Abstract
Context: Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson, Asteraceae (yacon) roots are a natural product recognized by the traditional medicine to treat diabetes-related problems. There are no reports concerning the potential of yacon roots to reduce oxidative stress and ameliorate diabetes complications in diabetic animals.
Objective: This work analyzes the in vivo antioxidant activity and beneficial effects of yacon roots, using a model of streptozotocin-induced diabetes in rats.
Materials and methods: Lipid peroxidation and other indicators of oxidative stress were determined in liver and kidney homogenates from non-diabetic rats, untreated diabetic rats, and diabetic rats treated orally with yacon flour (340 mg fructooligosaccharide/kg/d) as a diet supplement for 90 d. Biochemical parameters were determined in liver, kidney, and blood at the end of the experimental period.
Results: Yacon supplementation to diabetic rats produced a significant decrease in malondialdehyde levels in both liver (−30.97%) and kidney (−19.15%). Hepatic superoxide dismutase and catalase activities were significantly lower in diabetic-treated rats (−13.46 and −64.33%, respectively) compared with diabetic controls. Similar results were observed in kidney. The treatment of diabetic rats produced an increase of glutathione peroxidase and glutathione levels in liver (172.50 and 35.91%, respectively) and kidney (177.78 and 57.76%, respectively). Plasma cholesterol and triacylglycerol levels and liver fatty acid composition, which were altered in diabetic rats, reverted back to nearly normal with yacon treatment.
Conclusions: These results indicate that yacon root flour is a potential diet supplement with high in vivo antioxidant activity.
Introduction
Diabetes mellitus is one of the most common metabolic diseases in the world today, the main causes of mortality in diabetic patients being micro- and macrovascular complications. High blood glucose levels, which are characteristic of diabetes, can damage heart, eyes, kidneys, and peripheral nerves as well as other organs.
In addition to hyperglycemia, diabetes-associated metabolic disorders include dyslipidemia and excessively high levels of free radicals, resulting in peroxidation of membrane lipids and non-enzymatic protein glycation that lead to oxidative damage of cell membranes.
Glycated or oxidized low-density lipoproteins (LDL) impair the activities of mitochondrial respiratory chain complex enzymes, which leads to mitochondrial dysfunction and excess reactive oxygen species (ROS) production (Shen, Citation2012). There is considerable evidence that chronic hyperglycemia is the main cause of free radical-mediated lipid peroxidation (Likidlilid et al., Citation2010). Free radicals tend to attack different kinds of unsaturated fatty acids and lipids such as LDL. Oxidized products cause the start and the development of diabetes (Valko et al., Citation2007).
Diabetes is usually accompanied by a sharp reduction in endogenous antioxidant defenses (Oberley, Citation1988). Thus, in the absence of an appropriate response from antioxidant mechanisms, redox imbalance causes the activation of intracellular signaling pathways, transcription factors, protein kinase C, and oxidative stress. It has been demonstrated that reduced glutathione (GSH) is an essential cofactor for many antioxidant enzymes and is a direct free-radical scavenger, playing a crucial role in cellular antioxidant defense (Hayes et al., Citation2005; Maritim et al., Citation2003). In the light of the above, the management and the control of diabetes may require very high supplementation with functional foods or bioactive compounds that act as free radical scavengers and can, therefore, lower the risk of diabetic complications.
Several studies have indicated that many medicinal plants are a good source of natural antioxidant compounds with inhibitory activity on oxidative-induced cell damage (Haslam, Citation1996; Parejo et al., Citation2002).
The antioxidant properties of medicinal plants depend on the plant, its variety, climatic factors, and geographical regions of growth, degree of ripeness, cultivation practices, and many other factors such as processing. Yacon, Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson (Asteraceae), is a traditional Andean crop. Its roots are used by the local population as an important dietary component because of its sweet taste and high water content (Grau & Rea, Citation1997). Yacon tubers contain β (2 → 1)-fructooligosaccharides (FOS) with a low degree of polymerization (3–10 fructoses) as storage carbohydrates (Goto et al., Citation1995). Moreover, like all members of the Asteraceae family, yacon contains considerable amounts of phenolic compounds. Five caffeic acid derivatives were found in yacon roots. Two of these were chlorogenic acid (3-caffeoylquinic acid and 3,5-dicaffeoylquinic acid) and the other family compounds were 2,4- or 3,5-dicaffeoylaltraric acid, 2,5-dicaffeoylaltraric acid, and 2,3,5- or 2,4,5-tricaffeoylaltraric acid (Takenaka et al., Citation2003). Yacon roots also accumulate significant quantities of potassium and tryptophan (Yan et al., Citation1999).
The beneficial activities of yacon roots on diabetes-associated alterations have been widely demonstrated. In previous work (Genta et al., Citation2005), we found that subchronic-rich FOS-yacon root dietary supplementation resulted in significantly reduced post-prandial serum triacylglycerol levels in normal rats with no negative response, toxicity, or adverse nutritional effects. Even more, we determined that the administration of yacon root flour to diabetic rats had a significant lipid-lowering effect, mainly a decrease in plasma triacylglycerol and very low-density lipoprotein levels, which are regarded as important factors in the development of diabetic macrovascular disease (Habib et al., Citation2011). Because of its low caloric content and high proportion of non-digestible oligosaccharides with soluble fiber properties, in recent years, there has been increased interest in yacon as a natural dietary supplement for weight reduction in patients with metabolic syndrome or diabetes (Genta et al., Citation2009).
In view of the above findings, investigation of the in vivo antioxidant activity of yacon constituents could be very important to increase its dietary and pharmacological values. Consequently, the aim of the present work was to find out if a subchronic treatment with yacon flour as a dietary supplement could improve antioxidant defense mechanisms and reduce lipid peroxidation in STZ-diabetic rats and, in this way, contribute to the prevention of diabetic complications.
Materials and methods
Plant material and root flour preparation
Smallanthus sonchifolius (yacon) (Clone LIEY97-1) roots were obtained from the 2007 harvest of an experimental field at the Instituto de Ecología Regional, Horco Molle, province of Tucumán, 26° 47′ S, 65° 19′ W Argentina. Voucher specimens were deposited in the herbarium of “Instituto Miguel Lillo”, San Miguel de Tucumán, Tucumán, Argentina (no. 607173LIL).
Roots were peeled, sliced, and dried at 40 °C in a forced air circulation oven. Partially dried slices were subjected to 60 °C for 2 h to reduce the water content to a minimum and then milled. The flour obtained was stored at 4 °C until required.
Animals
Adult male Wistar rats weighing 150–220 g were used for the study. They were obtained from the colony bred at the Department of Developmental Biology, INSIBIO (CONICET-UNT), Tucumán, Argentina. All animals were maintained under standard laboratory conditions [temperature (23 ± 1 °C) and humidity (approximately 60%)] with a 12 h day/night cycle. The animals were given free access to a standard diet obtained from a commercial source (Standard Food – Asociación de Cooperativas Argentinas – S.E.N.A.S.A. N° 2706). Water was also available without restriction. There were no known contaminants in the food or water that could interfere with the results of the study. All the experimental procedures were in strict accordance with institutional animal ethical committee guidelines for the care and use of laboratory animals.
Experimental induction of diabetes
Diabetes was induced in overnight-fasted rats by intraperitoneal injection of streptozotocin (STZ, Sigma Chemical Company, St. Louis, MO) at a dose of 45 mg/kg body weight dissolved in 10 mM cold sodium citrate buffer (pH 4.5). STZ can induce fatal hypoglycemia as a result of massive pancreatic insulin release, so in order to prevent this hypoglycemic effect, the rats were given 5% dextrose solution after 6 h of STZ administration for the next 24 h. Control rats were injected with citrate buffer alone. Diabetes induction was verified after 48 h and the animals were allowed 10 d for the stabilization of blood glucose levels. Only rats with glycemia >350 mg/dl 10 d after STZ treatment were considered diabetic and used for the experiments. Treatment with yacon flour was started on the 15th day after STZ injection, which was considered as the first day of treatment.
Experimental design for antioxidant activity
The rats were divided into three different groups of six animals each: NC group (non-diabetic control rats), DC group (diabetic control rats), and DY340 group (diabetic rats treated with yacon flour equivalent to 340 mg FOS/kg body weight).
The feeding technique used involved daily dietary supplementation with yacon root flour over a 90-d period. The NC and DC groups were given the standard diet, whereas the yacon-fed diabetic rats’ group received the same diet plus a yacon flour tablet containing the desired FOS intake levels (a dose of 340 mg FOS/kg body weight/d). Controlled administration was achieved as each animal was trained to eat the yacon tablet every day at approximately 07:00 p.m. before food administration. The standard diet was available ad libitum. At the end of the experimental period, the animals were fasted overnight and euthanized with ketamine (150 mg/kg body weight). Blood samples were collected in fasting (12 h fast) conditions. Serum was separated by centrifugation at 5000 rpm for 10 min and biochemical parameters were analyzed. Liver and kidney were surgically removed, washed with ice-cold saline solution, and stored immediately at −70 °C until use for biochemical assays.
Tissue preparation
Weighed tissue samples were homogenized on ice using a sonic dismembrator (Vibra-Cell, Sonics and Materials, Inc., Newtown, CT) in an appropriate 0.1 M phosphate buffer, pH 7.4, containing 1 mM EDTA (1:40 w/v). The homogenates were centrifuged at 18 000 × g for 15 min at 4 °C. The supernatants were immediately used for enzyme assay and protein estimation by the method of Lowry et al. (Citation1951), using bovine serum albumin as a standard.
Biochemical analysis
All the enzymes and biochemical reagents were purchased from Sigma (St Louis, MO). Blood glucose was measured with an Accu-chek® Active tester (Roche Diagnostics GnbHD-68 298, Mannheim, Germany) based on the glucose dye oxidoreductase mediator reaction.
Plasma insulin was determined by an enzyme-linked immune-sorbent assay (Rat/Mouse Insulin ELISA Kit, Linco Research, Inc., St. Charles, MO). Blood for clinical chemistry studies was collected into tubes without anticoagulant, allowed to clot, and centrifuged to obtain the serum. An EFM method (Hitachi 902, Roche Diagnostics, Mannheim, Germany) was employed to measure the following parameters: creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), and triacylglycerol (TG).
Free fatty acid and cholesterol in liver homogenates
Total lipid was extracted from the liver tissue according to the modified method of Folch et al. (Citation1957). The hydrolysis of glycerides and the obtainment of fatty acids methyl esters for identification of fatty acids by GC-MS were performed in two successive operations. During the first step, the lipids were extracted by homogenizing the tissue with 2:1 chloroform–methanol (v/v) and filtering the homogenate. During the second step, derivatization of fatty acids was performed according to the method described by Chin et al. (Citation1992), where lipids were converted to methyl esters by adding 15 ml of methanol solution of KOH 2 N.
Determination of oxidation products
Lipid peroxidation was assessed by determining the levels of malondialdehyde (MDA) in liver and kidney homogenates by the spectrophotometric method of Beuge and Aust (Citation1978). MDA has been identified as the product of lipid peroxidation that reacts with the thiobarbituric acid to give a red species absorbing at 535 nm. The MDA concentration of the sample was calculated with an extinction coefficient of 1.56 × 105 M−1 cm−1. The results were expressed as nmoles of MDA formed/mg protein.
Estimation of non-enzymatic antioxidants
GSH was measured as described by Ellman (Citation1959) using the 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent. Tissue homogenates were deproteinized by the addition of 1 M HClO4 containing 2 mM EDTA. The acid extracts were centrifuged at 5000 × g for 5 min to remove protein. An aliquot of the deproteinized extract was neutralized with a solution containing 2 M KOH and 0.3 M N-morpholinopropanesulfonic acid (MOPS). The neutralized sample (1 ml) was added to 0.5 ml of Ellman's reagent. Development of yellow color was read at 412 nm in a spectrophotometer. A series of GSH standards were treated in a similar way along with a blank containing buffer. GSH levels were expressed as mg GSH/100 g of tissue.
Assessment of enzymatic antioxidants
Superoxide dismutase (SOD) activity was determined using the method of Ukeda et al. (Citation1997). This is an indirect method that involves the generation of superoxide anions using xanthine oxidase/nitroblue tetrazolium salt (XO/NBT). The superoxide anion radical () is generated by the xanthine/XO system, and then detected with a chromogenic solution (NBT). In the presence of SOD, superoxide anion concentration is reduced, thereby decreasing the colorimetric signal. About 2.5 ml of 50 mM sodium carbonate buffer (pH 9.4 and 10.2) was added to 0.1 ml to each of the followings: 3 mM xanthine, 3 mM EDTA, 0.75 mM NBT solution, 15% BSA solution, and a sample solution containing SOD or water (blank). The reaction was initiated by the addition of XO. The changes in the absorbance at 560 nm were monitored at 25 °C for 20 min. In this assay, one unit of SOD (U SOD) is defined as the amount required to inhibit 50% NBT photo-reduction. Activity was expressed as U SOD/mg protein.
Catalase (CAT) activity was measured according to the modified method of Aebi (Citation1974). The method involves the decomposition of H2O2 which can be monitored directly by the decrease in the absorbance at 240 nm. About 20 μl of tissue homogenate (containing approximately 1.5 mg of protein) was added to 1 ml of 0.1 M phosphate buffer pH 7 containing 100 mM H2O2. The rate of H2O2 decomposition was followed spectrophotometrically at 240 nm for 1 min. Enzyme activity was calculated using a molar extinction coefficient of 0.043 M−1 cm−1 and expressed in International Units (IU) μmoles H2O2 destroyed/min/mg protein, at 25 °C.
Glutathione peroxidase (GPx) activity was assayed by a modified method of Flohé and Günzler (Citation1984). The GSSG formed during the reaction of GPx was instantaneously and continuously reduced by an excess of glutathione-reductase (GR). This reduction was monitored spectrophotometrically by the concomitant oxidation of NADPH to NADP. The procedure was as follows: 500 μl of 0.1 M phosphate buffer (pH 7.0) containing 1 mM EDTA, 100 μl of sample treated with 1 mM sodium azide, 100 μl of glutathione reductase 0.24 U/ml, and exactly 100 μl of 10 mM of GSH were added. The mixture was preincubated for 10 min at 37 °C. Thereafter, 100 μl of 2 mM NADPH was added and the hydroperoxide-independent consumption of NADPH was monitored at 340 nm for 3 min. The overall reaction began with the addition of 100 μl of prewarmed hydroperoxide solution and the decrease in the absorbance at 340 nm was monitored for about 5 min. The specific activity of the enzyme was expressed as mmoles NADPH consumed per min/mg of protein.
Statistical analysis
All the data were expressed as mean ± SD and evaluated by one-way analysis of variance (ANOVA) followed by unpaired Student`s t-test using the Statistical Package for the Social Sciences version 12.0 (SPSS) program (SPSS Inc., Chicago, IL). Values of p < 0.05 were considered as statistically significant.
Results
Effects of yacon root flour on plasma insulin and glucose levels
shows fasting plasma glucose and insulin levels in control and treated animals after 90 d of yacon supplementation. STZ injection caused a significant initial rise in plasma glucose levels in experimental animals compared with the non-diabetic group that continued to increase during the whole experimental period (p < 0.05). In contrast, we found that the 90-d dietary enrichment with yacon root flour did not cause a significant variation in plasma glucose levels.
Table 1. Effect of yacon treatment (90 d) on blood glucose and plasma insulin levels under fasting conditions.
Interestingly, treatment with yacon root flour produced a significant increase in fasting plasma insulin levels compared with the diabetic animals. However, this increase was not sufficient to maintain glycemia within normal ranges ().
Effects of treatment on serum cholesterol, triacylglycerol, ALT, AST, and creatinine levels after 90 d of yacon supplement feeding
shows serum TC and TG levels in control and yacon-treated animals after 90 d of experimental procedure. There was a significant increase in serum TC and TG levels in diabetic rats compared with non-diabetic rats (p < 0.05). TG levels were significantly lower in diabetic-treated animals compared with the diabetic control group (p < 0.05) in fasting conditions. In contrast, there was no significant TC-lowering response to the treatment at the dose assayed.
Table 2. Terminal clinical chemistry in rats after 90 d of yacon-supplement feeding.
In addition, shows the levels of fasting ALT, AST, and creatinine in control and treated animals after 90 d of yacon treatment. Diabetes resulted in a significant increase in ALT, AST, and creatinine concentration compared with non-diabetic control rats (p < 0.05). Yacon treatment for 90 d caused a significant decrease in fasting ALT, AST, and creatinine levels compared with diabetic animals (p < 0.05).
Effects of yacon supplementation on fatty acid composition in liver homogenates
Comparison of total cholesterol and free fatty acids (FFAs) contents in control and experimental groups of rats is shown in . A significant increase (p < 0.05) in total cholesterol levels and FFAs was observed in diabetic animals compared with non-diabetic control rats. Administration of yacon root flour as a dietary supplement to diabetic rats tends to bring hepatic lipid levels near to normal levels.
Table 3. Levels of total cholesterol and free fatty acids in liver homogenates at the end of the experimental period.
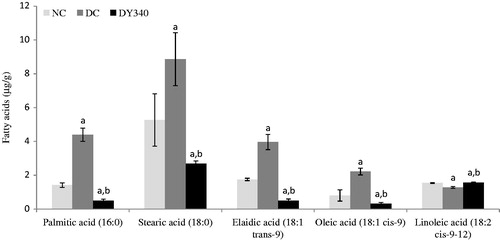
shows the alterations in liver fatty acid composition in control and experimental rats. There was a significant increase in palmitic acid (16:0), stearic acid (18:0), elaidic acid (18:1 trans-9), and oleic acid (18:1 cis-9) in liver homogenate of diabetic rats (p < 0.05) compared with non-diabetic control rats. In contrast, a significant decrease in linoleic acid (18:2 cis-9-12) was observed in this experimental group (p < 0.05). The altered fatty acid composition was restored to almost normal in diabetic rats treated with yacon root flour (340 mg FOS/kg body weight).
Figure 1. Effect of yacon root flour (340 mg FOS/kg body weight) on fatty acid composition (μg/g) in the liver of STZ-diabetic rats. Data are the mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
Effects of yacon root on lipid peroxidation in liver and kidney homogenates
Effects of yacon root flour on MDA concentration
Diabetic control rats showed a significant increase (p < 0.05) in the MDA concentration compared with the non-diabetic control group in liver and kidney homogenates (). The increase in MDA levels in the diabetic control group compared with the non-diabetic control group was 57.22% in liver and 33.03% in kidney ( and ). It is interesting to note that diabetic rats treated with yacon root flour showed a significant decrease (p < 0.05) of −30.97% in liver and of −19.15% in kidney in the MDA concentration compared with the diabetic control group ( and ).
Figure 2. Effect of yacon root flour (340 mg FOS/kg body weight) on malondialdehyde (MDA) concentrations in (a) liver and (b) kidney of normal and STZ-diabetic rats. Data are the mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
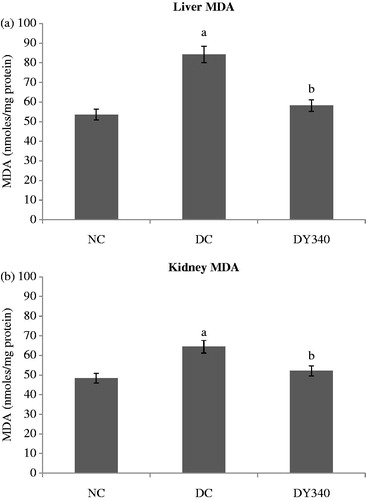
Table 4. Effects of treatment with yacon root flour for 90 d on liver CAT, SOD and GPx activities as well as GSH and MDA levels of normal and diabetic rats.
Table 5. Effects of treatment with yacon root flour for 90 d on kidney CAT, SOD, and GPx activities as well as GSH and MDA levels of normal and diabetic rats.
Effects of yacon root on enzymatic antioxidants in liver and kidney homogenates
Effects of yacon root flour on CAT activity
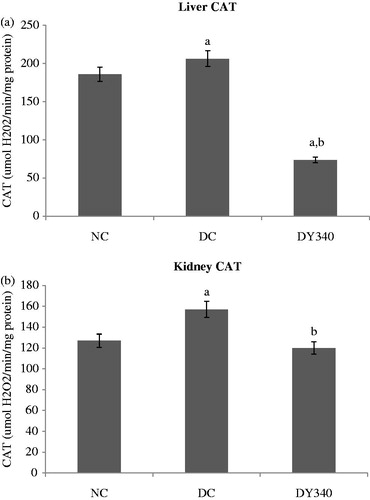
Diabetic rats showed a significant increase (p < 0.05) in the CAT activity in liver (10.92%) and kidney (23.84%) compared with the non-diabetic control group ( and ). There was a significant decrease (p < 0.05) in the CAT activity of diabetic rats treated with yacon flour compared with the diabetic control group in liver and kidney homogenates (). It should be noted that the CAT activity in diabetic rats treated with yacon flour showed a significant decrease of −64.33% in liver and of −23.70% in kidney compared with the diabetic control group ( and ).
Figure 3. Effect of yacon root flour (340 mg FOS/kg body weight) on catalase (CAT) activity in (a) liver and (b) kidney of normal and STZ-diabetic rats. Data are the mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
Effects of yacon root flour on SOD activity
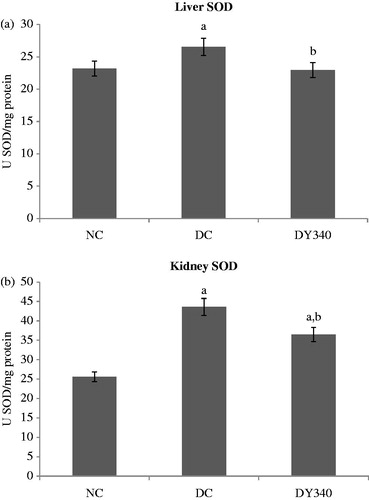
SOD activity was significantly higher in diabetic animals compared with the non-diabetic control group (p < 0.05) in both liver and kidney homogenates (). The increase in SOD activity was 14.46% in liver homogenate and 70.30% in kidney homogenate ( and ). Treatment with yacon root flour caused a significant decrease (p < 0.05) in liver and kidney SOD levels in diabetic rats (). Diabetic rats treated with yacon flour showed a significant decrease of −13.46% in liver and of −16.22% in kidney compared with the diabetic control group ( and ).
Figure 4. Effect of yacon root flour (340 mg FOS/kg body weight) on superoxide dismutase (SOD) activity in (a) liver and (b) kidney of normal and STZ-diabetic rats. Data are the mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
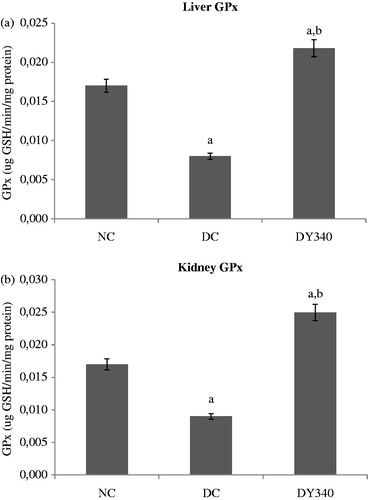
Effects of yacon root flour on GPx activity
GPx activity in diabetic control rat liver and kidney homogenates was significantly lower (p < 0.05) than that observed in the non-diabetic control group (). The decrease in GPx activity of diabetic control animals was −54.28% compared with the non-diabetic control group in liver and −46.11% in kidney homogenates ( and ). Treatment with yacon flour produced a significant increase in the liver enzyme activity in diabetic animals compared with the diabetic control group (p < 0.05). The same effect was demonstrated in kidney (). The activity of liver and kidney GPx in diabetic rats treated with yacon flour exhibited significant increases of 172.50 and 177.78%, respectively, compared with the diabetic control group ( and ).
Figure 5. Effect of yacon root flour (340 mg FOS/kg body weight) on glutathione peroxidase (GPx) activity in (a) liver and (b) kidney of normal and STZ-diabetic rats. Data are the mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
Effects of yacon root on non-enzymatic antioxidant in liver and kidney homogenates
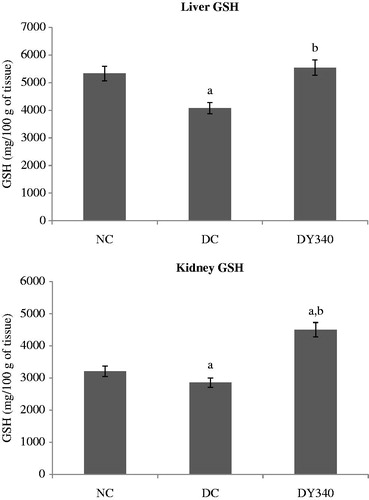
Effects of yacon root flour on GSH concentration
GSH concentration in the diabetic control group was significantly lower (p < 0.05) than in the non-diabetic control animals in liver and kidney homogenates (). In both organs, treatment with yacon flour significantly increased (p < 0.05) non-enzymatic antioxidant levels in the diabetic group. It is important to note that there was a significant increase (p < 0.05) in liver and kidney GSH levels in diabetic-treated animals (35.91 and 57.76%, respectively) compared with the diabetic control group ( and ).
Figure 6. Effect of yacon root flour (340 mg FOS/kg body weight) on reduced glutathione (GSH) concentration in (a) liver and (b) kidney of normal and STZ-diabetic rats. Data are mean ± SD. ap < 0.05 compared with non-diabetic control animals; bp < 0.05 compared with diabetic control animals. n = 6 animals per group. NC, non-diabetic control animals; DC, diabetic control animals; DY340, diabetic animals treated with yacon root flour (340 mg FOS/kg body weight).
Discussion
Several studies have shown that chronic hyperglycemia-induced oxidative stress primarily contributes to the development and progression of diabetes and its secondary complications (Folli et al., Citation2011; Shen, Citation2012). There is emerging evidence that diabetes leads to increased levels of ROS and depletion of the cellular antioxidant defense system. These findings suggest an interesting therapeutic option for the treatment of diabetes and its complications by using antioxidants or nutrients with high antioxidant activity. In the present study, we demonstrated that treatment with yacon root flour as a dietary supplement produced a significant decrease in the level of MDA and SOD and CAT activities in the liver and kidney of diabetic rats, while the levels of GPx and GSH increased significantly.
It is known that compounds such as phenolic acids, polyphenols, and flavonoids scavenge free radicals such as peroxide, hydroperoxide, or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to diabetes complications. In yacon roots, we found five caffeic acid derivatives as the major water-soluble phenolic compounds. Two of them were chlorogenic acid (3-caffeoylquinic acid and 3.5-dicaffeoylquinic acid), which are common phenolic compounds in plants of the Asteraceae family (Takenaka et al., Citation2003). In agreement with this finding, it was to be expected that yacon should exhibit high antioxidant activity. Yan et al. (Citation1999) demonstrated the in vitro antioxidant activity of yacon roots, l-tryptophan, and chlorogenic acid being the major antioxidant compounds. In the present work, several biochemical assays were used to determine the in vivo antioxidant properties of yacon roots flour: inhibition of lipid peroxidation, measurement of antioxidant enzymatic activities (SOD, CAT, and GPx), and determination of GSH levels in liver and kidney tissues.
Under our experimental conditions, the subchronic treatment (90 d) of diabetic rats with yacon flour produced a significant decrease in MDA levels in liver and kidney homogenates, indicating an effective inhibition of lipid peroxidation. MDA is one of the lipid peroxidation products frequently used to determine the oxidant/antioxidant balance in diabetic patients (Cheeseman & Slate, Citation1993). Our results revealed that liver and kidney MDA levels significantly increased in rats with experimental diabetes in comparison with the non-diabetic control group. In agreement with our study, Kakkar et al. (Citation1997), Ugochukwu et al. (Citation2003), Punitha et al. (Citation2006), Selvan et al. (Citation2008), Chakraborty and Das (Citation2010), Kamel et al. (Citation2011), and Ramachandran et al. (Citation2011) reported that STZ administration increased hepatic and renal MDA levels. The rise in hepatic MDA levels may be due to the high concentration of FFAs in diabetic animals compared with the non-diabetic control group as a consequence of the decrease in plasma insulin levels. It is known that hypoinsulinemia increases the activity of the enzyme fatty acyl coenzyme A oxidase, which initiates beta-oxidation of fatty acids and lipid peroxidation in liver (Baynes, Citation1991). Treatment with yacon root flour caused a significant decrease in lipid peroxidation in diabetic rats. We believe that this result may be attributed to the phenol compounds in the flour. These compounds contain phenolic hydroxyl groups with the ability to donate electrons and thereby to reduce the oxidized intermediates of lipid peroxidation induced by free radicals (Zhao et al., Citation2011). Besides, phenol compounds can provide another anti-oxidative mechanism directly by scavenging the free radical species (Arti et al., Citation2009)
GPx, which detoxifies H2O2 to H2O through the oxidation of GSH, is a sensitive enzyme to low H2O2 concentrations (Bagri et al., Citation2009). Our results revealed a significant decrease in liver and renal GPx activities in diabetic animals compared with the non-diabetic control group, treatment with yacon flour being effective at increasing GPx activity in both organs under study. Non-enzymatic glycation of proteins is a possible mechanism to explain inactivation of the enzyme in diabetes (Baynes, Citation2003). In this study, although treatment with yacon produced a significant decrease in fasting glucose levels, they were still elevated, indicating that non-enzymatic glycosylation can persist. Some studies have suggested that the depletion of GSH content may also lower GPx activity since GSH is the substrate in the reaction catalyzed by GPx (Arthur, Citation2000). The present study revealed that GSH levels in the liver and kidney of diabetic rats increased significantly after treatment with yacon root flour, which could contribute to the increase observed in GPx activity.
GSH is a non-enzymatic antioxidant that is essential to prevent damage to cellular components caused by ROS such as free radicals and peroxides (Bagri et al., Citation2009). In the present work, low GSH levels found in the liver of animals after STZ administration may be probably due to its increased utilization by GSH-related enzymes, as suggested by Kamel et al. (Citation2011). Some authors reported that GSH concentration is significantly reduced in the diabetic kidney (Lee et al., Citation2000; Obrosova et al., Citation2003), suggesting that this decrease may play a role in the development of renal complications. Under our experimental conditions, the increase in GSH levels observed in diabetic rats that received yacon root flour could have a cumulative effect over time and may be one of the factors responsible for the reduced oxidative stress found in the treated animals.
This study shows that treatment of diabetic rats with yacon roots decreased the activities of SOD and CAT enzymes in liver and kidney homogenates to the near-normal values obtained in the non-diabetic control group. These enzymes are regarded as the first line of the antioxidant defense system and work together to eliminate ROS generated during oxidative stress (Matés et al., Citation1999). Under our experimental conditions, it is possible that the increased activity of these enzymes in liver and kidney could result from an increase in ROS levels as a consequence of diabetes. Our results are consistent with the findings of Bray et al. (Citation1974), who showed that the increase in oxygen radicals during diabetes could increase the CAT activity, which would protect SOD from inactivation by H2O2 and consequently cause an increase in the SOD activity. We think that antioxidant therapy with yacon roots could reduce ROS levels through direct trapping of the free radicals. This in turn may result in a decrease in SOD and CAT activities. In agreement with these results, Ugochukwu and Cobourne (Citation2003) reported that the administration of ethanol plant extracts in diabetic rats reduces ROS concentration in kidney, resulting in decreased SOD activity.
Hyperglycemia not only increases ROS production but also affects antioxidant reactions catalyzed by ROS-scavenging enzymes, CAT and SOD. Increased oxidative stress in diabetes appears to be related to the underlying metabolic abnormalities rather than to the complications of this disease (Nourooz-Zadeh et al., Citation1997). In the present work, we demonstrated that yacon treatment caused a slight increase in the fasting plasma insulin levels of diabetic rats, while it was not enough to keep blood glucose within normal ranges. These findings strongly support the idea that the polyphenols present in yacon roots would act as free radical scavengers rather than as hypoglycemic compounds.
In a previous work, we demonstrated that yacon-supplemented diabetic rats showed an increased glucagon-like peptide-1 (GLP-1) content in the cecum accompanied by an important cecal tissue enlargement. These findings lead us to suggest that this incretin could be an effective mediator of the lipid-lowering effects of FOS present in the yacon flour (Habib et al., Citation2011). A recent review suggests that GLP-1 could be involved in the antioxidant effect (Tomas & Habener, Citation2010). While GLP-1 (7–36) amide stimulates glucose-dependent insulin secretion, these authors propose that GLP-1 (9–36) amide, a cleavage product of GLP-1 (7–36) amide by the action of diaminopeptidyl peptidase-4 (DPP-4), suppresses hepatic glucose production, exerts antioxidant cardioprotective actions, and reduces oxidative stress in vasculature tissues. In view of the above, additive and synergistic effects of natural phytochemicals present in the yacon root could contribute to achieve health benefits.
The present investigation revealed increased concentrations of palmitic, stearic, elaidic, and oleic acids in liver homogenates from STZ-diabetic rats. This finding agrees with other reports that show a preferential synthesis of stearic acid and other total saturated fatty acids in type 1 diabetic patients (Tilvis & Miettinen, Citation1985) and an increased concentration of oleic acid in the membranes of both type 1 and type 2 diabetic patients (Seigneur et al., Citation1994). It is known that the high serum concentration of FFAs observed in diabetes induces impairment of the proper function of liver cell organelles (Cazanave & Gores, Citation2010). Under our experimental conditions, when diabetic rats were treated with yacon root for 90 d, a significant decrease in FFA contents, particularly saturated fatty acid and oleic acid, was detected in liver homogenates. Although the lipotoxic effects of FFAs are multiple and complex, we think that this finding, together with its antioxidant properties, could account for the protective effects of yacon flour on hepatocytes against saturated FFA-induced toxicity.
In agreement with the above results, the present study shows that the treatment of diabetic rats with yacon root flour caused a significant decrease in ALT serum levels. It is known that diabetic molecular complications such as increased gluconeogenesis and ketogenesis can be accompanied by elevated transaminase activity (Ghosh & Suryawansi, Citation2001). AST is not a highly specific indicator of liver injury as its elevation can occur as a result of other injured tissues. In contrast, ALT is normally found largely in the liver and is released into the bloodstream as a result of liver injury. Thus, it serves as a fairly specific indicator of liver conditions (Vozarova et al., Citation2002). In this work, the decrease observed in ALT levels could be the consequence of improved carbohydrate, fat, and protein metabolism due to yacon root treatment as a result of an increase in the insulin secretion. In addition, the variation in ALT levels indicates an improvement in liver function.
Increased oxidative stress is a common finding in tissues affected with diabetes, including the kidney. Reddi and Bollineni (Citation2001) showed that transforming growth factor β1 (TGF-β1) is a pro-oxidant factor involved in renal fibrosis (Liu, Citation2011). In agreement with this fact, in a previous work, we observed increased TGF-β 1 expression in the kidney of STZ-diabetic rats (Honoré et al., Citation2012). In the present work, we determined that diabetic rats supplemented with yacon showed normalization in serum creatinine levels, indicating an improvement in renal function. Further studies are needed to clarify the involvement of the cytokine in the attenuation of diabetes-induced renal dysfunction.
Conclusion
In spite of the widespread recognition of the plant, this is the first report concerning the in vivo antioxidant activity of yacon root flour. The results obtained demonstrate a great potential for the application of these roots, traditionally considered as important food products in the Andean region, which could be included in foods with remarkable benefits for human and animal health, particularly in diabetes.
Acknowledgements
We wish to thank Dr. César A. Catalán for his assistance with the chemical analyses of fatty composition in liver homogenates. We are also grateful to Dr. Alfredo Grau, Regional Ecology Institute, U.N.T. (Argentina) for the supply of yacon roots.
Declaration of interest
The authors report that there are no declarations of interest.
References
- Aebi H. (1974). Catalase. Methods Enzyme Anal 2:673–84
- Arthur JR. (2000). The glutathione peroxidases. Cell Mol Life Sci 57:1825–35
- Arti RV, Vijayakumar M, Chandra SM, Chandana VR. (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol 47:2196–201
- Bagri P, Ali M, Aeri V, et al. (2009). Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol 47:50–4
- Baynes JW. (1991). Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–12
- Baynes JW. (2003). Chemical modification of proteins by lipids in diabetes. Clin Chem Lab Med 41:1159–65
- Beuge JA, Aust SD. (1978). The thiobarbituric acid assay. Methods Enzymol 67:119–24
- Bray RC, Cockle SA, Martin-Fielder E, et al. (1974). Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J 139:43–8
- Cazanave SC, Gores GJ. (2010). Mechanisms and clinical implications of hepatocyte lipoapoptosis. J Clin Lipid 5:71–85
- Chakraborty U, Das H. (2010). Antidiabetic and antioxidant activities of Cinnamomum tamala leaf extracts in STZ-treated diabetic rats. Global J Biotech Biochem 5:12–18
- Cheeseman KH, Slate TF. (1993). An introduction to free radical biochemistry. Br Med Bull 49:481–93
- Chin SF, Liu W, Storkson JM, et al. (1992). Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Comp Anal 5:185–97
- Ellman GC. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Flohé L, Günzler WA. (1984). Assays of glutathione peroxidase. Methods Enzymol 105:114–21
- Folch J, Lees M, Sloane Stanley GH. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
- Folli F, Corradi D, Fanti P, et al. (2011). The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev 7:313–24
- Genta S, Cabrera W, Habib N, et al. (2009). Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin Nutr 28:182–7
- Genta SB, Cabrera WM, Grau A, Sánchez SS. (2005). Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem Toxicol 43:1657–65
- Ghosh S, Suryawanshi SA. (2001). Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol 39:748–59
- Goto K, Fukai K, Hikida J, et al. (1995). Isolation and structural analysis of oligosaccharides form yacon (Polymnia sonchifolia). Biosci Biotech Biochem 59:2346–7
- Grau A, Rea J. (1997). Yacon Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson. In: Hermann M, Heller J, eds. Andean Root and Tubers: Ahipa, Arracacha, Maca and Yacon. Rome: IPGRI, 199–240
- Habib NC, Honoré SM, Genta SB, Sánchez SS. (2011). Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: Biochemical approach. Chem Biol Interact 194:31–9
- Haslam E. (1996). Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J Nat Prod 59:205–15
- Hayes JD, Flanagan JU, Jowsey IR. (2005). Glutathione transferases. Ann Rev Pharmacol Toxicol 45:51–88
- Honoré SM, Cabrera WM, Genta SB, Sánchez SS. (2012). Protective effect of yacon leaves decoction against early nephropathy in experimental diabetic rats. Food Chem Toxicol 50:1704–15
- Kakkar R, Mantha SV, Radhi J, et al. (1997). Antioxidant defense system in diabetic kidney: A time course study. Life Sci 60:667–9
- Kamel ZH, Daw I, Marzouk M. (2011). Effect of Cichorium endivia leaves on some biochemical parameters in streptozotocin-induced diabetic rats. Aust J Basic Appl Sci 5:387–96
- Lee YM, Kim H, Hong EK, et al. (2000). Water extract of 1:1 mixture of Phellodendron cortex and Aralia cortex has inhibitory effects on oxidative stress in kidney of diabetic rats. J Ethnopharmacol 73:429–36
- Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. (2010). Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai 93:682–93
- Liu Y. (2011). Cellular and molecular mechanisms of renal fibrosis. Nephrology 7:684–96
- Lowry OH, Rosenbrough NJ, Farr AI, Randall RJ. (1951). Protein measurement with Folin’s phenol reagent. J Biol Chem 193:265–75
- Maritim AC, Sanders RA, Watkins JB. (2003). Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol 17:24–38
- Matés JM, Pérez-Gómez C, Núñez de Castro I. (1999). Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
- Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, et al. (1997). Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetology 40:647–53
- Oberley LW. (1988). Free radicals and diabetes. Free Radic Biol Med 5:113–24
- Obrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J. (2003). Early oxidative stress in diabetic kidney: Effect of dl-α-lipoic acid. Free Radic Biol Med 34:186–95
- Parejo I, Viladomat F, Bastida J, et al. (2002). Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–90
- Punitha ISR, Shirwaikar A, Shirwaikar A. (2006). Antidiabetic activity of benzyl tetra isoquinoline alkaloid berberine in streptozotocin–nicotinamide induced type 2 diabetic rats. Diabetol Croat 34:117–28
- Ramachandran S, Asokkumar K, Uma Maheswari M, et al. (2011). Investigation of antidiabetic, antihyperlipidemic, and in vivo antioxidant properties of Sphaeranthuhs indicus Linn. in type 1 diabetic rats: An identification of possible biomarkers. Evid Based Complement Alternat Med 2011: 571721 (1--8)
- Reddi S, Bollineni JS. (2001). Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int 59:1342–53
- Seigneur M, Freyburger G, Gin H, Boisseass MR. (1994). Serum fatty acid profiles in type 1 and type 2 diabetes: Metabolic alterations of fatty acids of the main serum lipids. Diabetes Res Clin Pr 23:169–77
- Selvan VT, Manikandan L, Senthil Kumar GP. (2008). Antidiabetic and antioxidant effect of methanol extract of Artanema sesamoides in streptozotocin-induced diabetic rats. Int J Appl Res Nat Prod 1:25–33
- Shen GX. (2012). Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovasc Hematol Dis Drug Targets 12:106–12
- Takenaka M, Yan X, Ono H, et al. (2003). Caffeic acid derivatives in the roots of yacón (Smallanthus sonchifolius). J Agric Food Chem 51:793–6
- Tilvis RS, Miettinen TA. (1985). Fatty acid composition of serum lipids, erythrocytes and platelets in insulin-dependent diabetic women. J Clin Endocrinol Metab 61:741–5
- Tomas E, Habener JF. (2010). Insulin-like actions of glucagon-like peptide-1: A dual receptor hypothesis. Trends Endocrinol Metab 21:59–67
- Ugochukwu NH, Babady NE, Cobourne M, Gasset SR. (2003). The effect of Gangronema latifolium extracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats. J Biosci 28:1–5
- Ugochukwu NH, Cobourne MK. (2003). Modification of renal oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with extracts from Gongronema latifolium leaves. Clin Chim Acta 336:73–81
- Ukeda H, Maeda S, Ishii T, Sawamura M. (1997). Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis (4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xantine–xantine oxidase. Anal Biochem 251:206–9
- Valko M, Leibfritz D, Moncol J, et al. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
- Vozarova B, Stefan N, Lindsay RS, et al. (2002). High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51:1889–95
- Yan X, Suzuki M, Ohnishi-Kameyama M, et al. (1999). Extraction and identification of antioxidants in the root of yacon (Smallanthus sonchifolius). J Agric Food Chem 47:4711–13
- Zhao Y, Krishnamurthy B, Mollah ZU, et al. (2011). NF-κB in type 1 diabetes. Inflamm Allergy Drug Targets 10:208–17

