Abstract
Context: Rheumatoid arthritis fibroblast-like synoviocytes (RAFLSs) play an important role in the initiation and progression of RA, which are resistant to apoptosis and proliferate in an anchorage-independent manner.
Objective: The effects of arctigenin on the proliferation and apoptosis of RAFLSs were explored.
Materials and methods: Arctigenin (0–160 µM) was used to treat RAFLSs for 48 h. Cell viability and apoptosis were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay and annexin V/propidium iodide staining. Western blot analysis was performed to detect the changes in apoptosis-related genes.
Results and discussion: Arctigenin decreased cell viability by 23, 30, and 38% at the dose of 10, 20, and 30 µM, respectively. The half maximal inhibitory concentration (IC50) of arctignein on RAFLSs was about 38 µM. Moreover, 9, 15, and 21% of RAFLSs are induced apoptosis by 10, 20, and 30 µM of arctigenin. The apoptotic response was due to the loss of mitochondrial membrane potential, coupled with the release of cytochrome C into cytoplasm, the up-regulation of pro-apoptotic protein, Bax, and down-regulation of antiapoptotic protein, B cell lymphoma 2 (Bcl-2). The activation of mitochondrial pathway in arctigenin-treated RAFLSs induced the cleavage of caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP). Additionally, arctigenin inhibited the nuclear translocation of p65, decreased the degradation of inhibitor of kappa B alpha (IκBα), and attenuated the phosphorylation of Akt.
Conclusion: Our results reveal that arctigenin inhibits cell proliferation and induces mitochondrial apoptosis of RAFLSs, which is associated with the modulation of NF-κB and Akt signaling pathways.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the pseudotumoral expansion of synovial tissue (Huber et al., Citation2006). One of the major properties of RA synovium is the tumor-like growth of fibroblast-like synoviocytes (FLSs) (Huber et al., Citation2006). RAFLSs are resistant to apoptosis and proliferate in an anchorage-independent manner, which refers to the ability of cells to survive and proliferate in the absence of attachment to the extracellular matrix (Firestein, Citation2003). The overexpansion of RAFLSs results in the destruction of articular cartilage (Pattacini et al., Citation2010). Although the mechanism of RAFLS hyperplasia is not fully understood, several antiapoptotic molecules or signals involved in this process have been identified (Pattacini et al., Citation2010). The antiapoptotic apparatuses expressed in RAFLSs include Fas-associated death domain-like interleukin-1β-converting enzyme inhibitory protein (FLIP) (Schedel et al., Citation2002), mutated p53 (Aupperle et al., Citation1998; Firestein et al., Citation1997), sentrin (Franz et al., Citation2000), sentrin-1/small ubiquitin-like modifier (SUMO-1) (Franz et al., Citation2000), β-cell lymphoma 2 (Bcl-2) (Perlman et al., Citation2000), and the activation of the nuclear factor-kappa β (NF-κB) or the Akt signaling pathways or both (Aupperle et al., Citation1999; Morel et al., Citation2005).
Arctium lappa L. (Asteraceae/Compositae), a biennial plant known as burdock, is regarded as an effective Chinese medicine for alleviation of rheumatic pain and fever (Holetz et al., Citation2002). Arctigenin (C21H24O6; molecular weight: 372.41) is a phenylpropanoid dibenzylbutyrolactone lignan, extracted from the Arctium lappa (Awale et al., Citation2006; Lee & Kim, Citation2010; Sun et al., 2010). Arctigenin has various biological activities including anti-inflammatory, antioxidative, anticancer, and antiviral activities (Awale et al., Citation2006; Cho et al., Citation2004; Holetz et al., Citation2002; Lee & Kim, Citation2010; Matsumoto et al., Citation2006; Sun et al., 2010; Zhao et al., Citation2009). As a new anticancer agent, arctigenin induces apoptosis of estrogen receptor-negative breast cancer cells through the reactive oxygen species (ROS)/p38 mitogen-activated protein kinases (MAPKs) pathway and epigenetic regulation (Hsieh et al., Citation2014). In bladder cancer T24 cells, arctigenin induces cell-cycle arrest and apoptosis (Yang et al., Citation2012). Arctigenin enhances chemosensitivity of cancer cells to cisplatin through down-regulation of survivin expression and inhibition of the signal transducer and activator of transcription 3 (STAT3) signaling pathway (Wang et al., Citation2014; Yao et al., Citation2011). In the present study, we sought to investigate the effects of arctigenin on the proliferation and apoptosis of RAFLSs.
Materials and methods
Cell isolation and treatment
RAFLSs were obtained from 22 RA patients (6 males and 16 females; 51 ± 12 years) who underwent knee replacement surgery. All the patients fulfilled the American College of Rheumatology (ACR) criteria for the diagnosis of RA (Arnett et al., Citation1988). Written consent was obtained from all patients prior to study entry. This study was approved by the ethics committee of the 97th Hospital of People's Liberation Army (PLA2013012). Synovial tissues were minced into 2–3 mm pieces and treated for 4 h with 4 mg/ml of type I collagenase (Worthington, Freehold, NJ) in Dulbecoo's Modified Eagle Medium (DMEM) (Invitrogen Life Tech, Carlsbad, CA) at 37 °C in 5% CO2. After digestion, FLSs were filtered through a nylon cell strainer (BD Falcon, Franklin Lakes, NJ), washed extensively with DMEM, and cultured in DMEM supplemented with 10% v/v fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA), and 1% l-glutamine (Sigma, St. Louis, MO). Cells were kept at 37 °C in 5% CO2 and the medium was replaced every 3 d. Once the cells were 80–90% confluent, adherent cells were trypsinized and split in a 1:3 ratio. FLSs used in this study were from passage three to eight. Cells were frozen in 10% dimethyl sulfoxide (DMSO)/90% FBS and stored in liquid nitrogen until required.
Cells were seeded in 96-, 24-well plates, or 60-mm culture dishes (Corning Life Sciences, Acton, MA) in serum-free DMEM medium overnight, and then cultivated in DMEM complete medium in the presence or absence of arctigenin (10, 20, and 30 μM; Sigma-Aldrich, St. Louis, MO).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay
RAFLSs were seeded into 96-well plates at a density of 1 × 104 per well in triplicates. Cells were cultured under indicated treatment for 12, 24, or 48 h. Three hours before each time point, 20 μl of MTT solution (5 mg/ml in phosphate-buffered saline; PBS) was added into each well and cells were incubated at 37 °C for 3 h. The medium was removed and 100 µl of DMSO was added into each well. The plates were gently rotated on an orbital shaker for 10 min to completely dissolve the precipitation. Absorbance was measured at 570 nm in a micro-plate reader (Thermo Scientific, Fremont, CA).
Lactate dehydrogenase assay
RAFLSs were seeded into 96-well plates at a density of 1 × 104 per well in triplicates. Cells were cultured with indicated treatment for 48 h. Cytotoxicity of arctigenin was assessed by the release of Lactate dehydrogenase (LDH) into the medium (LDH Cytotoxicity Assay Kit, Cambridge Biosciences, Cambridge, UK).
Apoptosis assay
RAFLSs were seeded in 24-well plates at 5 × 104 per well in triplicates and treated as described above. Cells were washed with PBS and then stained with Annexin V-FITC Apoptosis Detection kit II (BD Biosciences, San Jose, CA) according to the manufacturer's instructions. The cells were subjected to flow cytometric analysis on a FACSCalibur™ (BD Biosciences, San Jose, CA) and the CELLQuest™ software (BD Biosciences, San Jose, CA). Propidium iodide (PI) negative and annexin V positive cells are considered apoptotic. PI and annexin V double positive cells are considered necrotic.
Measurement of mitochondrial membrane potential (△Ψm)
RAFLSs were stained with the fluorescent probe JC-1 (5,-5′,-6,-6′-tetrachloro-1,-1′,-3,-3′-tetraethylbenzi-midazolecarbocyanide iodide; BD Biosciences, San Jose, CA) at 1 μg/ml for 10 min at 37 °C, washed, and analyzed by flow cytometry (FACSCalibur™; BD Biosciences, San Jose, CA). For JC-1 monomers, the fluorimeter was set at a 490-nm excitation wavelength and 530-nm emission wavelength. For JC-1 aggregates, the fluorimeter was set at a 525-nm excitation wavelength and 590-nm emission wavelength. Fluorescence was measured using FACSCalibur™ (BD Biosciences, San Jose, CA). In healthy cells, JC-1 exists as a monomer in the cytosol (FL1-positive, green) and also accumulates as aggregates in the mitochondria (FL2-positive, red). In apoptotic cells, JC-1 exists exclusively in monomer form and produces a green cytosolic signal (Perelman et al., Citation2012).
Western blot analysis
Cells of 2 × 105 were seeded in a 60-mm culture dish. After the indicated treatment, cells were harvested. Total protein, cytoplasmic protein, and nuclear protein were extracted by ReadyPrep Protein Extraction Kit (Total Protein) and ReadyPre Protein Extraction Kit (Cytoplasmic/Nuclear) (Bio-Rad, Hercules, CA), respectively. The protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA). Protein samples were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were blocked with Tris buffered saline and 0.1% Tween 20 (TBST) containing 5% non-fat milk for 1 h at room temperature, followed by the incubation with primary antibodies, such as anti-Bcl-2 (Cell Signaling Technology Inc., Danvers, MA), anti-Bax (Cell Signaling Technology Inc., Danvers, MA), anti-cytochrome C (Cell Signaling Technology Inc., Danvers, MA), anti-cleaved caspase-9 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cleaved caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-p65 (Cell Signaling Technology Inc., Danvers, MA), anti-IκBα (Cell Signaling Technology Inc., Danvers, MA), anti-Akt (Santa Cruz Biotechnology, Santa Cruz, CA), anti-pAkt (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), overnight at 4 °C. After washed with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. Proteins were visualized with an enhanced chemiluminescence (ECL) detection kit and Hyperfilm-ECL reagents (Amersham Pharmacia Biotech, Piscataway, NJ). The bands were quantified by densitometry using Bio-Rad Quantity One software (Bio-Rad, Hercules, CA).
Statistical analysis
SPSS 16.0 program (SPSS Inc., Chicago, IL) was used for all statistical analysis. Results are shown as mean ± SD (n = 3). Analysis of variance (ANOVA) was used for statistical analysis of mean values of more than two groups. A p value of 0.05 or less was considered statistically significant.
Results
The effects of arctigenin on the cell viability of human RAFLSs
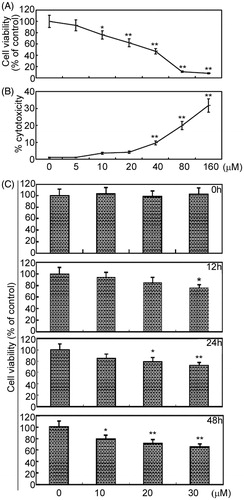
To test the effects of arctigenin on the cell viability of RAFLSs, RAFLSs were exposed to arctigenin at different concentrations and for different periods of time. The cell viability was measured by MTT assay and the cytotoxicity of arctigenin was analyzed by lactate dehydrogenase (LDH) assay (). As shown in , arctigenin inhibited the proliferation of RAFLSs. Arctigenin (10 μM) had no effect on the amount of viable cells at 12 and 24 h, but decreased the total cell count by about 23% at 48 h (p < 0.05, relative to control). Treatment with 20 μM of arctigenin for 12 h had no obvious effects on cell growth, but resulted in about 21% (p < 0.05) and 30% (p < 0.01) cell death at 24 and 48 h, respectively (). Moreover, arctigenin-induced cell growth inhibition had been observed at 12 h at the dose of 30 μM (p < 0.05; ). shows that 40–160 μM of arctigenin further decreased the proliferation of RAFLSs, and induced cytotoxicity in RAFLSs. In the present study, we chose 10–30 μM of arctigenin to investigate the effects of arctigenin on RAFLSs. These results demonstrate the proliferation inhibition effect of arctigenin on RAFLSs.
Figure 1. The effects of arctigenin on the cell viability of RAFLSs. RAFLSs (1 × 104) were seeded into 96-well plates. Cells were cultured in the presence or absence of arctigenin (0–160 μM) for 48 h (A) and (B) or in the presence or absence of arctigenin (0–30 μM) for 0, 12, 24, and 48 h (C). The cell viability was measured by the MTT assay. Arctigenin-induced cytotoxicity was assessed by LDH assay. Data are shown as mean ± SD (n = 3) from three independent experiments. *p < 0.05, **p < 0.01, relative to control RAFLSs.
Arctigenin induces apoptosis with RAFLSs
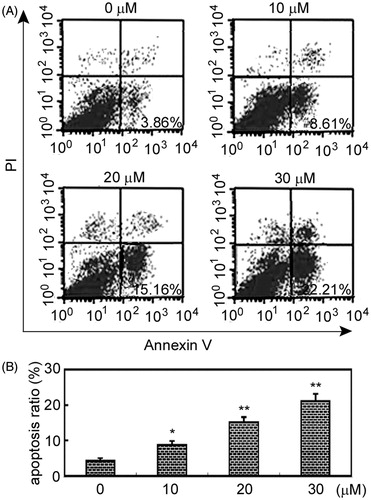
Beside growth inhibition, we asked whether arctigenin-mediated decrease of RAFLSs was related to apoptosis. Apoptosis was assessed by the measurement of phosphatidylserine externalization, a hallmark of membrane apoptosis (Martin et al., Citation1995). As shown in , apoptosis was significantly, but weakly, induced by 10 μM of arctigenin (p < 0.05, relative to control cells; ), and strongly induced by 20 and 30 μM arctigenin (p < 0.01; ).
Figure 2. Arctigenin induces RAFLS apoptosis. RAFLSs were treated with various concentrations of arctigenin (0–30 μM) for 48 h and examined by annexin V/ propidium iodide (PI) double staining. Apoptosis was analyzed using flow cytometry. Apoptotic cells were annexin V-positive and PI-negative cells (lower right quadrant). (A) Representative flow cytometry results for RAFLSs exposed to arctigenin (0–30 μM) for 48 h. (B) The histogram shows the apoptosis ratio (%) of RAFLSs. The data represent three independent experiments. *p < 0.05, **p < 0.01, relative to control RAFLSs.
Arctigenin-mediated apoptosis of RAFLSs is through mitochondrial pathway
To identify the apoptotic pathway being activated in response to arctigenin, we detected the mitochondrial membrane potential in RAFLSs exposed to arctigenin. Changes in mitochondrial membrane potential were analyzed by a fluorescent cationic dye 5,-5′,-6,-6′-tetrachloro-1,-1′,-3,-3′-tetraethyl-benzamidazolocarbocyanin iodide, known as JC-1. As shown in , arctigenin induced mitochondrial depolarization in RAFLSs. The percentages of cells with low mitochondrial membrane potential were 9.56 ± 1.59%, 16.85 ± 1.46%, and 23.28 ± 2.36% in RAFLSs under the treatment of 10, 20, and 30 μM of arctigenin, respectively ().
Figure 3. Arctigenin induces mitochondrial apoptosis of RAFLSs. (A) Cells were treated with indicated concentrations of arctigenin (0–30 μM) for 48 h and stained with JC-1. The mean JC-1 fluorescence intensity was detected by FACS analysis. Images are representative of three independent experiments (upper panel). Quantification of FACS analysis is shown in the lower panel. (B)–(D) Cells were treated with indicated concentrations of arctigenin (0–30 μM) for 48 h and subjected to Western blot analysis. Data are shown as mean ± SD (n = 3) from three independent experiments. *p < 0.05, **p < 0.01, relative to control RAFLSs.
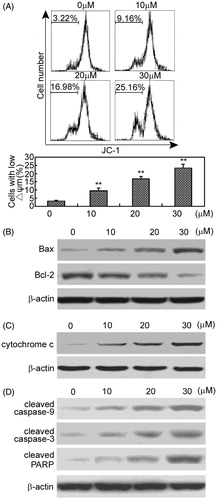
Consistently, Western blot analysis showed that arctigenin up-regulated the pro-apoptotic protein, Bax down-regulated the antiapoptotic protein, Bcl-2 increased the cytochrome C release into the cytoplasm, and induced the cleavage of caspase-9, caspase-3, and PARP ().
Arctigenin inhibits the activation of NF-κB and Akt signaling pathways
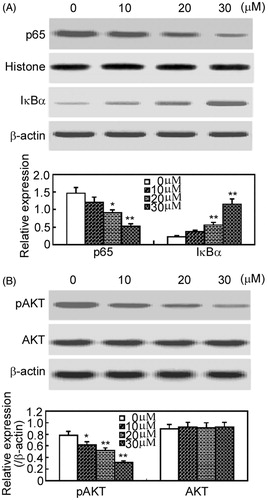
As the activation of the nuclear factor-kappa B (NF-κB) and Akt signaling pathways play important roles in the apoptosis of RAFLSs (Aupperle et al., Citation1999; Morel et al., Citation2005), the effects of arctigenin on the activation of NF-κB and Akt were analyzed by Western blot analysis (). Arctigenin inhibited p65 nuclear translocation and attenuated the degradation of IκBα, indicating that arctigenin decreases the activation of NF-κB (). Similarly, arctigenin attenuated the phosphorylation of Akt, but had no effects on the expression of total Akt ().
Figure 4. Arctigenin inhibits NF-κB and Akt signaling pathways in RAFLSs. RAFLSs were treated with the indicated concentrations of arctigenin (0–30 μM) for 48 h and subjected to Western blot analysis. The nuclear translocation of p65 and the degradation of IκBα (A) and the phosphorylation of Akt (B) are shown in the upper panel. Quantification of Western blot analysis is shown in the lower panel. Data are shown as mean ± SD (n = 3) from three independent experiments. *p < 0.05, **p < 0.01, relative to control RAFLSs.
Discussion
In the present study, we found that arctigenin inhibited the cell viability of RAFLSs and induced cell apoptosis. Arctigenin induced RAFLS apoptosis through the mitochondrial pathway, evidenced by the loss of mitochondrial membrane potential in arctigenin-treated RAFLSs. Consistently, arctigenin increased Bax expression, decreased Bcl-2 expression, enhanced the release of cytochrome C into cytoplasm, and raised the cleavage of caspase-9, caspase-3, and PARP. Furthermore, the activation of NF-κB and Akt, both of which play an important role in the apoptosis of RAFLSs, was attenuated in RAFLSs by arctigenin. It seems that arctigenin can induce cell apoptosis through mitochondrial pathway in human RAFLSs, and suppress the activation of NF-κB and Akt.
RAFLSs are one of the key effector cells in the initiation and pathology of RA (Bartok & Firestein, Citation2010), which are characterized by rapid proliferation and defective apoptosis (Bartok & Firestein, Citation2010). In the present study, we investigated the effects of arctigenin on the cell viability and apoptosis of RAFLSs. We found that arctigenin decreased the cell viability of RAFLSs and induced apoptosis. Our results indicate that arctigenin-induced cytotoxicity on RAFLSs might be one possible mechanism for Arctium lappa-mediated rheumatoid arthritis-relieving effects. Actually, arctigenin has been shown to inhibit the proliferation of human ovarian cancer OVCAR3 and SKOV3 cells (Huang et al., Citation2014), enhance the chemosensitivity of human lung cancer H460 cells to cisplatin (Wang et al., 2014), reduce the viability of bladder cancer T24 cells, and induce cytotoxicity of lung cancer A549 cells, liver cancer HepG2 cells, and stomach cancer KATO III cells (Susanti et al., Citation2012). Collectively, these results suggest that arctigenin has antiproliferation effects in various types of tumor or tumor-like cells.
The intrinsic apoptosis pathway involves non-receptor-mediated intracellular signals, leading to the loss of mitochondrial membrane potential which initiates apoptosis (Elmore, Citation2007). The mitochondrial pathway plays a critical role in the apoptosis of RAFLSs (García et al., Citation2010). To assess the mechanism of arctigenin-induced apoptosis of RAFLSs, we measured the mitochondrial membrane potential in arctigenin-treated RAFLSs. We found that arctigenin enhanced the percentages of RAFLSs with low mitochondrial membrane potential, indicating that arctigenin induces the loss of mitochondrial potential. Actually, it has been shown that arctigenin induces apoptosis in bladder cancer T24 cells through the mitochondrial pathway (Yang et al., Citation2012). Proteins of the Bcl-2 family are key regulators of the mitochondrial pathway of apoptosis (García-Sáez, Citation2012). Proteins in the Bcl-2 family are formed by proapoptotic and antiapoptotic members (García-Sáez, Citation2012). The expression of Bcl-2, an antiapoptotic protein in the Bcl-2 family, in synovial fibroblasts is essential for mitochondrial homeostasis and cell viability (Perlman et al., Citation2000). Bcl-2 expression was decreased in RAFLSs exposed to arctigenin. Bax, a pro-apoptotic protein in the Bcl-2 family, can homodimerize or heterodimerize with other pro-apoptotic members such as Bak, disrupt the integrity of the outer mitochondrial membrane, and lead to the release of apoptogenic factors, such as cytochrome C (Vyssokikh et al., Citation2002). Our results showed that arctigenin up-regulated the expression of Bax. The down-regulation of Bcl-2 and up-regulation of Bax in RAFLSs exposed to arctigenin indicate that Bcl-2 and Bax might be involved in arctigenin-mediated regulation of mitochondrial pathway in RAFLSs. Next, we examined the release of cytochrome C into the cytoplasm. Consistently, the release of cytochrome C was significantly enhanced in arctigenin-treated RAFLSs. Once released, cytochrome C binds apoptotic protease-activating factor 1 (Apaf1), pro-caspase-9, and ATP and induces the cleavage of caspase-9 (Rinkenberger & Korsmeyer, Citation1997). Cleaved and activated caspase 9 induces the activation of caspase-3 (Kuida et al., Citation1998), resulting in the cleavage of cytoskeletal, nuclear scaffold, DNA repair, and cell-cycle proteins (Rao & White, Citation1997). Exposure to arctigenin enhanced the cleavage of caspase-9, caspase-3, and PARP (a nuclear enzyme involved in DNA repair), revealing the involvement of capase-9 and caspase-3 in arctigenin-mediated apoptosis. All together, arctigenin induces apoptosis through the mitochondrial pathway in RAFLSs.
NF-κB expression and activation in RA synovium has been extensively documented (Makarov, Citation2001). NF-κB activation facilitates synovial hyperplasia by promoting proliferation and inhibiting apoptosis of RAFLS (Makarov, Citation2001). Arctigenin treatment inhibited the activation of NF-κB, evidenced by less nuclear translocation of p65 and IκBα degradation, indicating that arctigenin induces apoptosis through suppression of NF-κB activation. Akt also plays an important role in the survival of RAFLSs (García et al., Citation2010; Morel et al., Citation2005). Similarly, we analyzed the effects of arctigenin on the phosphorylation of Akt. We found that arctigenin attenuated Akt phosphorylation in RAFLSs. Collectively, suppression of NF-κB and Akt offers another strategy for arctigenin-induced apoptosis in RAFLSs.
In summary, our results suggest that arctigenin exhibits cytotoxic and apoptotic effects in RAFLSs through the activation of the mitochondrial pathway and suppression of NF-κB and Akt signaling pathways. Arctigenin seems to be a new therapeutic agent for the treatment of RA in vitro. Further investigation of its antiproliferation and anti-inflammatory effects in RA animal models is essential before proceeding to clinical trials. Additionally, it is also worth to explore the synergistic, additive, or antagonistic effects of arctigenin with other standard chemotheraputic drugs for assessing the values of arctigenin in the treatment of RA.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Arnett FC, Edworthy SM, Bloch DA, et al. (1988). The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–24
- Aupperle KR, Bennett BL, Boyle DL, et al. (1999). NF-kappa B regulation by I kappa B kinase in primary fibroblast-like synoviocytes. J Immunol 163:427–33
- Aupperle KR, Boyle DL, Hendrix M, et al. (1998). Regulation of synoviocyte proliferation, apoptosis, and invasion by the p53 tumor suppressor gene. Am J Pathol 152:1091–8
- Awale S, Lu J, Kalauni SK, et al. (2006). Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 66:1751–7
- Bartok B, Firestein GS. (2010). Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol Rev 233:233–55
- Cho MK, Jang YP, Kim YC, Kim SG. (2004). Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent KK inhibition: The role in TNF-alpha inhibition. Int Immunopharmacol 4:1419–29
- Elmore S. (2007). Apoptosis: A review of programmed cell death. Toxicol Pathol 35:495–516
- Firestein GS. (2003). Evolving concepts of rheumatoid arthritis. Nature 423:356–61
- Firestein GS, Echeverri F, Yeo M, et al. (1997). Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA 94:10895–900
- Franz JK, Pap T, Hummel KM, et al. (2000). Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum 43:599–607
- García S, Liz M, Gómez-Reino JJ, Conde C. (2010). Akt activity protects rheumatoid synovial fibroblasts from Fas-induced apoptosis by inhibition of bid cleavage. Arthritis Res Ther 12:R33
- García-Sáez AJ. (2012). The secrets of the Bcl-2 family. Cell Death Differ 19:1733–40
- Holetz FB, Pessini GL, Sanches NR, et al. (2002). Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 97:1027–31
- Hsieh CJ, Kuo PL, Hsu YC, et al. (2014). Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol Med 67:159–70
- Huang K, Li LA, Meng YG, et al. (2014). Arctigenin promotes apoptosis in ovarian cancer cells via the iNOS/NO/STAT3/survivin signalling. Basic Clin Pharmacol Toxicol 115:507--11, doi: 10.1111/bcpt.12270
- Huber LC, Distler O, Tarner I, et al. (2006). Synovial fibroblasts: Key players in rheumatoid arthritis. Rheumatology 45:669–75
- Kuida K, Haydar TF, Kuan CY, et al. (1998). Reduced apoptosis and cytochrome C-mediated caspase activation in mice lacking caspase 9. Cell 94:325–37
- Lee JY, Kim CJ. (2010). Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I-IV allergic inflammation and pro-inflammatory enzymes. Arch Pharm Res 33:947–57
- Makarov SS. (2001). NF-kappa B in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res 3:200–6
- Martin SJ, Reutelingsperger CP, McGahon AJ, et al. (1995). Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–56
- Matsumoto T, Hosono-Nishiyama K, Yamada H. (2006). Antiproliferative and apoptotic effects of butyrolactone lignans from Arctium lappa on leukemic cells. Planta Med 72:276–8
- Morel J, Audo R, Hahne M, Combe B. (2005). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem 280:15709–18
- Pattacini L, Boiardi L, Casali B, Salvarani C. (2010). Differential effects of anti-TNF-alpha drugs on fibroblast-like synoviocyte apoptosis. Rheumatology (Oxford) 49:480–9
- Perelman A, Wachtel C, Cohen M, et al. (2012). JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis 3:e430
- Perlman H, Georganas C, Pagliari LJ, et al. (2000). Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol 164:5227–35
- Rao L, White E. (1997). Bcl-2 and the ICE family of apoptotic regulators: Making a connection. Curr Opin Genet Dev 7:52–8
- Rinkenberger JL, Korsmeyer SJ. (1997). Errors of homeostasis and deregulated apoptosis. Curr Opin Genet Dev 7:589–96
- Schedel J, Gay RE, Kuenzler P, et al. (2002). FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum 46:1512–8
- Sun S, Wang X, Wang C, et al. (2011). Arctigenin suppresses unfolded protein response and sensitizes glucose deprivationmediated cytotoxicity of cancer cells. Planta Med 77:141–5
- Susanti S, Iwasaki H, Itokazu Y, et al. (2012). Tumor specific cytotoxicity of arctigenin isolated from herbal plant Arctium lappa L. J Nat Med 66:614–21
- Vyssokikh MY, Zorova L, Zorov D, et al. (2002). Bax releases cytochrome c preferentially from a complex between porin and adenine nucleotide translocator. Hexokinase activity suppresses this effect. Mol Biol Rep 29:93–6
- Wang HQ, Jin JJ, Wang J. (2014). Arctigenin enhances chemosensitivity to cisplatin in human nonsmall lung cancer H460 cells through downregulation of survivin expression. J Biochem Mol Toxicol 28:39–45
- Yang S, Ma J, Xiao J, et al. (2012). Arctigenin anti-tumor activity in bladder cancer T24 cell line through induction of cell-cycle arrest and apoptosis. Anat Rec (Hoboken) 295:1260–6
- Yao X, Zhu F, Zhao Z, et al. (2011). Arctigenin enhances chemosensitivity of cancer cells to cisplatin through inhibition of the STAT3 signaling pathway. J Cell Biochem 112:2837–49
- Zhao F, Wang L, Liu K. (2009). In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J Ethnopharmacol 122:457–62

