Abstract
Context: Effective drugs to treat osteoarthritis (OA) and inflammatory bowel disease (IBD) are needed.
Objective: To identify essential oils (EOs) with anti-inflammatory activity in cell models of OA and IBD.
Materials and methods: EOs from Eryngium duriaei subsp. juresianum (M. Laínz) M. Laínz (Apiaceae), Laserpitium eliasii subsp. thalictrifolium Sennen & Pau (Apiaceae), Lavandula luisieri (Rozeira) Rivas-Martínez (Lamiaceae), Othantus maritimus (L.) Hoff. & Link (Asteraceae), and Thapsia villosa L. (Apiaceae) were analyzed by GC and GC/MS. The anti-inflammatory activity of EOs (5–200 μg/mL) was evaluated by measuring inducible nitric oxide synthase (iNOS) and nuclear factor-κB (NF-κB) activation (total and phosphorylated IκB-α), in primary human chondrocytes and the intestinal cell line, C2BBe1, stimulated with interleukin-1β (IL-1β) or interferon-γ (IFN-γ), IL-1β and tumor necrosis factor-α (TNF-α), respectively.
Results: The EO of L. luisieri significantly reduced iNOS (by 54.9 and 81.0%, respectively) and phosphorylated IκB-α (by 87.4% and 62.3%, respectively) in both cell models. The EO of E. duriaei subsp. juresianum caused similar effects in human chondrocytes, but was inactive in intestinal cells, even at higher concentrations. The EOs of L. eliasii subsp. thalictrifolium and O. maritimus decreased iNOS expression by 45.2 ± 8.7% and 45.2 ± 6.2%, respectively, in C2BBe1 cells and were inactive in chondrocytes. The EO of T. villosa was inactive in both cell types.
Discussion and conclusion: This is the first study showing anti-inflammatory effects of the EOs of L. luisieri and E. duriaei subsp. juresianum. These effects are specific of the cell type and may be valuable to develop new therapies or as sources of active compounds with improved efficacy and selectivity towards OA and IBD.
Introduction
An increasing number of reports describes anti-inflammatory properties of many natural products and compounds. Nonetheless, very few studies have been dedicated to evaluate their therapeutic potential on chronic inflammatory diseases. These remain important therapeutic targets due to their high prevalence and lack of effective therapies. Indeed, most studies concerning anti-inflammatory properties of natural products have been performed in cell or animal models of acute inflammation. These models do not entirely reflect pathological mechanisms and cells involved in chronic inflammatory diseases.
Among natural products, essential oils (EOs) are particularly interesting to look for compounds with pharmacological activities, since they are complex mixtures of a huge diversity of low molecular weight (<300 Da) lipophilic compounds. These characteristics may represent favorable pharmacokinetic properties (Miguel, Citation2010).
Recent reviews on the anti-inflammatory potential of EOs fully highlight that these plant extracts and some of their components are useful therapeutic alternatives, modulating several molecular targets of the acute inflammation cascades, namely in cells of the immune system, like monocytes and macrophages (Adorjan & Buchbauer, Citation2010; Miguel, Citation2010). There is, however, an evident lack of information on the potential of EOs as modulators of chronic inflammatory diseases, especially involving cells unrelated to the immune system. Exceptions are two reports from our group (Neves et al., Citation2010; Rufino et al., Citation2014) describing anti-inflammatory effects of the EO of Juniperus oxycedrus L. subsp. oxycedrus (Cupressaceae) and α-pinene in a cell model of osteoarthritis (OA).
Chronic inflammatory diseases, in general, lead to the up-regulation of a series of enzymes and signaling molecules that bring about the characteristic inflammation and tissue destruction. Likewise, OA and inflammatory bowel disease (IBD) share many features and mechanisms and are largely driven and perpetuated by pro-inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) (Goldring et al., Citation2008; Goldring & Otero, Citation2011; Jobin & Sartor, Citation2000; Wielockx et al., Citation2004). In chronic inflammatory diseases, the onset and perpetuation of inflammatory responses and tissue destruction are primarily dependent on the transcription factor, nuclear factor-κB (NF-κB) (Tak & Firestein, Citation2001). This promotes the expression of inflammation-related genes, including cytokines (IL-1β, TNF-α, IL-6, etc.) and enzymes. One of these, the inducible nitric oxide synthase (iNOS), produces large amounts of nitric oxide (NO), a potent and destructive inflammatory mediator that plays an important role in the development and progression of OA and IBD. For instance, our previous study showed that iNOS expression, NO production, and NF-κB activity are spontaneously increased in chondrocytes isolated from OA patients compared with those isolated from non-affected human cartilage (Rosa et al., Citation2008). Increased mucosal and plasma NO concentrations associated with augmented iNOS activity have also been demonstrated in active IBD (Quenon et al., Citation2013). Moreover, NF-κB promotes the expression of specific proteases that degrade the extracellular matrix, causing the characteristic tissue destruction of OA and IBD (Goldring & Otero, Citation2011; Jobin & Sartor, Citation2000; Wielockx et al., Citation2004).
Therefore, this transcription factor constitutes an attractive target for anti-inflammatory therapeutic interventions both in OA and in IBD (Berenbaum, Citation2004; Goldring & Otero, Citation2011; Jobin & Sartor, Citation2000; Marcu et al., Citation2010). Hence, we proposed to study the ability of EOs to inhibit NF-κB activation in cell models of these diseases.
To address this purpose, EOs to be screened were selected considering two major criteria: (i) to collectively ensure the highest diversity of compounds from the chemical classes usually found in EOs; and (ii) the availability of etnopharmacological information or previous evidence of anti-inflammatory activity. For this, several EOs, isolated at laboratory from native or endemic species of the Iberian flora, were first fully characterized by identification and quantification of their components. Upon composition elucidation, the EOs were assayed in cellular models relevant for the study of OA and IBD (Csaki et al., Citation2009; Megias et al., Citation2007). Since we found previously that α-pinene inhibits inflammatory and catabolic responses in human chondrocytes, namely IL-1β-induced NF-κB activation (Neves et al., Citation2010; Rufino et al., Citation2014), we also tested this compound in the cellular model of IBD.
Primary human chondrocyte cultures stimulated with IL-1β were used as an in vitro cartilage degradation model that emulates the damage seen in OA, and cultures of the human colorectal adenocarcinoma cell line, C2BBe1, stimulated with a mixture of pro-inflammatory cytokines composed of IFN-γ, TNF-α, and IL-1β, were used as a model of IBD.
Materials and methods
Essential oils
EOs from the aerial parts of Eryngium duriaei subsp. juresianum (M. Laínz) M. Laínz (Apiaceae), from the aerial parts of Laserpitium eliasii subsp. thalictrifolium Sennen & Pau (Apiaceae), from the leaves and flowers of Lavandula luisieri (Rozeira) Rivas-Martínez (Lamiaceae), from the aerial parts of Othantus maritimus (L.) Hoff. & Link (Asteraceae), and from the aerial parts of Thapsia villosa L. (Apiaceae) were picked from the collection of plant extracts of the Faculty of Pharmacy, University of Coimbra. Plant materials, collected at different locations in the center region of Portugal, were identified by plant taxonomists (Ana Cristina Tavares, PhD and Celia Cabral, PhD, University of Coimbra). Voucher specimens of plant material were deposited at the Herbarium of the Botanic Garden, University of Coimbra (COI) or at the Herbarium of the Faculty of Pharmacy, University of Coimbra. All EOs were prepared at laboratory by water distillation using a Clevenger-type apparatus (EDQM, Citation2007). After chemical analysis, as described below, the EOs were stored at −70 °C in hermetically sealed amber glass vials. α-Pinene (purity ≥ 98%) was purchased from Sigma Chemical Co. (St. Louis, MO).
Analysis of essential oils
The composition of each EO was established immediately after extraction following a combined methodology of GC and GC/MS. Analytical GC was performed in a Hewlett-Packard 6890 (Agilent Technologies, Palo Alto, CA) gas chromatograph with a HP GC ChemStation Rev. A.05.04 data handling system (Agilent Technologies, Palo Alto, CA), equipped with a single injector and two-flame ionization detectors (FID). A graphpak divider (Agilent Technologies Palo Alto, CA, part no. 5021-7148) was used for simultaneous sampling to two Supelco (Supelco, Bellefonte, PA) fused silica capillary columns with different stationary phases: SPB-1 (polydimethylsiloxane 30 m × 0.20 mm i.d., film thickness 0.20 μm) and SupelcoWax-10 (polyethyleneglycol 30 m × 0.20 mm i.d., film thickness 0.20 μm). Oven temperature program: 70–220 °C (3 °C/min), 220 °C (15 min); injector temperature: 250 °C; carrier gas: helium adjusted to a linear velocity of 30 cm/s; split ratio 1:40; detectors temperature: 250 °C. GC-MS was performed in a Hewlett-Packard 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA) fitted with a HP1-fused silica column (polydimethylsiloxane 30 m × 0.25 mm i.d., film thickness 0.25 μm), interfaced with an Hewlett-Packard mass selective detector 5973 (Agilent Technologies, Palo Alto, CA) operated by HP Enhanced ChemStation software (Agilent Technologies, Palo Alto, CA), version A.03.00. GC parameters were as described above; interface temperature: 250 °C; MS source temperature: 230 °C; MS quadrupole temperature: 150 °C; ionization energy: 70 eV; ionization current: 60 μA; scan range: 35–350 units; scans/s: 4.51.
Components of each EO were identified considering their retention indices (RIs) on both SPB-1 and SupelcoWax-10 columns, and their mass spectra. RIs, calculated by linear interpolation relative to retention times of C8–C23 n-alkanes (Vandendool & Kratz, Citation1963), were compared with those of authentic samples or data available in digital banks (Acree, Citation2004; El-Sayed, Citation2012; Linstrom, Citation2013). Acquired mass spectra were compared with reference spectra from the laboratory database, Wiley/NIST database (EDQM, Citation2007) and literature data (Adams, Citation1995; Joulain, Citation1998). Relative amounts of individual components were calculated based on GC raw data without further correction.
Before beginning the cell assays, each EO was re-analyzed and the composition was compared with that obtained on the first analysis. No significant differences, either qualitative or quantitative, were observed. shows the composition of each essential oil obtained in the second analysis, preformed immediately before the pharmacological assays. For these assays, the EOs were diluted in dimethylsulfoxide (DMSO) and then dispersed in the culture medium to achieve final concentrations of 5–200 µg/mL. The final DMSO concentration did not exceed 0.1% (v/v).
Table 1. Classes of compounds and major constituents of the essential oils tested.
Cartilage samples and cell cultures
Human chondrocytes were isolated by enzymatic digestion (Rosa et al., Citation2009) of knee cartilage from the distal femoral condyles of multi-organ donors (20–67 years old, mean = 48.5, n = 17) and, with informed consent, of patients (59–71 years old, mean = 64.0, n = 4) undergoing total knee arthroplasty at the Orthopedic Department of the University and Hospital Center of Coimbra (CHUC). The cartilage samples presented variable degrees of degradation, ranging from intact to severely damaged. All procedures were approved by the Ethics Committee of CHUC (protocol approval numbers 8654/DC and HUC-13-05).
Chondrocyte cultures were established from non-pooled cartilage samples. Before each experiment, the cells were serum-starved for at least 6 h and maintained thereafter in serum-free culture medium. The human colorectal adenocarcinoma cell line, C2BBe1 (ATCC CRL-2102), was cultured as recommended by the American Type Culture Collection. Before treatments, C2BBe1 cells were cultured for 7–9 d until reaching a hyperconfluent state to induce differentiation.
Primary chondrocyte and C2BBe1 cultures were treated with 10 ng/mL IL-1β (Peprotech EC, London, UK) or a cytokine mixture (CytMix, 1000 U/mL IFN-γ, 10 ng/mL IL-1β and 10 ng/mL TNF-α, Peprotech EC), respectively, in the presence or absence of the EOs or the positive control compound, Bay 11-7082 (EMD Millipore, Billerica, MA), for the periods indicated in figure legends. In all cases, the EOs, α-pinene, or Bay 11-7082 were added to the cell cultures 30 min before the respective pro-inflammatory stimulus.
Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT; Sigma, St. Louis, MO) reduction assay, a quantitative colorimetric method based on the reduction of the MTT salt by the mitochondrial enzymes of viable cells (Mosmann, Citation1983), was used to determine the cytotoxicity of the EOs in the cell models used and to select non-cytotoxic concentrations of each EO. Briefly, the cells were incubated with each EO in concentrations ranging from 5 to 200 μg/mL, for 24 h in the presence or absence of the inflammatory stimulus. Then, the culture medium was replaced with fresh medium containing 5 μg/mL MTT and the cells further incubated for 30 min. The resulting dark blue crystals of formazan were then dissolved in acidified isopropanol and the absorbance of the corresponding solution, which is directly proportional to the number of living cells, was measured in an automatic plate reader (SLT, St Anton, Austria) set at a test wavelength of 570 nm and a reference wavelength of 620 nm. The absence of cytotoxic effects in each cell model was defined as the concentration of EO eliciting an absorbance reading of at least 80% of the value measured in the respective control untreated cells or cells treated with the inflammatory stimulus for the same time period.
Nitric oxide production
The concentration of nitrite, which reflects NO production, was measured in the cell-free supernatants collected from chondrocyte or C2BBe1 cell cultures treated for 24 h with IL-1β or CytMix, respectively, in the presence or absence of different concentrations of each EO. Nitrite concentration was measured using the spectrophotometric method based on the Griess reaction (Green et al., Citation1982).
Western blot
Total and cytoplasmic cell extracts were prepared and subjected to western blots as described previously (Rosa et al., Citation2009). The membranes were probed with the following antibodies: mouse monoclonal anti-human iNOS (R&D Systems, Minneapolis, MN), rabbit polyclonal anti-human IκB-α or mouse monoclonal anti-human phospho-IκB-α (Cell Signaling Technology, Inc., Beverly, MA), and anti-rabbit or anti-mouse alkaline phosphatase-conjugated secondary antibodies (GE Healthcare, Little Chalfont, UK). Mouse anti-human β-tubulin (Sigma, St. Louis, MO) or anti-human actin monoclonal antibodies (EMD Millipore Corporation, Billerica, MA) were used to detect β-tubulin or actin as loading controls. Immune complexes were detected with the Enhanced ChemiFluorescence reagent (GE Healthcare, Little Chalfont, UK) and the bands were analyzed using ImageQuant™ TL (version 7.0, GE Healthcare, Little Chalfont, UK). The results were normalized by calculating the ratio between the intensities of the bands corresponding to the protein of interest and the protein used as a loading control.
Statistical analysis
Results are presented as mean ± SEM. Statistical analysis was performed using the GraphPad Prism (version 5.00, GraphPad Software Inc., San Diego, CA). SPSS software (version 17.0, SPSS Inc., Chicago, IL) was used to assess the normality (Kolmogorov–Smirnov test) and homogeneity of variances to determine whether the conditions required to apply parametric tests were satisfied. As in all cases such conditions were observed, the statistical analysis was performed using the paired t-test for comparison of each condition with its respective control and one-way ANOVA for comparison of all conditions. Results were considered statistically significant at p < 0.05.
Results
Composition of the essential oils
Chemical characterization of the EOs of L. luiseri, E. duriaei subsp juresinanum, O. maritimus, L. eliasii, and T. villosa allowed the identification and quantification of 94, 25, 30, 42, and 14 compounds, respectively, comprising at least 85% of the respective composition. summarizes the composition of each EO, indicating the relative amounts of grouped components and the most representative compounds (≥2%).
Thirty-eight compounds, comprising seven monoterpene hydrocarbons, 12 oxygen-containing monoterpenes, including four necrodane derivatives, eight sesquiterpene hydrocarbons, nine oxygen-containing sesquiterpenes, the phenylpropanoid, methyleugenol, and the aliphatic, n-nonane, were found in concentrations over 2.0% in, at least, one EO.
Evaluation of cytotoxicity and selection of non-cytotoxic concentrations of the essential oils
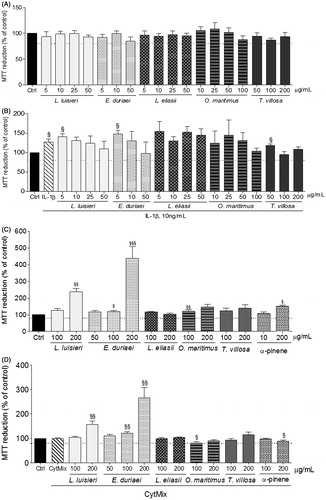
Neither the EOs nor the α-pinene was cytotoxic to C2BBe1 cells in concentrations up to 200 µg/mL, either in the presence or in the absence of the pro-inflammatory cytokine mixture (). On the contrary, in human chondrocytes, only the EO of T. villosa had no significant cytotoxic effects at that concentration (). In concentrations up to 50 μg/mL for 24 h, the EOs of E. duriaei subsp. juresianum, L. eliasii, and L. luisieri did not affect chondrocyte viability either in the presence or in the absence of IL-1β. The EO of O. maritimus showed no cytotoxicity at concentrations up to 100 μg/mL (). Therefore, subsequent experiments were performed using the non-cytotoxic concentrations identified for each EO in each cell model, as shown in .
Figure 1. Viability of human chondrocytes (A and B) and C2BBe1 cells (C and D) treated with the EOs for 24 h in the absence (A and C) or presence (B and D) of the respective pro-inflammatory stimulus. Each column represents, at least, four independent experiments. The dotted line represents the limit below which cell viability is impaired. §p < 0.05 and §§p < 0.01 relative to the respective control (untreated) cells.
Effect of the EOs on cytokine-induced iNOS expression and NO production
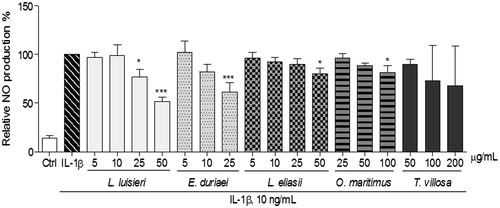
Treatment of human chondrocytes () and C2BBe1 cells () with IL-1β or CytMix, for 24 h, strongly induced the expression of iNOS. Accordingly, human chondrocytes stimulated with IL-1β produced almost six-fold more NO than control cells, as shown by the concentration of nitrite accumulated in the respective culture medium (15.6 ± 1.9 µM and 2.7 ± 0.8 µM, respectively, p < 0.001, ).
Figure 2. Effect of EOs on iNOS protein expression in human chondrocytes left untreated (Ctrl) or treated with IL-1β, 10 ng/mL, for 24 h, after pre-treatment with each EO. The images shown are representative of, at least, three independent experiments. **p < 0.01 and ***p < 0.001 relative to cells treated with IL-1β. #p < 0.05 and ##p < 0.01 between different concentrations of the same essential oil (one-way ANOVA).
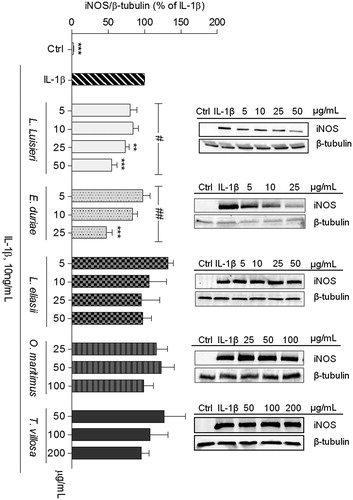
Figure 3. Effect of EOs on iNOS protein expression in C2BBe1 cells left untreated (Ctrl) or treated with CytMix, for 24 h, after pre-treatment with each EO. The images shown are representative of, at least, three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to cells treated with CytMix.
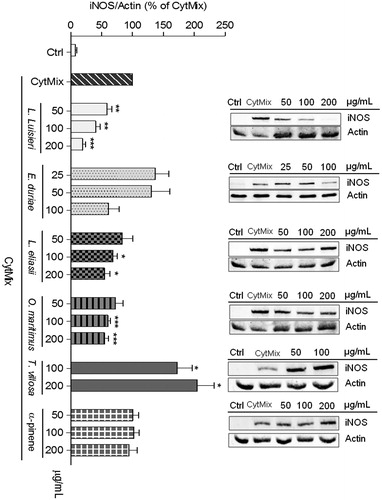
Figure 4. Effect of EOs on IL-1β-induced NO production in human chondrocytes left untreated (Ctrl) or treated with IL-1β, 10 ng/mL, for 24 h, after pre-treatment with each EO. Each column represents, at least, four independent experiments. *p < 0.05, and ***p < 0.001 relative to IL-1β-treated cells.
Surprisingly, no differences in nitrite concentration were detected in C2BBe1 cell cultures treated with the CytMix for various periods (6–48 h) in comparison with untreated cells (data not shown). As measurement of NO production was used just as a rapid screening assay and iNOS protein was readily identified, no other methods were used to detect NO production in C2BBe1 cells.
Of the EOs tested, only those of L. luisieri and E. duriaei subsp. juresianum were effective in decreasing iNOS expression () and the subsequent NO production () in human chondrocytes. The EOs of L. eliasii and O. maritimus produced a statistically significant, but very modest decrease of NO production (), even though no significant effects were detected on iNOS protein levels (). The EO of T. villosa had no significant effects, either on iNOS protein levels () or on NO production ().
In C2BBe1 cells, the EO of L. luisieri, at a concentration of 200 μg/mL, achieved the highest inhibition of iNOS expression (81.0 ± 5.2%). At a concentration of 50 μg/mL, iNOS levels were similarly reduced in C2BBe1 cells and in chondrocytes (). The EOs of L. eliasii and O. maritimus significantly reduced iNOS protein levels (by 45.2 ± 8.7% and 45.2 ± 6.2%, respectively) in C2BBe1 cells, but were much less effective than the EO of L. luisieri (). Noticeably in these cells, the EO of E. duriaei subsp. juresianum had no significant effect on iNOS expression (), even at a concentration of 100 μg/mL which is several fold higher than those tested in human chondrocytes. Neither the EO of T. villosa nor the α-pinene had any significant effect ().
Effect of the EOs of L. luisieri and E. duriaei subsp. juresianum on NF-κB activation
In response to appropriate stimuli, the NF-κB inhibitory protein, IκB-α, is phosphorylated and subsequently degraded, which releases NF-κB and allows its translocation to the nucleus to induce the expression of target genes (Hayden & Ghosh, Citation2008; O'Dea & Hoffmann, Citation2009). To assess NF-κB activation, we evaluated the cytoplasmic levels of phosphorylated and total IκB-α.
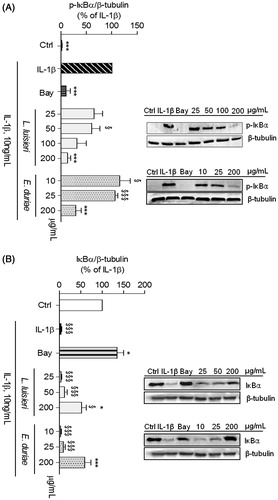
The EOs more effective in inhibiting iNOS expression and NO production were selected to evaluate their ability to inhibit IL-1β-induced NF-κB activation in human chondrocytes and C2BBe1 cells. In human chondrocytes, the EOs of L. luisieri and E. duriaei subsp. juresianum, at the concentration of 200 µg/mL, completely inhibited IL-1β-induced IκB-α phosphorylation, achieving an effect similar (p = 0.82 and p = 0.25, respectively) to that elicited by Bay 11-7082, a specific inhibitor of IκBα-phosphorylation (Mendes Sdos et al., Citation2009) used as a positive control (). Total IκB-α levels relative to those in control cells increased from 4.3 ± 1.5% (n = 4) in IL-1β-treated chondrocytes to 47.9 ± 10.7% (n = 4) and 55.6 ± 9.9% (n = 4) in cells treated with 200 μg/mL of the EOs of L. luisieri and E. duriaei subsp. juresianum, respectively. Moreover, total IκB-α levels in chondrocytes treated with 200 μg/mL of the EO of E. duriaei subsp. juresianum were not significantly different from those observed in control cells, indicating that this concentration completely prevented the response induced by IL-1β.
Figure 5. Effect of EOs of L. luisieri and E. duriaei subsp. juresianum on IL-1β-induced IκB-α phosphorylation and degradation in chondrocytes left untreated (Ctrl) or treated with IL-1β, 10 ng/mL, for 5 (A) or 30 min (B) after pre-treatment with the EOs or Bay 11-7082 (5 µM). The images shown are representative of, at least, four independent experiments. *p < 0.05 and ***p < 0.001 relative to IL-1β-treated cells. §p < 0.05 and §§§p < 0.001 relative to Ctrl.
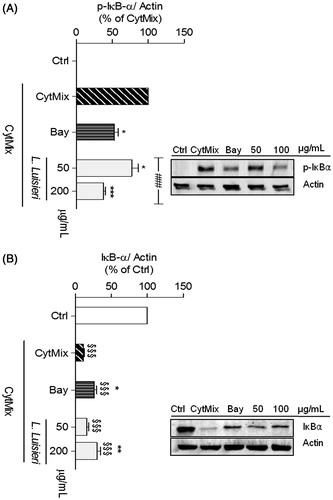
In C2BBe1 cells, the EO of L. luisieri, at concentrations of 50 and 200 µg/mL, significantly reduced IκB-α phosphorylation to 77.3 ± 8.7% (n = 7) and to 37.7 ± 3.0% (n = 7), respectively, of the CytMix-induced response (). Total IκB-α levels increased from 11.7 ± 1.2% in CytMix-treated cells to 30.4 ± 4.3% (n = 8) in L. luisieri-treated cells relative to the control, indicating that the EO effectively decreased CytMix-induced NF-κB activation. Moreover, 200 μg/mL of this EO inhibited IκB-α phosphorylation to even a larger extent than the positive control, Bay 11-7082 (p = 0.028), while IκB-α degradation was similarly inhibited (p = 0.31).
Figure 6. Effect of EOs of L. luisieri on CytMix-induced IκB-α phosphorylation and degradation in intestinal C2BBe1 cells left untreated (Ctrl) or treated with CytMix for 5 min (A) or 30 min (B) after pre-treatment with the EO or Bay 11-7082 (5 µM). The images shown are representative of, at least, four independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to CytMix-treated cells. §§§p < 0.001 relative to Ctrl and ###p < 0.001 between different concentrations of the same essential oil (one-way ANOVA).
Discussion
The results presented show clear differences in the ability of the EOs tested to inhibit relevant mediators of inflammation in the two cell models of OA and IBD used. Such differences are evident among distinct EOs, as well as comparing each one in the two cell models. Indeed, only the EO of L. luisieri was capable of significantly inhibiting inflammatory markers (iNOS expression and NF-κB activation) both in human chondrocytes and intestinal C2BBe1 cells. These results confirm our previous observation that this EO inhibits IL-1β-induced NO production in human chondrocytes (Neves et al., Citation2010). On the other hand, the EO of E. duriaei subsp. juresianum did not significantly inhibit iNOS expression in intestinal cells over a wide range of concentrations, but was effective in human chondrocytes, even at much lower concentrations. Conversely, the EOs of O. maritimus and L. eliasii showed some activity in intestinal cells, but were inactive in chondrocytes. These results indicate that the EOs of L. luisieri and E. duriaei subsp. juresianum have cell type-specific anti-inflammatory effects.
To our knowledge, this is the first study showing anti-inflammatory effects of the EOs of L. luisieri and E. duriaei subsp. juresianum. Indeed, EOs from Lavandula ssp, namely L. angustifolia, L. stoechas, L. multifida, and L. viridis, have demonstrated antibacterial, antifungal, analgesic, and anti-inflammatory effects (Amira et al., Citation2012; Ghelardini et al., Citation1999; Kirmizibekmez et al., Citation2009; Zuzarte et al., Citation2011a,Citationb), while the EO of L. luisieri has only been reported to have antifungal effects (Zuzarte et al., Citation2012). On the other hand, EOs from Eryngium ssp, including E. duriaei subsp. juresianum, have been shown to have anti-fungal and anti-bacterial properties (Cavaleiro et al., Citation2011; Celik et al., Citation2011), whereas anti-inflammatory effects were only reported for aqueous or alcoholic extracts from Eryngium species (Dawilai et al., Citation2013; Kupeli et al., Citation2006) not including E. duriaei subsp. juresianum.
α-Pinene from a commercial source (purity ≥ 98%) was unable to decrease iNOS expression in intestinal cells, while our previous studies showed that both commercial α-pinene (Rufino et al., Citation2014) and the EO of J. oxycedrus subsp. oxycedrus (76.4% α-pinene) and one of its fractions (93% α-pinene) (Neves et al., Citation2010) were effective in human chondrocytes. Interestingly, the EOs of L. luisieri, O. maritimus, and L. eliasii, the only ones containing significant amounts of α-pinene (2.3, 6.7, and 30.5%, respectively), were all effective in intestinal cells, but only that of L. luisieri was also effective in human chondrocytes, even though it presents the lowest α-pinene content. Taken together, these results suggest that α-pinene is unlikely the compound responsible for the inhibitory activities of the EOs of L. luisieri, O. maritimus, and L. eliasii observed in this study.
Similar considerations can be made about other compounds present in two or more of the EOs tested. Limonene, for instance, represents 58.8% of the EO of T. villosa, but only 2.7% of the EO of L. eliasii. Nevertheless, this EO was effective in intestinal cells while that of T. villosa was inactive in both cell types.
In contrast, the EOs of L. luisieri and E. duriaei subsp. juresianum have distinct compositions, the first being rich in oxygenated monoterpenes, while the second is mainly composed of oxygenated and non-oxygenated sesquiterpenes. Nonetheless, they were both effective in reducing markers of inflammation in human chondrocytes, suggesting that distinct compounds are involved in the observed activities of these EOs.
Moreover, we cannot discount the possibility that different components of the EOs act in synergy or in antagonism to modulate their overall activity, contributing to the apparent discrepancies described above. This possibility is even more plausible as some of these compounds, like limonene (Chi et al., Citation2012), have been shown to exert anti-inflammatory effects in various cell and animal models.
Taken together, the results presented indicate that two of the EOs studied, those of L. luisieri and E. duriaei subsp. juresianum, have significant cell type-specific anti-inflammatory effects that can be useful for the development of tissue-selective anti-inflammatory therapies.
The results obtained also show that the EOs of L. luisieri and E. duriaei subsp. juresianum which were the most effective in inhibiting iNOS expression in human chondrocytes and/or intestinal epithelial cells also decreased cytokine-induced NF-κB activation. Since NF-κB is essential for iNOS expression (Taylor et al., Citation1998), these results strongly suggest that the observed inhibition of iNOS is mediated, at least in part, by the inhibitory effects of these EOs on NF-κB activation. The concentrations of the EOs of L. luisieri and E. duriaei subsp. juresianum effective in inhibiting IL-1β-induced NF-κB activation in human chondrocytes were substantially higher than those that inhibited iNOS expression and NO production, suggesting that other mechanisms may contribute to the inhibitory activity of these EOs, at least in human chondrocytes. Since none of the EOs showed NO scavenging activity (data not shown), this can be discarded as a potential contributing mechanism. Other possibilities include direct inhibition of iNOS activity and inhibition of other signaling pathways that are required for iNOS expression in human chondrocytes (Mendes et al., Citation2002). More studies are required to identify the specific molecular targets of the EOs of L. luisieri and E. duriaei subsp. juresianum. On the contrary, since EOs are complex mixtures of chemically diverse compounds, it is possible that distinct components have different targets, so that the effects observed result from the combined actions of individual compounds. Future work will be directed at fractionating each of these EOs and elucidating the chemical composition and pharmacological activity of each fraction in order to identify the active compound(s) and their specific molecular targets, as well as potential pharmacological interactions.
In summary, this study shows for the first time that the EOs of L. luisieri and E. duriaei subsp. juresianum efficiently inhibit NF-κB activation and the expression of its target genes, namely iNOS, in cells unrelated to the immune system. Moreover, these EOs display differential effects in relevant cell models of OA and IBD and in response to distinct inflammatory stimuli. These properties may be of great value in the development of new therapies with improved efficacy and selectivity towards distinct chronic inflammatory diseases, namely OA and IBD.
Declaration of interest
The authors report that there no declarations of interest. This work was supported by Grants CENTRO-07-ST24-FEDER-002006, PEst-C/SAU/LA0001/2011, Pest-OE/SAU/UI0177/2011, and PTDC/EME-TME/113039/2009 and the PhD fellowship, SFRH/BD/47470/2008, to Rufino A. T. from FEDER through the programs COMPETE and QREN and by national funds through the Portuguese Foundation for Science and Technology (FCT).
References
- Acree T, Arn HX. (2004). Flavornet and human odor space. Datu Inc. [Online] Available from: http://www.flavornet.org [last accessed 20 Mar 2013]
- Adams RP. (1995). Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream (IL): Allured Publishing Corp
- Adorjan B, Buchbauer G. (2010). Biological properties of essential oils: An updated review. Flavour Frag J 25:407–26
- Amira S, Dade M, Schinella G, Rios JL. (2012). Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak J Pharm Sci 25:65–72
- Berenbaum F. (2004). Signaling transduction: Target in osteoarthritis. Curr Opin Rheumatol 16:616–22
- Cavaleiro C, Goncalves MJ, Serra D, et al. (2011). Composition of a volatile extract of Eryngium duriaei subsp. juresianum (M. Lainz) M. Lainz, signalised by the antifungal activity. J Pharm Biomed Anal 54:619–22
- Celik A, Aydinlik N, Arslan I. (2011). Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem Biodivers 8:454–9
- Chi G, Wei M, Xie X, et al. (2012). Suppression of MAPK and NF-kappaB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 36:501–11
- Csaki C, Mobasheri A, Shakibaei M. (2009). Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther 11:R165
- Dawilai S, Muangnoi C, Praengamthanachoti P, Tuntipopipat S. (2013). Anti-inflammatory activity of bioaccessible fraction from Eryngium foetidum leaves. Biomed Res Int 2013:958567
- European Directorate for the Quality of Medicines (EDQM). (2007). European Pharmacopoeia. 7th ed. Council of Europe, Strasbourg
- El-sayed AM. (2012). The pherobase: Database of pheromones and semiochemicals. [Online] Available from: http://www.pherobase.com [last accessed 20 Mar 2013]
- Ghelardini C, Galeotti N, Salvatore G, Mazzanti G. (1999). Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med 65:700–3
- Goldring MB, Otero M. (2011). Inflammation in osteoarthritis. Curr Opin Rheumatol 23:471–8
- Goldring MB, Otero M, Tsuchimochi K, et al. (2008). Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis 67:iii75–82
- Green LC, Wagner DA, Glogowski J, et al. (1982). Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–8
- Hayden MS, Ghosh S. (2008). Shared principles in NF-kappaB signaling. Cell 132:344–62
- Jobin C, Sartor RB. (2000). NF-kappaB signaling proteins as therapeutic targets for inflammatory bowel diseases. Inflamm Bowel Dis 6:206–13
- Joulain D, Konig AW, Verlag EB. (1998). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg: EB Verlag
- Kirmizibekmez H, Demirci B, Yesilada E, et al. (2009). Chemical composition and antimicrobial activity of the essential oils of Lavandula stoechas L. ssp. stoechas growing wild in Turkey. Nat Prod Commun 4:1001–6
- Kupeli E, Kartal M, Aslan S, Yesilada E. (2006). Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J Ethnopharmacol 107:32–7
- Linstrom PJ, Mallard WG. (2013). NIST Chemistry WebBook, NIST Standard Reference Database. 69, Gaithersburg, MD. National Institute of Standards and Technology [Online]. Available from: http://webbook.nist [last accessed 20 Mar 2013]
- Marcu KB, Otero M, Olivotto E, et al. (2010). NF-kappaB signaling: Multiple angles to target OA. Curr Drug Targets 11:599–613
- Megias J, Busserolles J, Alcaraz MJ. (2007). The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol 150:977–86
- Mendes AF, Caramona MM, Carvalho AP, Lopes MC. (2002). Role of mitogen-activated protein kinases and tyrosine kinases on IL-1-Induced NF-kappaB activation and iNOS expression in bovine articular chondrocytes. Nitric Oxide 6:35–44
- Mendes SDos S, Candi A, Vansteenbrugge M, et al. (2009). Microarray analyses of the effects of NF-kappaB or PI3K pathway inhibitors on the LPS-induced gene expression profile in RAW264.7 cells: Synergistic effects of rapamycin on LPS-induced MMP9-overexpression. Cell Signal 21:1109–22
- Miguel MG. (2010). Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 15:9252–87
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Neves A, Rosa S, Gonçalves J, et al. (2010). Screening of five essential oils for identification of potential inhibitors of IL-1-induced NF-κB activation and NO production in human chondrocytes: Characterization of the inhibitory activity of alpha-pinene. Planta Med 76:303–8
- O'Dea E, Hoffmann A. (2009). NF-kappaB signaling. Wiley Interdiscip Rev Syst Biol Med 1:107–15
- Quenon L, Hindryckx P, De Vos M, et al. (2013). Hand-held fractional exhaled nitric oxide measurements as a non-invasive indicator of systemic inflammation in Crohn's disease. J Crohn's Colitis 7:644–8
- Rosa SC, Goncalves J, Judas F, et al. (2009). Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther 11:R80
- Rosa SC, Judas F, Lopes MC, Mendes AF. (2008). Nitric oxide synthase isoforms and NF-kappaB activity in normal and osteoarthritic human chondrocytes: Regulation by inducible nitric oxide. Nitric Oxide 19:276–83
- Rufino AT, Ribeiro M, Judas F, et al. (2014). Anti-inflammatory and chondroprotective activity of (+)-α–pinene: Structural and enantiomeric selectivity. J Nat Prod 77:264–9
- Tak PP, Firestein GS. (2001). NF-kappaB: A key role in inflammatory diseases. J Clin Invest 107:7–11
- Taylor BS, De Vera ME, Ganster RW, et al. (1998). Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem 273:15148–56
- Vandendool H, Kratz PD. (1963). A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr 11:463–71
- Wielockx B, Libert C, Wilson C. (2004). Matrilysin (matrix metalloproteinase-7): A new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev 15:111–15
- Zuzarte M, Goncalves MJ, Cavaleiro C, et al. (2011a). Chemical composition and antifungal activity of the essential oils of Lavandula viridis L'Her. J Med Microbiol 60:612–18
- Zuzarte M, Goncalves MJ, Cruz MT, et al. (2012). Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem 135:1505–10
- Zuzarte M, Vale-Silva L, Goncalves MJ, et al. (2011b). Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur J Clin Microbiol Infect Dis 31:1359–66

