Abstract
Context: Rumex vesicarius L. (Polygonaceae), an edible plant, is reported to have many bioactive phytochemicals, especially flavonoids and anthraquinones with antioxidant and detoxifying properties.
Objective: This study evaluated the methanolic extract of R. vasicarius (MERV) for hepatoprotective activity in rats against CCl4-induced liver damage.
Materials and methods: The whole plant extract was prepared and investigated for its hepatoprotective activity. Rats were pretreated with MERV (100 and 200 mg/kg, p.o.) for 7 d prior to the induction of liver damage by CCl4. Animals were then sacrificed 24 h after CCl4 administration for the biochemical (AST, ALT, and ALP activity in serum; lipid peroxidation (LPO) and glutathione (GSH) levels in liver tissue) and histological analyses.
Results: CCl4-induced hepatotoxicity was confirmed by an increase (p < 0.05) in serum AST (4.55-fold), ALT (3.51-fold), and ALP (1.82-fold) activities. CCl4-induced hepatotoxicity was also manifested by an increase (p < 0.05) in LPO (3.88-fold) and depletion of reduced glutathione (3.14-fold) activity in liver tissue. The multiple dose MERV administration at 200 mg/kg showed promising hepatoprotective activity as evident from significant decrease levels of serum AST (230.01 ± 13.21), serum ALT (82.15 ± 5.01), serum ALP (504.75 ± 19.72), hepatic LPO (3.38 ± 0.33), and increased levels of hepatic glutathione (0.34 ± 0.04) towards near normal. Further, biochemical results were confirmed by histopathological changes as compared with CCl4-intoxicated rats.
Discussion and conclusion: The results obtained from this study indicate hepatoprotective activity of Rumex plant against CCl4-induced liver toxicity; hence, it can be used as a hepatoprotective agent.
Introduction
The liver is a vital organ and plays a very important role in the maintenance of metabolic function and detoxification from the exogenous and endogenous challenges, such as xenobiotic, drugs, viral infection, and chronic alcoholism. Every year about 20 000 deaths are due to liver disorders (Sharma & Sharma, Citation2010). Liver damage is always associated with cellular necrosis, increase in tissue liquid peroxidation, and depletion in the glutathione (GSH) levels in tissue. In addition, serum levels of many biochemical markers such as AST, ALT, ALP, and billirubin are elevated (Mascolo et al., Citation1998; Mossa et al., Citation1991). Drug-induced liver injury is one of the most common causative factors that poses a major clinical and regulatory challenge (Russmann et al., Citation2009).
Recently there has been a large volume of work aimed at scientific validation of the efficacy of herbal drugs used in the traditional medicine. Modern medicine does not have suitable answers for many health conditions such as liver disorder, asthma, cardiovascular disorder, etc. Many active plant extracts are frequently utilized to treat a wide variety of clinical diseases including liver disease (Chattopadhyay, Citation2003). Therefore, searching for effective and safe drugs for liver disorders continues to be an area of interest. In our field survey, we found that a widely grown plant Rumex vesicarius L. (Polygonaceae), an annual herb, is a wild edible plant eaten fresh or cooked (Mostafa et al., Citation2011). It grows throughout desert and semi-desert areas of North Africa, Asia, and Australia (Rechinger, Citation1984). In Saudi Arabia, R. vesicarius is widely used as food, as a medicinal herb, and as an antidote to scorpion stings (Al-Yahya et al., Citation1990). The leaves are used as asperient, diuretic, and considered as an antidote for snake venom; the seeds are considered as an antidote for scorpion venom (Joshi, Citation2000). Antibacterial and antioxidant activities of R. vesicarius have been performed (Mostafa et al., Citation2011; Panduraju et al., Citation2009). Despite its importance, only few studies have been conducted on R. vesicarius. However, there are no scientific bases or reports in the modern literature regarding its usefulness as a hepatoprotective agent. Thus, the present study was conducted to evaluate the hepatoprotective activity of the methanol extract of R. vesicarius (MERV) by using CCl4-induced hepatic damage in male Wistar albino rats.
Materials and methods
Chemicals
All the chemicals and solvents used were of highest purity, commercially available, and analytical grade.
Plant material
Whole plants of R. vesicarius were collected in the Spring of 2012 from the Al-Kharj area, Kingdom of Saudi Arabia. The plants were cleaned, dried under shade, ground to a coarse powder, and stored in an air-tight container at 25 °C for further use. Plant specimens were botanically identified and authenticated by expert taxonomist Mr. Osman Ali Elmakki. A voucher specimen was deposited at the herbarium of the Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Extraction
Air-dried and fine powder of R. vesicarius was subjected to extraction with methanol by using the percolation method, three times, with an interval of 24 h each. The container with its contents was sealed and kept for a period of 4 d accompanying occasional shaking and stirring. The extract was filtered in the Buchner funnel and the filtrate was concentrated with a rotary evaporator at bath temperature not exceeding 40 °C to have a gummy concentrate of greenish black extract (yield approx. 16.55%).
Animals
Eight week old adult male Wistar rats (150–200 g) were obtained from the Animal House Facility of the College of Pharmacy, Salman Bin Abdulaziz University, Al-Kharj and were housed in a ventilated room at 25 ± 2 °C under a 12-h light/dark cycle. The animals were acclimatized for 1 week before the study and had free access to standard laboratory feed and water ad libitum.
Hepatoprotective activity
Rats were randomly divided into six groups (N = 6 animals/group). Groups I (normal control) and II (toxic control) were given normal saline orally (2 ml/kg) for 7 d. Groups III, IV, and V were treated with R. vesicarius in a dose of 100, 200, and 200 mg/kg body weight, respectively, orally for 7 d. Group VI was administered with silymarin (100 mg/kg) for 7 d. On the 7th day, 30-min post-dose of normal saline, extract, and silymarin administration, animals of groups II, III, IV, and VI received CCl4 at a dose of 1 ml/kg (1:1 v/v of CCl4 in olive oil) orally. Group V was not treated with CCl4, and serves as the Rumex per se group. On the 8th day, after an overnight fasting, the rats were anesthetized with ether and blood samples were collected in tubes for biochemical analysis by the cardiac puncture method. Blood samples were centrifuged for 10 min at 3000 rpm to separate the serum. After blood collection, the animals were sacrificed using ether anesthesia and the liver tissue was harvested for biochemical and histopathological studies.
Serum AST and ALT levels were determined by the method of Reitman and Frankel (Citation1957). Each substrate (0.5 ml) (2 mM α-ketoglutarate and either 200 mM α-l-alanine or l-aspartate) was incubated for 5 min at 37 °C in a water bath. Serum (0.1 ml) was then added and the volume was adjusted to 1.0 ml with sodium phosphate buffer. The reaction mixture was incubated for exactly 30 min and 60 min for ALT and AST, respectively. Then, to the reaction mixture, 0.5 ml of DNPH (1 mM) was added and left for another 30 min at room temperature. Finally, the color was developed by the addition of 5.0 ml of NaOH (0.4 N) and the product was read at 505 nm. Serum alkaline phosphatase was estimated following the method of Bessey et al. (1976).
Liver tissue (1 g) was homogenized in nine volumes of phosphate buffer and centrifuged at 12 000 × g for 30 min at 4 °C. The supernatant was collected and used for the estimation of lipid peroxidation (LPO) (Esterbauer & Cheeseman, Citation1990) and GSH (Jollow et al., Citation1974).
The liver samples from different groups were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Sections of a thickness of about 5 μm were cut and stained with hematoxylin and eosin. Then, the samples were observed under microscope for degeneration, fatty changes, necrotic changes, and evidence of hepatotoxicity if any, by an experienced histopathologist.
Statistical analysis
All values were expressed as mean ± SE (n = 6 in each group). One-way ANOVA was applied to test for the significance of biochemical data of the different groups. Significance is set at p < 0.05.
Results
Administration of CCl4 (1 ml/kg, p.o.) induced a marked increase in the serum hepatic enzyme levels, AST, ALT and ALP, as compared with normal controls indicating liver damage. Pretreatment of the rats with MERV for 7 d prior to CCl4 administration caused a significant reduction in the levels of AST, ALT, and ALP in serum (p < 0.01), which were almost comparable with silymarin ().
Table 1. Effect of the methanolic extract of R. vesicarius on AST, ALT, and ALP levels in serum.
shows that administration of CCl4 results in a marked increased in LPO levels and a decrease in the GSH level in liver homogenate. The toxic effect of CCl4 was controlled in the animals treated with MERV (100 and 200 mg/kg, p.o.) by restorating LPO and GSH levels in liver.
Table 2. Effect of the methanolic extract of R. vesicarius on LPO and GSH levels in liver.
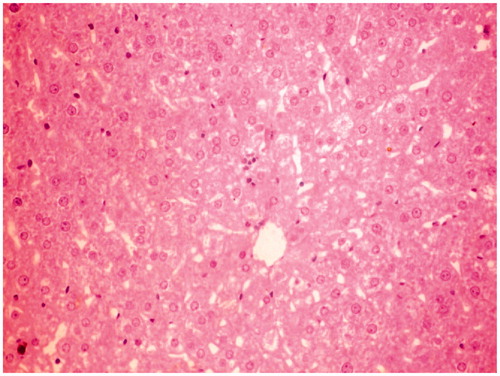
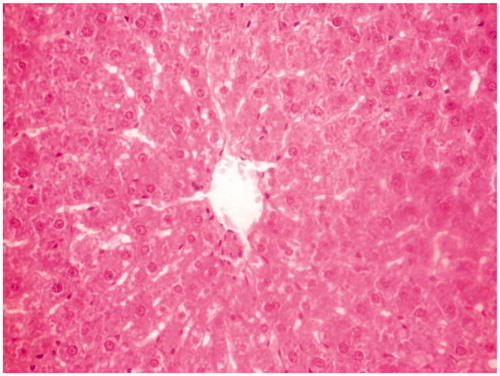
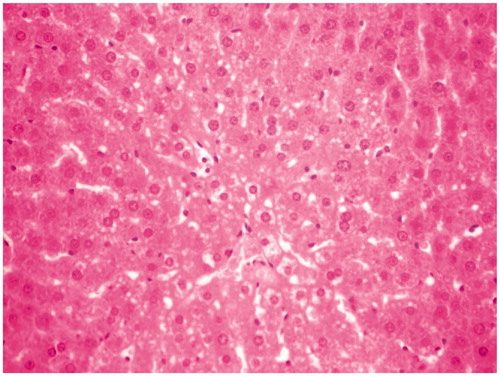
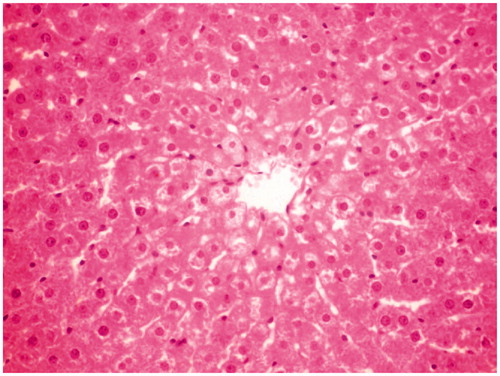
Histological studies of the liver tissue of control and treated animals also confirmed the hepatoprotective effect of the MERV. The histological architecture of liver sections of normal rats showed normal cellular architecture with distinct hepatic cells and sinusoidal space (). In the liver section of rats intoxicated with CCl4, there was disarrangement and degeneration of normal hepatic cells with intense centrilobular necrosis, cloudy swelling, and fatty degeneration of hepatocytes (). The histopathological profile of the rats treated with MERV showed no visible changes, confirming the safety of the extract at selected dose ( and ). Rats treated with MERV only in a dose of 200 mg/kg showed normal pattern of histological architecture (). While silymarin treatment in CCl4-intoxicated rats showed less disarrangement and degeneration of hepatocytes, indicating marked regeneration activity ().
Figure 1. Normal control group rat liver section showing normal cellular architecture with distinct hepatic cells and sinusoidal space.
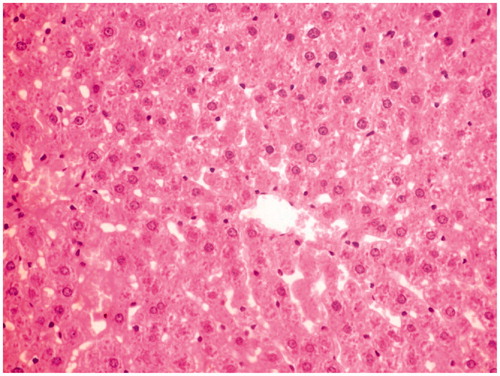
Figure 2. Toxic control group rat liver section showing disarrangement and degeneration of normal hepatic cells with intense centrilobular necrosis.
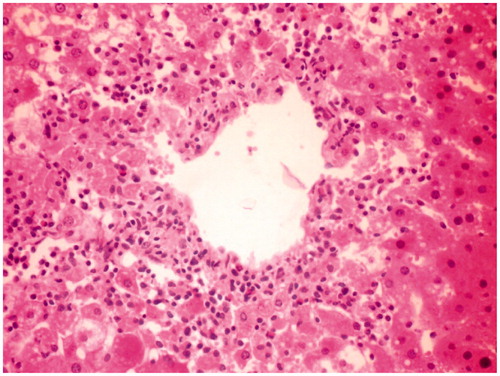
Figure 3. CCl4 + MERV-100 mg/kg-treated group rat liver section showing moderate hepatoprotective activity.
Discussion
In general, CCl4 has been extensively used as a model to study hepatic damage and used as an indicator of protective activity of newly discovered drugs (Clawson, Citation1989). In the present study, CCl4 was used as a toxic agent and the hepatoprotective effect of MERV was studied against the CCl4-induced hepatotoxicity. The extent of toxicity was estimated by biochemical markers like AST, ALT, and ALP levels in serum; LPO and GSH levels in liver; and histopathological studies of liver tissues. The present study reports the potential hepatoprotective activity of rumex against hepatic injury produced by CCl4 in Wistar albino rats.
It is well known that CCl4 is biotransformed by the Cyt P450 system to produce a trichloromethyl-free radical, which further undergoes reduction to form a trichloromethylperoxyl radical, which leads to LPO and finally leads to cell death (Clawson, Citation1989; Recknagel et al., Citation1989). Therefore, leakage of large quantities of enzymes into the blood stream is often associated with massive necrosis of the liver (Ha et al., Citation2005; Rees & Spector, Citation1961). Our study corroborates previous findings and elicited a significant increase in the activities of serum enzymes (AST, ALT, and ALP in serum); hepatic LPO levels and decreased levels of antioxidant marker (GSH) on exposure of rats to CCl4, indicating considerable hepatocellular injury (Ha et al., Citation2005; Rawat et al., Citation1997). GSH is an important endogenous antioxidant system, known to have key functions in protective mechanism. Excessive production of CCl3 radical results in the oxidation of reduced glutathione GSSG and, therefore, the GSH levels in CCl4-treated groups are decreased as compared with the normal group.
Treatment with MERV reversed the changes produced by CCl4. MERV significantly decreased hepatic LPO levels and increased the levels of above-mentioned biochemical markers to near normal levels. Pretreatment with MERV also increased the levels of GSH. Hence, the hepatoprotective effect of MERV may be due to the prevention of depletion in the GSH levels in tissue.
As expected, silymarin maintained normal architecture with minimal injuries and better protection than Rumex extract alone. Chronic treatment with silymarin also illustrated marked recovery in serum enzyme levels and oxidative parameters in liver tissue. Photomicrograph clearly reveals that the MERV works as a hepatoprotectant. The histopathological profile reveals a major damage in the toxic control group. Thus, it clearly states that, toxicity is due to either of the above mechanisms such as depletion of glutathione store or free radical generation or LPO. Simultaneous treatment of the extract and silymarin with CCl4 exhibits less damage to the hepatic cells as compared to the rats treated with the toxic group.
The MERV showed a significant result. Previous reports show flavonoids and steroids are responsible for hepatoprotective effect (Khatri et al., Citation2009; Panigrahi et al., Citation2010; Pattanayak et al., Citation2011; Singh & Gupta, Citation2011). The plant contains many bioactive substances such as flavonoids (vitexin, isovitexin, orientin, and isorientin), anthraquinones, particularly in roots (emodin and chrysophanol), carotenoids, vitamins (especially vitamin C), proteins, lipids, and organic acids. Flavonoids present in the plant may be responsible for the marked hepatoprotective effects, as observed in the present study.
Conclusion
With the aid of enzyme levels and histopathological studies of rat liver, we can conclude that MERV has potent hepatoprotective action against CCl4-induced hepatic damage in rats. The present study, thus, justifies the traditional use of R. vesicarius in the treatment of liver diseases and also points out that R. vesicarius warrants future detailed investigation as a promising hepatoprotective agent. However, the exact mechanism(s) and the active compound(s) involved in these effects are still not clear and need to be clarified in future studies.
Declaration of interest
The authors declare that they have no conflict of interest. The authors are thankful to Deanship of Scientific Research, Salman bin Abdulaziz University, Al-Kharj Saudi Arabia, for providing the funds to carry out this study under research Grants no. 4/H/1432.
References
- Al-Yahya MA, Al-Meshal IA, Mossa JS, et al. (1990). Saudi Plants – A Phytochemical and Biological Approach. Riyadh: General Directorate of Research Grants Programs, KACST
- Bessey OA, Lowery DH, Brock MJ. (1964). A method for the rapid determination of alkaline phosphatase with five cubic meters of serum. J Biol Chem 164:321–9
- Chattopadhyay RR. (2003). Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J Ethnopharmacol 89:217–19
- Clawson GA. (1989). Mechanism of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res 8:104–12
- Esterbauer H, Cheeseman KH. (1990). Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–21
- Ha KT, Yoon SJ, Choi DY, et al. (2005). Protective effect of Lycium Chinese fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol 96:529–35
- Jollow DJ, Mitchell JR, Zampagline N, Gillette JR. (1974). Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4-bromobenzene as the hepatic metabolite. Pharmacology 11:151–69
- Joshi SG. (2000). Medicinal Plants. New Delhi, Kolkata: Oxford and IBH Publishing Co. Pvt. Ltd
- Khatri A, Garg A, Agrawal SS. (2009). Evaluation of hepatoprotective activity of aerial part of Tephrosia purpuria L. and stem bark of Tecomella undulata. J Ethnopharmacol 122:1–5
- Mascolo N, Sharma R, Jain SC, Capasso F. (1998). Ethnopharmacology of Calotropisprocera flowers. J Ethnopharmacol 22:211–44
- Mossa JS, Tariq M, Mohsin A, et al. (1991). Pharmacological studies on aerial parts of Calotropisprocera. Am J Clin Med 19:223–31
- Mostafa HAM, El-Bakry AA, Eman AA. (2011). Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L. (Polygonaceae). Int J Pharm Pharm Sci 3:109–18
- Panduraju T, Rao PRS, Kumar VS. (2009). A study on antimicrobial activity of Rumex vesicarius L. Int J Pharm Tech 1:21–5
- Panigrahi BB, Panda PK, Patro VJ. (2010). Comparative hepatoprotective activity of different extracts of spirulina against CCl4 induced liver damage in rats. Inter J Pharma Sci Rev Res 4:200–202
- Pattanayak S, Nayak SS, Panda DP, et al. (2011). Hepatoprotective activity of crude flavonoids extract of Cajanus scarabaeoides (L) in paracetamol intoxicated albino rats. Asian J Pharm Biol Res 1:22–7
- Rawat AK, Mehrotra SC, TripathiShome U. (1997). Hepatoprotective activity of Boerhaavia diffusa L. roots a popular Indian ethnomedicine. J Ethnopharmacol 56:61–6
- Rechinger KH. (1984). Rumex (Polygonaceae) in Australia: A reconsideration. Nuytsia 5:75–122
- Recknagel RO, Glende EA, Dolak JA, Waller RL. (1989). Mechanism of carbon tetrachloride toxicity. Pharmacol Ther 43:139–54
- Rees KR, Spector WG. (1961). Reversible nature of liver cell damage due to carbon tetrachloride as demonstrated by the use of Phenergan. Nature 189:821–9
- Reitman S, Frankel SA. (1957). Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol 28:56–63
- Russmann S, Kullak-Ublick GA, Grattagliano I. (2009). Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem 16:3041–53
- Sharma B, Sharma UK. (2010). Hepatoprotective activity of some indigenous plants. Int J Pharm Tech Res 2:568–72
- Singh D, Gupta RS. (2011). Hepatoprotective activity of methanol extract of Tecomella undulate against alcohol and paracetamol induced hepatotoxicity in rats. Life Sci Med Res 26:1–8