Abstract
Context: 3,4-Dihydroxyacetophenone (DHAP) has been reported to possess cardiovascular pharmacological effects.
Objective: This study was designed to determine whether DHAP could improve endothelial function in obese rats.
Materials and methods: Wistar rats were randomly divided into control, obesity, and DHAP groups and fed a normal, high-fat, and high-fat plus DHAP (10 mg kg−1 d−1) diet, respectively, for 8 weeks. Endothelial-dependent vasodilatation was assessed. Endothelial nitric oxide synthase (eNOS) activity and nitric oxide (NO) production in endothelial cells were determined. Nuclear transcription factor kappa B (NF-κB) expression and superoxide production in aorta were evaluated.
Results: DHAP treatment significantly decreased plasma triglycerides (0.94 ± 0.31 mmol/l versus 1.36 ± 0.29 mmol/l, p < 0.05) and free fatty acids (0.53 ± 0.15 mmol/l versus 0.99 ± 0.24 mmol/l, p < 0.05), reduced serum tumor necrosis factor α (35.56 ± 9.28 pg/ml versus 68.3 ± 10.24 pg/ml, p < 0.05) and malondialdehyde (2.94 ± 0.58 pg/ml versus 6.45 ± 0.70 pg/ml, p < 0.05), and increased serum adiponectin levels (164.5 ± 34.5 μg/l versus 84.5 ± 20.4 μg/l, p < 0.05). DHAP enhanced endothelial-dependent vasodilatation and improved endothelial function in obese rats (p < 0.05). eNOS activity and NO production in endothelial cells significantly decreased and NF-κB activation and superoxide production in aorta significantly increased in obese rats compared with the control group (p < 0.05). However, DHAP treatment significantly up-regulated the eNOS–NO pathway and decreased NF-κB activation and superoxide production (p < 0.05).
Conclusion: DHAP improved endothelial function in obese rats. This beneficial effect may be associated with up-regulation of the eNOS–NO pathway by improving lipid metabolism and reducing oxidative stress and inflammation activity.
Introduction
Many clinical observations and animal experiments have shown that obesity is associated with ischemic cardiovascular disease (Calabro et al., Citation2009; Lteif et al., Citation2005). Endothelial dysfunction is an early marker for the initiation and progression of atherosclerosis and has been regarded as a key mediator that links obesity with cardiovascular disease (Han et al., Citation2012; Sun et al., Citation2013; Wang et al., Citation2012). Numerous mechanisms have been proposed to explain this endothelial dysfunction in obesity, such as hypertension, hyperinsulinemia, and high free fatty acid (FFA) levels. All of these abnormal changes could reduce endothelial nitric oxide (NO) production by inducing chronic inflammation and oxidative stress (Davel et al., Citation2011; Wang et al., Citation2013a,Citationb). Currently, some drugs, such as statins, peroxisome proliferator-activated receptor γ agonist or renin–angiotensin system blockade therapy, have been confirmed to improve endothelial function (Li et al., Citation2014; Mather, Citation2001; Sun & Yu, Citation2014). However, in some obese patients, cardiovascular risk remains high in spite of treatment with these drugs. Therefore, more effective drugs need to be identified and tested in future studies.
Recently, some traditional Chinese herbs have been approved to be emerging new alternatives for the treatment of cardiovascular disease (Chen et al., Citation2011; Fu et al., Citation2011; Hu et al., Citation2012; Jia & Hu, Citation2010). 3,4-Dihydroxyacetophenone (DHAP) is an active component isolated from leaves of Tumaodongqing (Ilex Pubescens Hook. et Arn. var glaber Chang). DHAP has been reported to possess wide cardiovascular pharmacological effects because of its antioxidant and anti-inflammatory effects (Zhang, Citation2013). It was initially used to promote blood circulation and remove blood stasis, and recent clinical studies have suggested that it could also inhibit the proliferation of vascular smooth muscle cells and platelet aggregation (Dai-juan, Citation2009; Wu, Citation2001). However, there are no reports on whether DHAP has protective effects on endothelial function in obesity. Therefore, the objective of this study was to explore the effects of DHAP on endothelial dysfunction of the thoracic aorta isolated from high-fat diet-induced obese rats.
Materials and methods
Experimental animal
Six-week-old male Wistar rats were purchased from the Experimental Animal Center of Shandong University. Rats were randomly divided into a normal control group (NC group), an obesity group (OB group), and a DHAP group. The NC group was fed regular chow and the other groups a high-fat diet (493 kcal 100 g−1) (Sun et al., Citation2013). Four weeks later, the DHAP group was administered DHAP (10 mg kg−1 d−1; Beijing Pharmaceutical Research Institute, Beijing, China) by oral gavage (Dai-juan, Citation2010). The NC group and the OB group were given the same amount of physiological saline in the same manner. The rats were housed under standard laboratory conditions. Body weight and food intake were monitored weekly. The study was conducted according to the Guide for the Care and Use of Laboratory Animals published by the Belgian Regulations.
Determination of endothelial function
After treatment for 8 weeks, rats were anesthetized with sodium pentobarbital (60 mg/kg) by intraperitoneal injection. The thoracic aorta was collected, cut into rings (3 mm in length), and fixed in an organ bath linked with a force transducer (PowerLab, AD Instruments, Castle Hill, NSW, Australia) containing 20 mL of Krebs–Henseleit bicarbonate buffer (K–H solution, 95% oxygen and 5% carbon dioxide at 37 °C) for the determination of endothelial function (Han et al., Citation2012). All rings were equilibrated for 60 min at a preload of 1.0 g. Before testing endothelium-dependent and independent vascular relaxation, each aorta ring was incubated with norepinephrine (1 μM; Sigma-Aldrich, St. Louis, MO) to induce vasoconstriction. Acetylcholine (Ach, 10−8–10−4 M, Sigma, St. Louis, MO) was then added to the organ bath to detect endothelium-dependent vasodilatation (EDV), and SNP (10−8–10−4 M, Sigma) to assess endothelium-independent vasodilatation (EIV). After organ bath experimentation, the aorta was weighed and collected for immunohistochemistry, as described below.
Plasma measurements
Blood was collected from the heart. Plasma insulin and glucose levels were measured by radioimmunoassay and the glucose oxidase method, respectively. Serum triglyceride (TG), total cholesterol, and malondialdehyde (MDA) were measured using colorimetric assays (the inter-assay and intra-assay CV of MDA <10%). Serum tumor necrosis factor-α (TNF-α) was measured with an enzyme-linked immunosorbent assay kit (USCN Life Sciences and Technology, Wuhan, China; the intra- and interassay CV were 3.9% and 6.3%, respectively).
NO production and eNOS activity in endothelial cells
After organ bath experimentation, endothelial cells of the aorta were collected by mechanical separation. Total NO production reflected by nitrite and nitrate was measured by high-performance liquid chromatography (HPLC) (Han et al., Citation2012; Jobgen et al., Citation2007). K–H solution (100 µL) from the organ bath and 100 µL of acetonitrile were mixed and centrifuged (13 000 g, 4 min) and the supernatant injected into the HPLC system. The eluent was 0.15 M (NH4)2HPO4 (pH 5.2) and the flow rate was 1.0 mL/min. The concentration of NO production was detected using a Shimadzu UV–VIS detector (Shimadzu Corp., Canby, OR) at a wavelength of 214 nm. Endothelial nitric oxide synthase (eNOS) activity was measured by the conversion of l-arginine to l-citrulline, as described previously (Jiang et al., Citation2005; Yu et al., Citation2008).
Measurement of superoxide anion production in the aorta
Superoxide anion production in the rat aorta was determined by the lucigenin chemiluminescence method, as described previously (Gupte et al., Citation2005; Mohazzab et al., Citation1996).
Immunohistochemistry
Formalin-fixed aorta were sectioned and used for nuclear transcription factor kappa B (NF-κB) immunohistochemistry. Tissue sections of 5 µm were deparaffinized in xylene and rehydrated in ethanol and were then quenched in 3% H2O2 solution for 10 min. The sections were incubated with the NF-κB p65 antibody (Abcam, Cambridge, UK) followed by the secondary antibody after restoring antigen retrieval. The slides were stained with 3,3′-diaminobenzidine and counterstained with hematoxylin. Numbers of NF-κB p65 positive nuclei and cells were calculated using Image-Pro plus 6.0 software (Media Cybernetics Inc, Bethesda, MD).
Statistical analysis
All data are expressed as mean ± standard deviation. SPSS 16.0 (SPSS Inc., Chicago, IL) was used for all analyses. Multiple comparisons were evaluated by one-way ANOVA. A Student–Newman–Keul test was used to detect significant differences between groups. A p value <0.05 was considered statistically significant.
Results
Biometric and blood parameters of rats in the studied groups
Rats in the OB group exhibited increased body weight, visceral fat mass, and visceral fat weight to body weight compared with rats in the NC group (p < 0.01). DHAP treatment did not reduce body weight or visceral fat mass (p > 0.05). As expected, levels of plasma FFA and serum TG, MDA, and TNF-α levels were elevated in obese rats compared with the NC group, which were reduced by DHAP treatment (p < 0.01). No significant difference in plasma glucose was found among the three groups (p > 0.05). Plasma insulin levels were significantly higher and serum adiponectin levels were significantly lower in the OB group compared with the NC group (p < 0.01). DHAP treatment did not reduce insulin levels (p > 0.05) but significantly enhanced serum adiponectin levels (p < 0.01) ( and ).
Table 1. Biometric parameters of rats in the two groups (n = 7–9).
Table 2. Blood parameters of rats in the studied groups (n = 7–9).
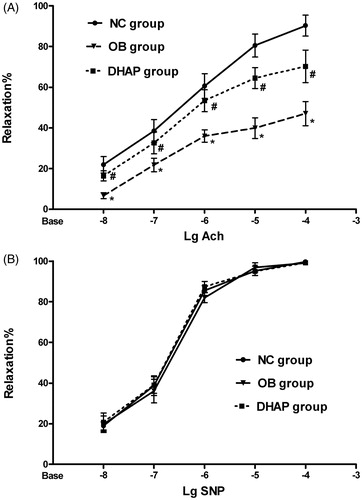
Endothelial function of rats in the studied groups
Consistent with previously reported results (Hou et al., Citation2014), Ach-induced concentration-dependent vasorelaxation was impaired in aorta segments isolated from obese rats (p < 0.05). SNP-induced concentration-dependent vasorelaxation remained unchanged in these vessels, which indicated that there was obvious endothelial dysfunction in obese rats (p > 0.05). However, DHAP treatment enhanced Ach-induced vasorelaxation and improved endothelial function (p < 0.05) ().
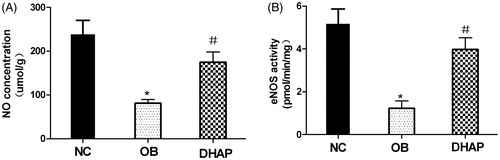
Total NO production and eNOS activity in endothelial cells
To prove that DHAP treatment may increase eNOS activity and endothelium-derived NO production in obese rats, we measured eNOS activity and NO levels in endothelial cells. As shown in , eNOS activity and NO levels showed a significant decrease in obese rats compared with the NC group (p < 0.05). However, DHAP treatment significantly increased eNOS activity and NO levels (p < 0.05).
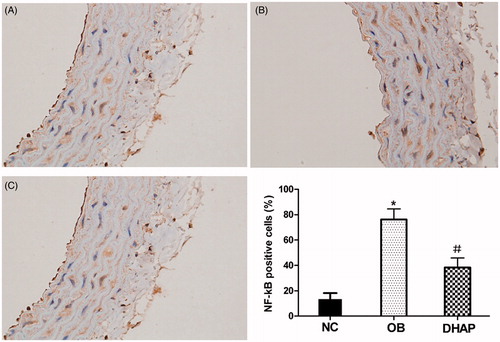
NF-κB expression in the aorta
Immunohistochemistry showed increased NF-κB activation in the aorta of obese rats (p < 0.05). However, DHAP intervention partly decreased NF-κB activation (p < 0.05) ().
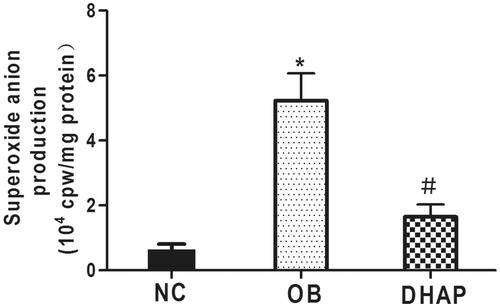
Superoxide anion production in the aorta
Superoxide anion production in the aorta was significantly enhanced in obese rats compared with the NC group (p < 0.05). However, DHAP treatment significantly reduced superoxide anion production (p < 0.05) ().
Discussion
In this study, we demonstrated that DHAP could improve endothelial function of the thoracic aorta isolated from high-fat diet-induced obese rats. This beneficial effect may be associated with up-regulation of the eNOS–NO signal pathway by improving lipid metabolism and reducing oxidative stress and inflammation activity. This result raises the possibility that therapeutic application of DHAP may be a useful treatment for vascular complications in obesity.
Cardiovascular disease is the leading cause of mortality worldwide, and obesity is one of the most important risk factors for cardiovascular disease. The endothelium plays a crucial role in regulating vascular function by producing vasoactive mediators, among which NO is the most important. In obesity, FFA-induced overproduction of reactive oxygen species (ROS) and inflammatory factors impair endothelial NO production and cause impaired EDV (Davel et al., Citation2011; Mellor et al., Citation2013; Sun et al., Citation2013). In our study, there was a significant pathological change in the rats of the OB group, including increased body weight, visceral fat mass, and serum levels of FFA and TG, which are similar to obesity and indicated that a model of obesity was established successfully. As expected, Ach-induced concentration-dependent vasorelaxation was impaired in aorta segments isolated from obese rats, which indicated that there was obvious endothelial dysfunction in obese rats.
DHAP is an active component isolated from leaves of Tumaodongqing (Ilex Pubescens Hook. et Arn. var glaber Chang) that has been used as a traditional Chinese herbal medicine. It has gradually shown good pharmacologic effects in patients with cardiovascular diseases such as pregnancy-induced hypertension and coronary heart disease (Huang, Citation1996; Wazir et al., Citation2004; Zhang, Citation2013). This might be partly because of its antioxidant and anti-inflammatory effects. In the present study, obese rats treated with DHAP showed significantly enhanced Ach-induced vasodilation and thus improvement in endothelial function. However, SNP-induced EIV was not affected. These results indicated that DHAP could improve endothelial function in obese rats. It is known that an altered eNOS–NO signal pathway is a common feature observed in animal models exhibiting endothelial dysfunction (Krishna et al., Citation2012). In our study, eNOS activity and NO levels significantly decreased in obese rats compared with the NC group, and treatment of obese rats with DHAP resulted in increases in both eNOS activity and totals NO production in endothelial cells. These results indicated that DHAP could improve endothelial function by enhancing the eNOS–NO signal pathway in obese rats.
The improvement of the eNOS–NO signal pathway in obesity by DHAP treatment may be explained in the following ways. First, in the endothelium, the phosphatidylinositol 3-kinase(PI3K)–Akt signal pathway mostly acts as a positive regulator of eNOS, which generates NO through the oxidation of l-arginine (Morello et al., Citation2009). In obesity, the eNOS–NO pathway is down-regulated by blocking the PI3K-Akt signal pathway induced by high FFA levels (Kawakami, Citation2008; Li et al., Citation2011; Varma, Citation2005). In our study, DHAP treatment could reduce plasma FFA levels and enhance eNOS–NO production in obese rats. Furthermore, previous studies have shown that in obesity high FFA levels and chronic inflammation can cause endothelial dysfunction by increasing oxidative stress, which can increase oxidation of tetrahydrobiopterin, leading to l-arginine producing superoxide rather than NO by eNOS uncoupling (Hou et al., Citation2014). In our study, we found that treatment of obese rats with DHAP reduced circulating TNF-α and MDA, and also reduced NF-κB activation and superoxide anion production in the aorta, which indicated that DHAP could reduce oxidative stress and inflammation activity. Finally, adiponectin has been regarded as a beneficial adipokine protecting endothelial function in obesity (Wang et al., Citation2012).
Hypoadiponectinemia has also been proved to be closely related to endothelial dysfunction and is thought to be an independent risk factor for cardiovascular disease. Adiponectin reverses endothelial dysfunction through enhancing eNOS–NO production and decreasing NO inactivation by blocking superoxide production. DHAP was unexpectedly found to increase serum adiponectin levels in this study. This beneficial effect of DHAP and its specific mechanism requires further evaluation in future studies.
In summary, the current study suggests that DHAP is likely able to reduce the risk of cardiovascular disease, as it improves endothelial function in obese rats. This beneficial effect may be associated with up-regulation of the eNOS–NO signal pathway by improving lipid metabolism and reducing oxidative stress and inflammation activity. These novel findings may help to increase the possibility of DHAP being used to combat vascular dysfunction in obesity.
Declaration of interest
All authors declare that there are no known conflicts of interests related to this publication.
References
- Calabro P, Golia E, Maddaloni V, et al. (2009). Adipose tissue-mediated inflammation: The missing link between obesity and cardiovascular disease. Intern Emerg Med 4:25–34
- Chen J, Liu L, Hou R, et al. (2011). Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett 308:144–51
- Dai-juan Z. (2009). Effect of 3, 4-dihydroxyacetophenone (DHAP) on the expression of TLR4 in atherosclerotic plaque of mice. China Pharm 33:2567–9
- Dai-juan Z. (2010). Preventive effect of 3, 4-dihydroxyacetophenone on atherosclerosis and role of visfatin expression. Chin J Pathophysiol 26:1700–3
- Davel AP, Wenceslau CF, Akamine EH, et al. (2011). Endothelial dysfunction in cardiovascular and endocrine-metabolic diseases: An update. Braz J Med Biol Res 44:920–32
- Fu JY, Qian LB, Zhu LG, et al. (2011). Betulinic acid ameliorates endothelium-dependent relaxation in l-NAME-induced hypertensive rats by reducing oxidative stress. Eur J Pharm Sci 44:385–91
- Gupte SA, Kaminski PM, Floyd B, et al. (2005). Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288:H13–21
- Han L, Yu Y, Sun X, Wang B. (2012). Exendin-4 directly improves endothelial dysfunction in isolated aortas from obese rats through the cAMP or AMPK-eNOS pathways. Diabetes Res Clin Pract 97:453–60
- Hou N, Han F, Wang M, et al. (2014). Perirenal fat associated with microalbuminuria in obese rats. Int Urol Nephrol 46:839–45
- Hu Y, Ehli EA, Kittelsrud J, et al. (2012). Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 19:861–7
- Huang Y. (1996). The effects of 3,4-dihydroxyacetophynone on the activity of nitric oxide synthetase in placental vascular endothelial cells and smooth muscle cells and on the level of endothelin-1 in plasma from pregnancy-induced hypertension patients. Zhonghua Fu Chan Ke Za Zhi 31:667–9
- Jia S, Hu C. (2010). Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules 15:1873–81
- Jiang X, Yang F, Tan H, et al. (2005). Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol 25:2515–21
- Jobgen WS, Jobgen SC, Li H, et al. (2007). Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 851:71–82
- Kawakami A. (2008). Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation 118:731–42
- Krishna SM, Seto SW, Moxon JV, et al. (2012). Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am J Pathol 181:706–18
- Li H, Li H, Bao Y, et al. (2011). Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-kappaB pathway in rat aorta. Int J Cardiol 152:218–24
- Li S, Wu Y, Yu G, et al. (2014). Angiotensin II receptor blockers improve peripheral endothelial function: A meta-analysis of randomized controlled trials. PLoS One 9:e90217
- Lteif AA, Han K, Mather KJ. (2005). Obesity, insulin resistance, and the metabolic syndrome: Determinants of endothelial dysfunction in whites and blacks. Circulation 112:32–8
- Mather KJ. (2001). Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 37:1344–50
- Mellor DD, Madden LA, Smith KA, et al. (2013). High-polyphenol chocolate reduces endothelial dysfunction and oxidative stress during acute transient hyperglycaemia in Type 2 diabetes: A pilot randomized controlled trial. Diabetic Med 30:478–83
- Mohazzab-H KM, Kaminski PM, Fayngersh RP, Wolin MS. (1996). Oxygen-elicited responses in calf coronary arteries: Role of H2O2 production via NADH-derived superoxide. Am J Physiol 270:H1044–53
- Morello F, Perino A, Hirsch E. (2009). Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 82:261–71
- Sun X, Yu Y, Han L. (2013). High FFA levels related to microalbuminuria and uncoupling of VEGF-NO axis in obese rats. Int Urol Nephrol 45:1197–207
- Sun XD, Yu YR. (2014). The protective effects of rosiglitazone on kidney in diet-induced obese rats. Sichuan Da Xue Xue Bao Yi Xue Ban 45:24–8, 33
- Varma S. (2005). Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol 289:H1744–55
- Wang B, Yu Y, Han L. (2012). Adiponectin improves endothelial dysfunction caused by elevated FFAs levels, partially through cAMP-dependent pathway. Diabetes Res Clin Pract 97:119–24
- Wang J, Toba H, Morita Y, et al. (2013b). Endothelial dysfunction, macrophage infiltration and NADPH oxidase-dependent superoxide production were attenuated by erythropoietin in streptozotocin-induced diabetic rat aorta. Pharmacology 91:48–58
- Wang JS, Yin HJ, Guo CY, et al. (2013a). Influence of high blood glucose fluctuation on endothelial function of type 2 diabetes mellitus rats and effects of Panax quinquefolius aponin of stem and leaf. Chin J Integr Med 19:217–22
- Wazir F, Wang D, Hu Q. (2004). Effects of 3,4-dihydroxyacetophenone on cytosolic calcium in pulmonary artery endothelial and smooth muscle cells during acute hypoxia. J Huazhong Univ Sci Technol Med Sci 24:550–1
- Wu P. (2001). Progress in studies on mechanism of promoting circulation and removing blood stasis of 3,4-hydroxyacetophenone. Chin Trad Herb Drugs (Chinese) 32:277
- Yu YR, Li HL, Zhang XX. (2008). Effects of free fatty acids on nitric oxide synthase activity and mRNA expression in endothelial cell of SD rat aorta. Sichuan Da Xue Xue Bao Yi Xue Ban 39:193–6
- Zhang D. (2013). Effects of 3, 4-dihydroxyacetophenone on the hypercholesterolemia-induced atherosclerotic rabbits. Biol Pharm Bull 36:733–40