Ecklonia cava and its antioxidant effect. J Funct Foods 4:158–66
- Kim SK, Wijesekara I. (2010). Development and biological activities of marine-derived bioactive peptides: A review. J Funct Foods 2:1–9
- King H, Aubert R, Herman W. (1998). Global burden of diabetes, 1995–2025: Prevalence, numerical estimates and projection. Diabetes Care 21:1414–31
- Kobayashi Y, Suzuki M, Satsu H, et al. (2000). Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem 48:5618–23
- Krentz AJ, Bailey CJ. (2005). Oral antidiabetic agents. Current role in type 2 diabetes mellitus. Drugs 65:385–411
- Kumar C, Ganesan P. (2008). In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem 107:707–13
- Kumar R, Balaji S, Uma TS, Sehgal PK. (2009). Fruit extracts of Momordica charantia potentiate glucose uptake and up-regulate Glut-4, PPAR gamma and PI3K. J Ethnopharmacol 126:533–7
- Lebovitz HE. (1997). α-Glucosidase inhibitors. Endocrinol Metab Clin 26:539–51
- Lee SH, Han JS, Heo SJ, et al. (2010). Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol In Vitro 24:375–81
- Lee SH, Min KH, Han JS, et al. (2012). Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem Toxicol 50:575–82
- Lee SH, Li Y, Karadeniz F, et al. (2009). α-Glucosidase and α-amylase inhibitory activities of phloroglucinol derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agric 89:1552–8
- Li Y, Qian ZJ, Ryu BM, et al. (2009). Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg Med Chem 17:1963–73
- Liu D, Zhen W, Yang Z, et al. (2006). Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes 55:1043–50
- Manach C, Scalbert A, Morand C, et al. (2004). Polyphenols: Food sources and bioavailability. Am J Clin Nutr 79:727–47
- Martin de Bock, Derraik GBJ, Cutfield SW. (2012). Polyphenols and glucose homeostasis in humans. J Acad Nutr Diet 112:808–15
- Matsui T, Tanaka T, Tamura S, et al. (2007). α-Glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem 55:99–105
- McDougall GJ, Shpiro F, Dobson P, et al. (2005). Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J Agric Food Chem 53:2760–6
- Min KH, Kim HJ, Jeon YJ, Han JS. (2011). Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/KsJ-db/db mice. Diabetes Res Clin Pr 93:70–6
- Nakai M, Kageyama N, Nakahara K, Miki W. (2006). Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Mar Biotechnol 8:409–14
- Nakamura T, Nagayama K, Uchida K, Tanaka R. (1996). Antioxidant activity of phlorotannins isolated from the brown alga Esinia bicyclis. Fish Sci 62:923–6
- Nwosu F, Morris J, Lund VA, et al. (2011). Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem 126:1006–12
- Okada Y, Ishimaru A, Suzuki R, Okuyama T. (2004). A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J Nat Prod 67:103–5
- Park CE, Kim MJ, Lee JH, et al. (2007). Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Mol Med 39:222–9
- Perez-Jimenez J, Neveu V, Vos F, Scalbert A. (2010). Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J Agric Food Chem 58:4959–69
- Prabhakar PK, Doble M. (2009). Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 16:1119–26
- Rangan MA, Glombitza KW. (1986). Phlorotannins, brown algal polyphenols. In: Hellebust JA, Craigie JS, eds. Progress in Physiological Research. NewYork: Cambridge University Press, 129–241
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. (2010). Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol 47:94–9
- Sailler B, Glombitza KW. (1999). Phlorethols and fucophlorethols from the brown alga Cystophora retroflexa. Phytochemistry 50:869–81
- Scalbert A, Manach C, Morand C, et al. (2005). Dietary polyphenols and the prevention of disease. Crit Rev Food Sci Nutr 45:287–306
- Shanthirani CS, Pradeepa R, Deepa R, et al. (2003). Prevalence and risk factors of hypertension in a selected South Indian population – The Chennai urban population study. J Assoc Physicians India 51:20–7
- Shibata T, Ishimaru K, Kawaguchi S, et al. (2008). Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J Appl Phycol 20:705–11
- Shibata T, Kawaguchi S, Hama Y, et al. (2004). Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol 16:291–6
- Shibata T, Nagayama K, Tanaka R, et al. (2003). Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2S, lipoxygenases and cyclooxygenases. J Appl Phycol 15:61–6
- Shibata T, Yamaguchi K, Nagayama K, et al. (2002). Inhibitory activity of brown algal phlorotannins against glycosidases from viscera of the turban shell Turbo cornutus. Eur J Phycol 37:493–500
- Singh I, Bharate S. (2006). Phlorogucinol compounds of natural origin. Nat Prod Rep 23:558–91
- Sugiura Y, Matsuda K, Yamada Y, et al. (2006). Isolation of a new anti-allergic phlorotannins, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci Biotechnol Biochem 70:2807–11
- Szablewski L. (eds.) (2011). Glucose Homeostasis and Insulin Resistance. Beijing, P.R. China: Bentham E-books, eISBN 978-1-60805-189-2. [Epub ahead of print]. doi:10.2174/97816080518921110101
- Szkudelski T, Szkudelska K. (2011). Anti-diabetic effects of resveratrol. Ann NY Acad Sci 1215:34–9
- Thomas NV, Kim SK. (2011). Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ Toxicol Pharmacol 32:325–35
- Torabian S, Haddad E, Rajaram S, et al. (2009). Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet 22:64–71
- Towler MC, Hardie DG. (2007). AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–41
- Unnikrishnan R, Rema M, Pradeepa R, et al. (2007). Prevalence and risk factors of diabetic nephropathy in an urban South Indian population. Diabetes Care 30:2019–24
- van de Laar FA, Lucassen PL, Akkermans RP, et al. (2005). α-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta analysis. Diabetes Care 28:154–63
- Vincent A, Russell J, Low P, Feldman E. (2004). Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–28
- Viswanathan M, Mohan V, Snehalatha C, Ramachandran A. (1985). High prevalence of type-2 (non-insulin-dependent) diabetes among the offsprings of conjugal type 2 diabetic parents in India. Diabetologia 28:907–10
- Waltner-Law ME, Wang XL, Law BK, et al. (2002). Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277:34933–40
- Wang T, Jonsdottir R, Liu H, et al. (2012). Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–83
- Wang T, Jonsdottir R, Olafsdottir G. (2009). Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–8
- WHO. (2010). Factsheet: Diabetes. Geneva: World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html [last accessed Aug 2012]
- Wijesekara I, Yoon NY, Kim SK. (2010). Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 36:408–14
- Yoon NY, Lee SH, Li Y, Kim SK. (2009). Phlorotannins from Ishige okamurae and their acetyl- and butylcholinesterases inhibitory effects. J Funct Foods 1:331–5
- Yoon NY. (2008). Cholinesterases and lens aldose reductase inhibitory activities of phlorotannins from Ecklonia stolonifera and their protective effects on tacrine-induced hepatotoxicity and hyperlipidemic rat models [PhD thesis]. Republic of Korea: Pukyong National University
- Zang M, Xu S, Maitland-Toolan KA, et al. (2006). Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55:2180–91
- Zaragoza MC, Lopez D, Saiz MP, et al. (2008). Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J Agric Food Chem 56:7773–80
- Zhang B, Kang M, Xie Q, et al. (2011). Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J Agric Food Chem 59:537–45
- Zhang J, Tiller C, Shen J, et al. (2007). Antidiabetic properties of polysaccharide- and polyphenol-enriched fractions from the brown seaweed Ascophyllum nodosum. Can J Physiol Pharmacol 85:1116–23
- Zhou G, Myers R, Li Y, et al. (2001). Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–74