Abstract
Context: The global surge in multi-drug resistant bacteria and the imminence of tuberculosis pandemic necessitate alternative therapeutic approaches to augment the existing medications. Pomegranate, the fruit of Punica granatum Linn. (Punicaceae), widely recognized for potency against a broad spectrum of bacterial pathogens, deserves further investigation in this respect.
Objective: This study determines the therapeutic potential of pomegranate juice, extracts of non-edible peel prepared with methanol/water, and its four polyphenolic constituents, namely caffeic acid, ellagic acid, epigallocatechin-3-gallate (EGCG) and quercetin, against drug-resistant clinical isolates.
Materials and methods: Phenotypic characterisation of Mycobacterium tuberculosis, extended-spectrum β-lactamase (ESBL) and KPC-type carbapenemase producing Klebsiella pneumoniae was performed by biochemical and molecular methods. Resistance profiles of M. tuberculosis and K. pneumoniae were determined using LJ proportion and Kirby–Bauer methods, respectively. Pomegranate fruit extracts, and the compounds, were evaluated at a dose range of 1024–0.5 µg/mL, and 512–0.25 µg/mL, respectively, to determine minimum inhibitory (MIC) and bactericidal concentrations (MBC) against the drug-resistant isolates by the broth micro-dilution method.
Results: The peel extracts exhibited greater antimycobacterial activity (MIC 64–1024 μg/mL) than the potable juice (MIC 256 - > 1024 μg/mL). EGCG and quercetin exhibited higher antitubercular (MIC 32–256 μg/mL) and antibacterial (MIC 64–56 μg/mL) potencies than caffeic acid and ellagic acid (MIC 64–512 μg/mL).
Discussion and conclusion: The pomegranate fruit peel and pure constituents were active against a broad panel of M. tuberculosis and β-lactamase producing K. pneumoniae isolates. EGCG and quercetin need further investigation for prospective application against respiratory infections.
Introduction
Pomegranate, the fruit of Punica granatum Linn. (Punicaceae), is well known for its nutritious and medicinal values since antiquity. It is recommended for the treatment of parasitic diseases, aphthae, diarrhea and ulcers in Ayurvedic medicine, and regarded as anti-diabetic in the Unani system practiced in the Middle East and India (Jurenka, Citation2008). Numerous studies on its antioxidant, anticarcinogenic, anti-inflammatory and antimicrobial properties were reported in the past decade (Dey et al., Citation2012; Jurenka, Citation2008; Naz et al., Citation2007). Pomegranate juice and peel could provide protection against hepatotoxicity, methicillin-resistant Staphylococcus aureus, human immunodeficiency virus (HIV), genital herpes virus, tumor and also exhibited estrogen-like activity, as well as hypolipidemic property (Naz et al., Citation2007). Various extracts derived from pomegranate were shown to possess antimicrobial activity (Dey et al., Citation2012; Jurenka, Citation2008; Naz et al., Citation2007). However, no significant study on its inhibitory activity against Mycobacterium tuberculosis has been reported.
Mycobacterium tuberculosis is the causative agent of the contagious respiratory disease known as tuberculosis (TB). World Health Organization (WHO) estimated that ∼9 million new infections and ∼1.4 million TB deaths had occurred worldwide during past 1 year (WHO, Citation2012). Thus, M. tuberculosis was responsible for more mortality than any single microbial species, and TB remains a global threat to health care systems even after 40 years of the introduction of antitubercular chemotherapy (Kolyva & Karakousis, Citation2012). Currently, the emergence of multi-drug resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB), in association with immuno-compromisation due to HIV, have aggravated the scenario which solicits an urgent need to develop new therapeutics to combat TB (Dey et al., Citation2014).
Antibacterial resistance has become one of the serious public health concerns, worldwide, over the last two decades. Penicillin- or macrolide-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA) and multi-drug resistant (MDR) enteric pathogens cause the majority of community associated infections (Davies & Davies, Citation2010). Furthermore, MRSA or vancomycin-resistant S. aureus and enterococci, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and MDR non-fermenters are considered as nosocomial pathogens around the world. New Delhi metallo-β-lactamase-1 (NDM-1) was found in almost every continent within a year of its emergence in India (Kang & Song, Citation2013).
Klebsiella pneumoniae, the most prevalent Gram-negative Enterobacteriaceae pathogen, is primarily responsible for the outbreaks of nosocomial infections in intensive care, burn, oncology and neonatal units (Correa et al., Citation2013). A strong correlation between β-lactam antibiotics used against these diseases and resistance development due to the β-lactamases (the related inactivating enzymes) has been observed over the past half-century (Davies & Davies, Citation2010). Then, the first report of plasmid-mediated extended-spectrum β-lactamases (ESBLs) capable of hydrolyzing third- and fourth-generation cephalosporins and monobactams was published in 1983 (Knothe et al., Citation1983). Moreover, ESBL-containing plasmids often carry resistance genes for other antibiotics, such as aminoglycosides and fluoroquinolones, also. Therefore, carbapenems were the treatment of choice for serious infections caused by ESBL-producers. However, in 1996, the first carbapenem-resistant K. pneumoniae carbapenemase (KPC)-producing K. pneumoniae isolate was reported in the Eastern USA. Since then, KPC-type carbapenemase producers had spread globally and are now endemic in US, Israel and Greece (Arnold et al., Citation2011). High mortality rate was documented (∼30–50%) in KPC-type carbapenemase producing K. pneumoniae infections (Correa et al., Citation2013), as most of these isolates were resistant to extended-spectrum cephalosporins, co-trimoxazole, fluoroquinolones and aminoglycosides, in addition to the carbapenem drugs (Arnold et al., Citation2011). Therefore, there is a necessity to explore the scope of complementary and alternative medicines in view of the limited therapeutic option available against the aforesaid isolates.
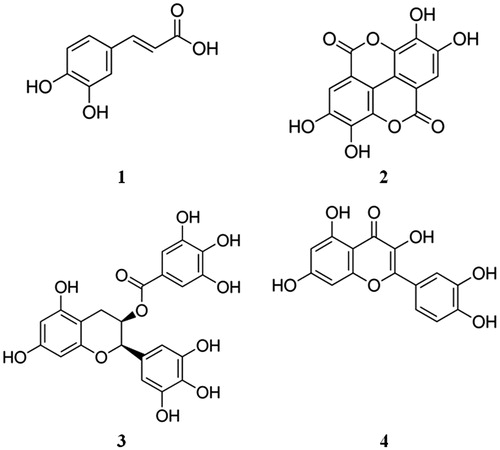
Asian countries are considered to be the epicenters of microbial resistance, with a high burden of tuberculosis (TB) and prevalence of Gram-negative-resistant bacteria (Kang & Song, Citation2013; WHO, Citation2012). Therefore, 30 drug-resistant isolates were selected for the present study in view of the high regional prevalence of M. tuberculosis, and ESBL- and KPC-type carbapenemase-producing K. pneumoniae (Kang & Song, Citation2013; WHO, Citation2012). Therefore, while searching for naturally occurring alternatives, we have now investigated the efficacy of pomegranate (peel) and juice against the aforesaid “superbugs”. Additionally, we tested the activity of some of its polyphenolic ingredients, namely caffeic acid, ellagic acid, epigallocatechin-3-gallate (EGCG) and quercetin (), against the aforesaid drug-resistant clinical isolates. Actually, these polyphenolic compounds are well-known nutraceutical constituents of several dietary plants including pomegranate. Several in vitro and in silico studies on the analysis of molecular interactions of these selected compounds against M. tuberculosis and β-lactamase-producing K. pneumoniae were reported by Plaper et al. (Citation2003), Zhang and Rock (Citation2004), Gradisar et al. (Citation2007), Sharma et al. (Citation2008), Suriyanarayanan et al. (Citation2013) and Biswas et al. (Citation2013). Here we are presenting the inhibitory activity of these selected compounds against these pathogenic organisms for the first time, to the best of our knowledge.
Materials and methods
Plant materials and test compounds
Ripe pomegranate (P. granatum) fruits were procured from a local market in September 2013. The plant material was duly authenticated at the Botanical Survey of India, Shibpur, Howrah. The voucher specimen was conserved in our laboratory for future reference. Four test compounds, namely caffeic acid (1), ellagic acid (2), epigallocatechin-3-gallate (3) and quercetin (4), were purchased from Sigma-Aldrich Co., St. Louis, MO ().
The non-edible pericarp of pomegranate fruit was separated from the edible arils and dried under shade. The fresh arils were crushed to extract the juice (15 mL) which was freeze dried in a lyophiliser (J; 7 g). A sample (10 g) of the pulverised pericarp was refluxed in methanol for 2 h, the extract was filtered and the solvent removed under vacuum (M; 2.9 g). Similarly, another sample (10 g) of the pericarp was extracted by boiling in water (2 h) to get the water extract which was dried in a lyophiliser (W; 4.8 g). Samples of “J”, “M” and “W”, and the pure compounds (1–4) were dissolved in dimethyl sulphoxide (DMSO; Merck, Mumbai, India) for preparing the respective stock solutions (2 mg/mL).
Isolation and identification of M. tuberculosis
Detection of mycobacteria in clinical specimen was performed through enrichment in Middlebrook 7H9 broth using BacT/ALERT 3D system (bioMérieux, Inc., Durham, NC) as described previously (Dey et al., Citation2014). The culture broth from the BacT/ALERT MP bottle with positive growth of mycobacteria was inoculated in Löwenstein–Jensen (LJ) slant (HiMedia, Mumbai, India), and the growth was identified by phenotypic tests and routine biochemical methods, using the kits procured from Himedia, Mumbai, India (Collee et al., Citation1996; Dey et al., Citation2014). Additionally, real-time DNA detection for genotypic confirmation of M. tuberculosis complex (MTC) was accomplished by using artus® M. tuberculosis RG PCR kit and Rotor-Gene Q analyzer (QIAGEN, Hilden, Germany) (Dey et al., Citation2014).
Antitubercular drug susceptibility
Susceptibility testing of nine M. tuberculosis isolates along with one American Type Culture Collection (ATCC) strain of M. tuberculosis (H37Ra) was carried out against first line (isoniazid, ethambutol, pyrazinamide, rifampicin and streptomycin) and second line (kanamycin, amikacin, ethionamide, d-cycloserine, clarithromycin, p-amino salicylic acid and rifabutin) antitubercular drugs (ATDs) on drug-incorporated LJ slants (HiMedia, Mumbai, India), following the LJ proportion method (Collee et al., Citation1996; Kolyva & Karakousis, Citation2012) as described previously by Dey et al. (Citation2014).
Antitubercular activity
The susceptibility of 10 selected M. tuberculosis isolates against the three extracts (J, M and W) and four compounds along with a standard drug ciprofloxacin was determined by tetrazolium microplate assay with 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO), according to the standard protocol (Dey et al., Citation2014). The tested range of concentrations for the extracts and pure compounds was 1024–0.5, and 512–0.25 μg/mL, respectively. The minimum inhibitory concentration (MIC) was the lowest concentration of the tested sample at which no tubercular growth was indicated by the colour change of MTT. To determine the minimum bactericidal concentration (MBC), a loop-full of culture from each well containing different dilutions of the tested antimycobacterials was streaked by a 4 mm loop (HiMedia, Mumbai, India; calibrated to 0.01 mL) on the solid LJ medium base containing glycerol (HiMedia, Mumbai, India). The lowest concentration of the tested compound that did not support the growth of visible mycobacterial colony on LJ medium was recorded as the MBC (Dey et al., Citation2014).
Isolation and characterization of K. pneumoniae isolates
Two ATCC strains and 18 pathogenic isolates of K. pneumoniae were identified by routine phenotypic and biochemical methods (Collee et al., Citation1996), and screened for their ESBL and KPC-type carbapenemase-producing capability by following standard procedures described below.
Double-disc synergy test
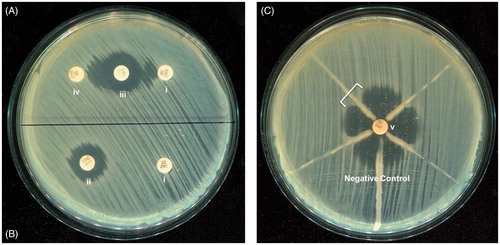
A third generation cephalosporin, namely ceftazidime or cefotaxime (30 μg), and a disc of co-amoxiclav (20 μg amoxicillin/10 μg clavulanic acid) were placed 20 mm apart on Mueller Hinton agar (MHA) plate on which 0.5 McFarland of test organism was swabbed. In case of an ESBL producer strain, the zone diameter of cephalosporin disc was found to extend by ≥5 mm towards the co-amoxiclav disc () (Collee et al., Citation1996; Dey et al., Citation2012).
Figure 2. (A) Double-disc synergy and (B) phenotypic confirmatory test for ESBL detection. (C) Modified Hodge Test of the four KPC-producing K. pneumoniae isolates and K. pneumoniae (ATCC 700603) as a negative control. All four KPC-producing isolates produced the characteristic cloverleaf-like indentation (indicated by a bracket), except the negative control. Antibiotic discs used for these procedures were (i) ceftazidime (30 μg), (ii) ceftazidime-clavulanic acid (30/10 μg), (iii) amoxy-clavulanic acid (20/10 μg), (iv) cefotaxime (30 μg) and (v) imipenem (10 μg).
Phenotypic confirmatory test
Disc tests were performed for phenotypic confirmation of the presence of ESBLs in K. pneumoniae by using cefotaxime (30 μg) and ceftazidime discs (30 μg) with and without clavulanate (10 μg) by following standard recommendation of Clinical and Laboratory Standards Institute (CLSI, Citation2013). Therefore, in case of ESBL-producing bacteria, the zone diameter of cephalosporin/clavulanate disc was at least 5 mm greater than the zone for cephalosporin disc alone. Klebsiella pneumoniae ATCC 700603 was used as a positive control for this test () (Dey et al., Citation2012).
The modified Hodge test
The 0.5 McFarland standard suspension of Escherichia coli ATCC 25922 in Mueller Hinton broth (MHB) was diluted to 1:10 in MHB and inoculated in MHA plates. An imipenem disc (10 μg) was placed at the centre of the plate. Then, 3–5 colonies of the test organism (grown separately in a blood agar plate) were picked with a 10 μL loop, and inoculated on the E. coli ATCC 25922 plate in a straight line from the edge of the disc. A characteristic clover leaf-like indentation, associated with the inoculation line of the KPC-type carbapenemase producer isolate, was observed at the periphery of the inhibition zone of E. coli ATCC 25922. A strain of K. pneumoniae (ATCC BAA 1705) was used as a positive control for this test () (CLSI, Citation2013; Dey et al., Citation2012).
Antibacterial drug susceptibility
Antibacterial susceptibility studies were carried out by Kirby and Bauer disk diffusion technique using commercially available antibiotic discs (HiMedia, Mumbai, India). The inhibition zone data were interpreted according to the CLSI guidelines (CLSI, Citation2013).
Antibacterial activity
The broth micro-dilution assay was used for the determination of MIC for three extracts (1024–0.5 μg/mL) and four pure compounds and a standard antibiotic ciprofloxacin (512–0.25 μg/mL) to find the lowest concentration at which no growth against each of the selected K. pneumoniae (two ATCC strains and 18 clinical isolates) was visible, as described before (Dey et al., Citation2012). To determine MBC, a loop-full of culture was taken from each well containing different dilutions of the tested compounds, and inoculated by a 4 mm loop (calibrated to 0.01 mL) on the MHA plate. The lowest concentrations of the tested compounds that did not support the growth of visible bacterial colony were recorded as the MBC of the respective compounds (Dey et al., Citation2014).
Results
Antitubercular activity
The pomegranate fruit extracts, namely J, M and W, along with the pure compounds (1–4) were evaluated for the antitubercular efficacy against one ATCC strain (H37Ra) and nine clinical isolates of M. tuberculosis, in terms of MIC and MBC (). Mycobacterium tuberculosis isolates were collected from the pulmonary tuberculosis cases and their ATD resistance pattern was determined using the LJ proportional method (Collee et al., Citation1996). Among the selected tubercle bacilli, a single isolate (Mtb-10) originated from multi-drug resistant tuberculosis (MDR-TB) infection, and the other one (Mtb-5) from extensively drug-resistant tuberculosis (XDR-TB). MDR-TB is caused by the strains of M. tuberculosis that are primarily resistant to isoniazid and rifampicin, while additional resistance to a fluoroquinolone (e.g. ciprofloxacin), as well as one second-line injectable agent (e.g. amikacin/kanamycin), is considered as XDR-TB (Kolyva & Karakousis, Citation2012; WHO, Citation2012).
Table 1. Drug resistance profile of M. tuberculosis isolates, and antitubercular efficacy of pomegranate extracts (J, M and W) and constituent compounds (1–4) against the isolates.
Data in showed that the methanol (M) and water (W) extracts of pomegranate fruit pericarp exhibited greater antitubercular activity (MIC 64–512, and 64–1024 µg/mL, respectively) than J, the lyophilised juice (MIC 256 - > 1024 µg/mL). Among the pure constituents studied here, EGCG and quercetin exhibited higher potency, with the MIC 32–256 µg/mL, than caffeic and ellagic acids (MIC 128–512 and 64–512 µg/mL, respectively). MIC of EGCG was found to be high (256 µg/mL) against isoniazid resistance isolates (Mtb-5, 8 and 10). Similarly, ciprofloxacin-resistant isolates (Mtb-5 and 8) exhibited greater resistance to quercetin, with an MIC of 256 µg/mL. The MBC/MIC ratio of the extracts and compounds against all tubercular isolates was found to be less than “32” ().
Antibacterial activity
The antibacterial efficacy of pomegranate extracts and constituents (1–4) was evaluated against K. pneumoniae, selected in the context of ESBL- and KPC-type carbapenemase production related to their multi-drug resistant phenotypes, through an extensive screening of available clinical specimens. Apart from the two ATCC strains, 18 clinical isolates were obtained from different patients suffering from pulmonary infections, superficial wounds, bacteremia, urinary and enteric infections caused by multi-drug resistant K. pneumoniae (), and were tested for individual antibiotic resistance pattern following CLSI guidelines (CLSI, Citation2013).
Table 2. Drug resistance profile of ESBL- and KPC type carbapenemase-producing K. pneumoniae isolates, and antibacterial efficacy of pomegranate extracts (J, M and W) and constituent compounds (1–4) against the isolates.
The results of antibacterial evaluation, expressed as MIC and MBC in , showed that pomegranate extracts were less responsive (MIC 256 - > 1024 µg/mL) than the pure compounds tested. EGCG and quercetin were found to be the most potent (MIC 64–256 μg/mL), while caffeic and ellagic acids were moderately effective (MIC 128–512 μg/mL) against the ESBL and KPC-type carbapenemase-producing K. pneumoniae isolates. However, the extracts as well as the pure constituents of pomegranate exhibited greater activity against M. tuberculosis () as compared with the activity against β-lactamase-producing K. pneumoniae isolates (). Here also the MBC/MIC ratio was observed to be less than “32”.
Discussion
All parts of pomegranate fruit, including the highly nutritious juice of its aril, have been used in various traditional remedies against acidosis, dysentery, microbial infections, diarrhoea, helminth infection, hemorrhage and respiratory pathologies around the world. Recently, many investigations on P. granatum fruit have been undertaken with a view to validate the age-old therapeutic application of this ancient plant for the treatment of infectious diseases caused by virus, bacteria, fungi and parasites (Jurenka, Citation2008; Naz et al., Citation2007). However, to the best of our knowledge, no scientific details were available on the prospective inhibitory activity of pomegranate fruits against M. tuberculosis and β-lactamase-producing K. pneumoniae, in particular. The present study clearly exhibited the positive response of both these organisms to the treatment with pomegranate fruit extracts. In an earlier study, we had indicated that pomegranate peel extracts, with a relative abundance of phenolic and flavonoid constituents, possess greater antibacterial efficacy than the potable juice (Dey et al., Citation2012). Similarly, in the present investigation, tubercle bacilli showed lower MIC values () for the peel extracts prepared with a polar solvent like methanol or water (MIC 64–1024 µg/mL) in comparison with the juice (MIC 256 - > 1024 µg/mL). However, the activity of the tested extracts was found to be comparatively weaker against the ESBL and KPC-type carbapenemase-producing K. pneumoniae isolates, as shown in (MIC 256 - > 1024 µg/mL).
The antitubercular efficacy of pomegranate observed in this study prompted us to select a few polyphenolic ingredients of this fruit, like caffeic acid, ellagic acid, EGCG and quercetin, which are also constituents of green tea, broccoli, sweet potato, berries, grapes, etc. (Dai & Mumper, Citation2010). Many such flavonoids and phenolic acids are common antioxidant constituents of fruits like apple, grape, pear, cherry and various berries, which contain up to 200–300 mg polyphenols per 100 g of fresh weight (Scalbert et al., Citation2005). EGCG is the major catechin abundant in green tea and several fruits including pomegranate. The results from and clearly confirmed the greater potency of EGCG and quercetin against M. tuberculosis and K. pneumoniae isolates (MIC 32–256 µg/mL) in comparison with both caffeic acid and ellagic acid (MIC 64–512 µg/mL). Gradisar et al. (Citation2007) also reported the antimicrobial activity of catechins including EGCG against Gram-negative bacteria at relatively high concentrations due to the poor penetration in the bacterial outer membrane. They further concluded that the catechins inhibited bacterial DNA gyrase by binding to the ATP binding site of the gyrase B subunit. Considering the great health impact of tea-catechins, NMR and molecular docking experiments were also performed by Gradisar et al. (Citation2007) to study the interaction of catechins with their molecular targets. In fact, target enzymes of various metabolic pathways specific to the pathogens are being explored extensively considering the emerging threat of multi-drug resistant isolates, and fatty acid biosynthesis pathway is one of them. Some of the studies concluded that EGCG could inhibit the component(s) of fatty acid synthase (FAS) type II, which is a characteristic of both bacteria and mycobacteria (Sharma et al., Citation2008; Zhang & Rock, Citation2004). Since isoniazid, a front-line drug for treating TB, is also known to inhibit FAS type II in mycobacteria, this enzyme has been recognised as a good target for antitubercular agents (Kolyva & Karakousis, Citation2012). It may be noted that, in the present study, isoniazid-resistant isolates (Mtb-5, 8 and 10) exhibited a greater MIC (256 µg/mL) against EGCG (). This might be due to the mutation and/or over-expression of the target protein(s) caused by a similar resistance mechanism induced by both isoniazid and EGCG (Kolyva & Karakousis, Citation2012).
Again, quercetin, one of the most abundant natural flavonoids found in onion, tea, and apple, was also shown to interact with DNA gyrase in E. coli (Plaper et al., Citation2003). Likewise, in silico analysis by Suriyanarayanan et al. (Citation2013) indicated subunit B of DNA gyrase in M. smegmatis and M. tuberculosis (H37Ra) as a probable target for quercetin, which inhibited the bacilli at MIC of 100 μg/mL, in vitro. So far, this was the only available report in the literature about assay of this flavonoid performed against M. tuberculosis (H37Ra). Our results showed similar activity not only on the same strain (MIC 64 μg/mL) but also on several clinical isolates of M. tuberculosis with tested drug-resistance profile (). Incidentally, resistance to fluoroquinolones in M. tuberculosis is commonly associated with mutations in the conserved quinolone resistance-determining region of gyrA and gyrB (Kolyva & Karakousis, Citation2012). In the present study, we found that quercetin was less sensitive (MIC 256 µg/mL) in ciprofloxacin-resistant tubercular isolates (Mtb-5 and 8) (), which implied a similar mode of resistance mechanism operating against quercetin and ciprofloxacin. However, such correlation was not found in the case of antibacterial data, probably due to the role played by additional non-specific resistance mechanisms in Gram-negative bacilli (Collee et al., Citation1996).
The other two polyphenols tested here, namely caffeic acid and ellagic acid, exhibited moderate activity (MIC 64–512 µg/mL) against M. tuberculosis and K. pneumoniae isolates. Recently, Biswas et al. (Citation2013) identified ellagic acid and two other compounds as prospective inhibitors of M. tuberculosis DNA primase by performing high-throughput screening of 2560 small molecules. In another study, caffeic acid was not found to be active at 74 µg/mL, the highest concentration tested against three M. avium subsp. paratuberculosis strains (Wong et al., Citation2008). Actually, the previous investigations regarding antibacterial/antitubercular activity of aforesaid plant-derived polyphenols were mostly limited to target-specific molecular interactions (Biswas et al., Citation2013; Gradisar et al., Citation2007; Sharma et al., Citation2008; Suriyanarayanan et al., Citation2013; Zhang & Rock, Citation2004). However, the present study was conducted in a clinical scenario to address the non-specific resistance mechanisms as well, operating in parallel, which might compromise the efficacy of the applied antimicrobial compounds (Collee et al., Citation1996; Kolyva & Karakousis, Citation2012). Over all, this study exhibited significant bactericidal potency of the tested extracts of pomegranate fruit as well as four of its pure constituents against the clinical isolates as indicated by the MBC/MIC ratio (Cockerill, Citation1998). Nevertheless, further scope remains to explore the polar extracts of this fruit-peel through bioassay-guided fractionation in order to find new antitubercular/antibacterial compounds, in future.
At this point, a caution has to be raised about the reactive polyphenols consisting of one or more catechol moieties. On one hand, these compounds are particularly prone to oxidation, thereby likely to form Michael adducts with non-specific host proteins. Candidates belonging to such functional groups were considered as “pan assay interference compounds” (PAINS) by Baell and Holloway (Citation2010), suggesting their exclusion from the ambit of high-throughput screening libraries implemented in drug discovery programs. On the other hand, such molecular entities could be suitably modified into products with desired pharmacological selectivity (Glaser et al., Citation2014). In fact, naturally occurring polyphenols like caffeic acid, widely consumed in human diet, have been explored as templates for designing new chemical entities with potential therapeutic function (Silva et al., Citation2014).
In summary, extracts of pomegranate fruit and its pure constituents were shown to be active against M. tuberculosis and β-lactamase-producing K. pneumoniae isolates for the first time. The catechins and flavonoids, like EGCG and quercetin, found in many plant foods including pomegranate need to be investigated further for prospective application against respiratory infections.
Acknowledgements
D. D. acknowledges technical and management support provided by Ashok Laboratory Clinical Testing Centre Private Limited, Kolkata.
Declaration of interest
The authors report that there are no declarations of interest. Research Scientist Grant from the University Grants Commission, New Delhi, is acknowledged by B. H.
References
- Arnold RS, Thom KA, Sharma S, et al. (2011). Emergence of Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria. South Med J 104:40–5
- Baell JB, Holloway GA. (2010). New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem 53:2719–40
- Biswas T, Resto-Roldán E, Sawyer SK, et al. (2013). A novel non-radioactive primase–pyrophosphatase activity assay and its application to the discovery of inhibitors of Mycobacterium tuberculosis primase DnaG. Nucleic Acids Res 41:e56
- Clinical and Laboratory Standards Institute. (2013). Performance Standards for Antimicrobial Susceptibility Testing; Twenty Third Informational Supplement. Wayne: CLSI
- Cockerill FR III. (1998). Conventional and genetic laboratory tests used to guide antimicrobial therapy. Mayo Clin Proc 73:1007–21
- Collee JG, Fraser AG, Marmion BP, Simmons A. eds. (1996). Mackie and McCartney Practical Medical Microbiology. London: Churchill Livingstone
- Correa L, Martino MD, Siqueira I, et al. (2013). A hospital-based matched case–control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis 13:80
- Dai J, Mumper RJ. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–52
- Davies J, Davies D. (2010). Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417–33
- Dey D, Debnath S, Hazra S, et al. (2012). Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram negative bacilli. Food Chem Toxicol 50:4302–9
- Dey D, Ray R, Hazra B. (2014). Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother Res. 28:1014–21
- Glaser J, Schultheis M, Hazra S, et al. (2014). Antileishmanial lead structures from nature: Analysis of structure-activity relationships of a compound library derived from caffeic acid bornyl ester. Molecules 19:1394–410
- Gradisar H, Pristovsek P, Plaper A, Jerala R. (2007). Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem 50:264–71
- Jurenka J. (2008). Therapeutic applications of pomegranate (Punica granatam L.): Review. Altern Med Rev 13:128–44
- Kang C-I, Song J-H. (2013). Antimicrobial resistance in Asia: Current epidemiology and clinical implications. Infect Chemother 45:22–31
- Knothe H, Shah P, Krcmery V, et al. (1983). Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315–17
- Kolyva AS, Karakousis PC. (2012). Old and new TB drugs: Mechanisms of action and resistance. In: Cardona P-J, ed. Understanding Tuberculosis – New Approaches to Fighting Against Drug Resistance. Europe: InTech, 209–32
- Naz S, Siddiqi R, Ahmad S, et al. (2007). Antibacterial activity directed isolation of compounds from Punica granatum. J Food Sci 72:341–5
- Plaper A, Golob M, Hafner I, et al. (2003). Characterisation of quercetin binding site on DNA gyrase. Biochem Biophys Res Commun 306:530–6
- Scalbert A, Manach C, Morand C, et al. (2005). Polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306
- Sharma SK, Kumar G, Kapoor M, Surolia A. (2008). Combined effect of epigallocatechin gallate and triclosan on enoyl-ACP reductase of Mycobacterium tuberculosis. Biochem Biophys Res Commun 368:12–17
- Silva T, Oliveira C, Borges F. (2014). Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin Ther Pat 24:1257–70
- Suriyanarayanan B, Shanmugam K, Santhosh RS. (2013). Synthetic quercetin inhibits mycobacterial growth possibly by interacting with DNA gyrase. Rom Biotechnol Lett 18:1587–93
- Wong SY, Grant IR, Friedman M, et al. (2008). Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl Environ Microbiol 74:5986–90
- World Health Organization. (2012). Global tuberculosis report (WHO/HTM/TB/2012.6.). Geneva, Switzerland: WHO [Online]. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf [last accessed 17 Nov 2014]
- Zhang YM, Rock CO. (2004). Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J Biol Chem 279:30994–1001