Abstract
Context: Chrysin, a natural flavonoid, has been shown to possess multiple pharmacological activities including anti-atherosclerosis.
Objective: The effects of chrysin on foam cell formation and cholesterol flow in RAW264.7 macrophages were investigated in this work to explore the potential mechanism underlying its anti-atherogenic activity.
Materials and methods: The inhibitive effect of chrysin on foam cell formation and cholesterol accumulation induced by oxidized low-density lipoprotein cholesterol (ox-LDL) was assessed by oil red O staining and intracellular total cholesterol and triglyceride quantification in RAW264.7 macrophages. The action of chrysin on cholesterol efflux and influx was tested by fluorescent assays. Real-time quantitative PCR was used to quantify the relative expression of cholesterol flow-associated genes and luciferase assay was applied to test the transcription activity of peroxisome proliferator-activated receptor gamma (PPARγ).
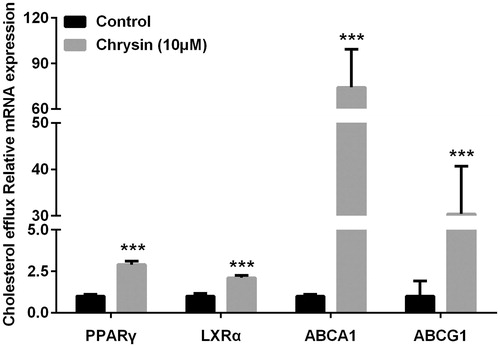
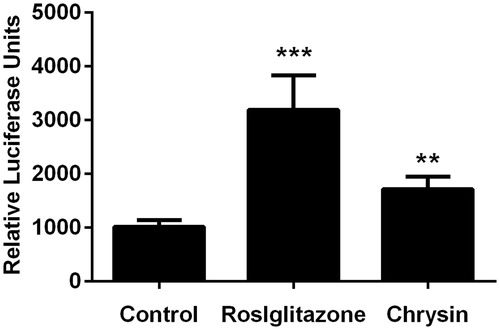
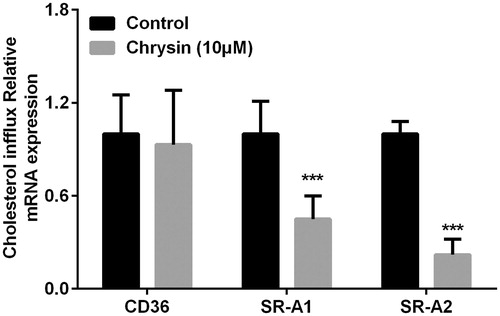
Results: Chrysin dose dependently inhibited the formation of foam cells and prevented the enhanced cholesterol accumulation by ox-LDL. Treatment with chrysin (10 μM) significantly enhanced cholesterol efflux and substantially inhibited cholesterol influx. Simultaneously, chrysin significantly increased the mRNA levels of PPARγ, liver X receptor alpha (LXRα), ATP-binding cassette, sub-family A1 (ABCA1), and sub-family G1 (ABCG1), decreased scavenger receptor A1 (SR-A1) and SR-A2, and increased the transcriptional activity of PPARγ.
Discussion and conclusion: Chrysin is a new inhibitor of foam cell formation that may stimulate cholesterol flow. Up-regulation of the classical PPARγ–LXRα–ABCA1/ABCG1 pathway and down-regulation of SR-A1 and SR-A2 may participate in its suppressive effect on intracellular cholesterol accumulation.
Introduction
Atherosclerosis, a kind of chronic disease characterized by the deposition of excessive cholesterol in the arterial intima (Yu et al., Citation2013a), continues to be a major cause of death worldwide (Glass & Witztum, Citation2001; Li et al., Citation2014). Among the various pathological factors, the foam cell formation, which results from the imbalanced cholesterol influx and efflux (Glass & Witztum, Citation2001; Pennings et al., Citation2006; Tabas, Citation2004; Yu et al., Citation2013a), plays a central role in the occurrence and development of atherosclerosis. Under atherogenic condition that inflow cholesterol exceeds outflow, excessive cholesterol accumulates in peripheral cells especially macrophages and endothelial cells, leading to foam cell formation (Reiss & Cronstein, Citation2012) and plaque appearance (Zhang et al., Citation2014). Therefore, inhibiting cholesterol influx and promoting efflux make great contribution to decrease foam cells formation.
Cholesterol efflux is the first step of reverse cholesterol transport (RCT) (Reiss & Cronstein, Citation2012; Yu et al., Citation2013a) through which accumulated cholesterol is transported from the vessel wall to the liver for excretion (Ohashi et al., Citation2005). A number of proteins are involved in the regulation of cholesterol efflux among which ATP-binding cassette, sub-family A1 (ABCA1), and sub-family G1 (ABCG1) have been best characterized (Liu et al., Citation2014; Zhang et al., Citation2014). ABCA1 and ABCG1, both belonging to integral membrane proteins that utilize ATP as a source of energy for transporting lipids and other metabolites across membranes (Dean et al., Citation2001), are completely or partially regulated by the proliferator-activated receptor gamma (PPARγ)/liver X receptor alpha (LXRα)-dependent pathway (Ozasa et al., Citation2011). In addition to impaired cholesterol efflux (Liu et al., Citation2014), dysregulated cholesterol uptake is also critical for foam cell formation. Scavenger receptors, such as CD36, scavenger receptor A1 (SR-A1), and SR-A2, are the principal receptors responsible for the binding and uptake of ox-LDL into macrophages (Yu et al., Citation2013a). Therefore, a drug that can regulate cholesterol flow and prevent cholesterol accumulation in macrophages may find promising utility in the treatment of atherogenesis.
Chrysin (5,7-dihydroxyflavone) is a flavonoid that is widely present in honey, propolis, and plant extracts (Ahad et al., Citation2014; Kandhare et al., Citation2014; Pietta, Citation2000). A bulk of studies have shown that chrysin possesses diverse pharmacological activities including antivirus (Wang et al., Citation2014), antioxidant (Sultana et al., Citation2012), anti-tumor (Yu et al., Citation2013b), anxiolytic (Zanoli et al., Citation2000), neuroprotection (Kandhare et al., Citation2014), and anti-diabetes (Ahad et al., Citation2014; Li et al., Citation2014). Various mechanisms are involved in chrysin-mediated health benefits such as attenuation of inflammation (Rehman et al., Citation2013), alleviation of oxidative stress (Mantawy et al., Citation2014), and induction of cell cycle arrest and apoptosis (Rashid et al., Citation2014). A recent report revealed that chrysin can inhibit inflammation through activation of PPARγ (Feng et al., Citation2014), a key regulator in glucose and lipid metabolism as well as in cholesterol efflux from macrophages. Furthermore, Anandhi et al. (Citation2014) indicated that chrysin significantly lowered the serum levels of total cholesterol, triglycerides, low-density, and very low-density lipoprotein cholesterol and exhibited anti-atherogenic potential in Wistar rats. In spite of such numbers of pharmacological activities of chrysin, further studies are needed to find out the dominated function and exact mechanism thereof.
The beneficial effects of chrysin on inflammation, antioxidant, and PPARγ expression make it a promising candidate for the prevention and treatment of atherosclerosis. However, the effect of chrysin on cholesterol flow and foam cell formation in macrophages has not been reported up to now. In this work, we performed an in vitro study investigating the effect of chrysin on foam cell formation and cholesterol flow in RAW264.7 macrophages. The potential mechanism of chrysin-stimulating cholesterol flow was explored. Our results provide evidence for the utility of chrysin for the prevention and treatment of atherosclerosis.
Materials and methods
Reagents
Chrysin was isolated from the fruits of Pandanus tectorius (purity > 98%) as previously reported (Wu et al., Citation2013b; Zhang et al., Citation2013). Lovastatin, 25-[N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-methyl]amino]-27-norcholesterol (25-NB D cholesterol), rosiglitazone, oil red O, and Dulbecco’s modified Eagle’s medium (DMEM) were procured from Sigma-Aldrich, Inc. (St Louis, MO). The intracellular cholesterol assay kit was purchased from Jian Cheng Biotechnology Company (Nanjing, China). Human ox-LDL and HDL were obtained from Yiyuan Biotechnologies (Guangzhou, China). A total RNA extraction reagent RNAiso Plus, a PrimeScript RT reagent kit, and a SYBR-Green PCR kit were purchased from Transgene Biotech, Inc. (Beijing, China).
Cell culture and cholesterol accumulation assay
RAW264.7 macrophages were originally obtained from the American Type Culture Collection (Manassas, VA) and cultured in DMEM containing 10% fetal bovine serum at 37 °C under 5% CO2. Cells were grown to 70–80% confluence and then treated with respective agents in indicated concentrations in serum-free DMEM containing ox-LDL (50 μg/mL) for 24 h. Cells were then washed three times and subjected to oil-red O staining or cholesterol determination as described previously (Wu et al., Citation2013a, Citation2014).
Cellular cholesterol efflux experiments
Cellular cholesterol efflux experiments were performed using 25-NBD-cholesterol in RAW264.7 macrophages. The cells were plated in 96-well clear-bottom black plates (Costar, Corning Inc., Corning, NY) at 4 × 104 cells/well. Six hours later, the medium was removed and the cells were labeled with 25-NBD-cholesterol (5 µg/mL) in serum-free DMEM for 24 h in a 37 °C, 5% CO2 incubator. After 24 h of labeling, cells were washed twice with PBS and incubated with 200 µL serum-free DMEM containing respective agents at indicated concentrations and HDL (50 µg/mL) for an additional 6 h. Then the amounts of cholesterol in medium and cells were measured using a Tecan Infinite M1000Pro Microplate Reader (TECAN Group Ltd, Shanghai, China; excitation 485 nm and emission 535 nm). The percentage of 25-NBD-cholesterol efflux was calculated as (medium)/(medium + cell) × 100%. Each efflux assay was performed in duplicate in three experiments.
Quantitative real-time PCR
The mRNA levels of cholesterol flow-related genes were determined by real-time quantitative PCR. Total RNA extraction, cDNA synthesis, and quantitative PCR assays were performed as described previously (Wu et al., Citation2013b). Samples were cycled 40 times using a Fast ABI-7500 Sequence Detector (Applied Biosystems, Foster City, CA). ABI-7500 conditions were as follows: 5 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 34 s at 60 °C, and 40 s at 72 °C. Cycle threshold (CT) was calculated under default setting for real-time sequence detection software (Applied Biosystems, Foster City, CA). At least three independent biological replicates were performed to check the reproducibility of the date. The gene-specific primers used for quantitative PCR are listed in .
Table 1. Primers used in real time quantitative PCR analysis.
25-NBD cholesterol uptake assay
Cholesterol uptake assay was performed using 25-NBD cholesterol in RAW264.7 macrophages. The cells were plated in 96-well clear-bottom black plates (Costar, Corning Inc., Corning, NY) at 4 × 104 cells/well. Six hours later, the medium was removed and the cells were labeled with 25-NBD-cholesterol (5 µg/mL) in serum-free DMEM containing indicated concentration of chrysin or equal volume of DMSO for indicated time. Then cells were washed twice with PBS and the amounts of cholesterol in cells were measured using a Tecan Infinite M1000Pro Microplate Reader (TECAN Group Ltd, Shanghai, China; excitation 485 nm and emission 535 nm). Each uptake assay was performed in duplicate in three experiments.
Luciferase assay
Cells were transiently transfected with PPARγ expression vector and DR-1 luciferase reporter vector. At 6 h after transfection, the transfection mixture was replaced with fresh medium containing the appropriate agonist. Luciferase assays were performed after 24 h using luciferase assay kit (Promega, Beijing, China) according to the instruction of the manufacturer.
Statistics analysis
The results were expressed as mean ± SD. A one-way analysis of variance (ANOVA) was done using SPSS13.0 software (SPSS Inc., Chicago, IL). Significance was accepted at p < 0.05.
Results
Chrysin inhibits foam cell formation induced by ox-LDL in RAW264.7 cells
The accumulation of foam cells (cholesterol-laden macrophages) in the arterial wall is an indication of plaque-build up or atherosclerosis (Ohashi et al., Citation2005). Therefore, we first assessed the inhibitive effect of chrysin on the formation of foam cell induced by ox-LDL. Enlargement of cell surface and accumulation of intracellular cholesterol are two signs of foam cell. As shown in , supplementation with ox-LDL largely increased the surface area of macrophages (control versus blank). Treatment with chrysin (10 µM) significantly decreased cell surface area. At the same time, supplementation with ox-LDL also resulted in an enormous accumulation of cholesterol in RAW264.7 macrophages (control versus blank) (). Treatment with chrysin significantly and dose dependently decreased intracellular cholesterol level with an efficiency comparable with the popular hypocholesterol drug lovastatin (), but weaker than rosiglitazone which can markedly stimulate cholesterol efflux (). These results suggest that chrysin efficiently prevents the formation of foam cells induced by ox-LDL in RAW264.7 macrophages.
Figure 1. The inhibitive effect of chrysin on foam cell formation induced by ox-LDL in RAW264.7 cells. (A) Typical photographs of RAW264.7 macrophages after oil-red O staining. Bar = 20 μm. (B) Cell surface area measured by Image-Pro Plus. Results are representative of four different experiments. ###p < 0.001 versus the blank group, **p < 0.01 versus the control group.
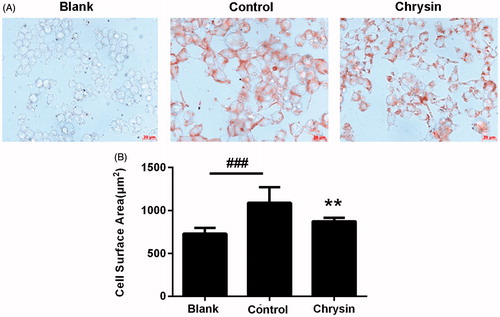
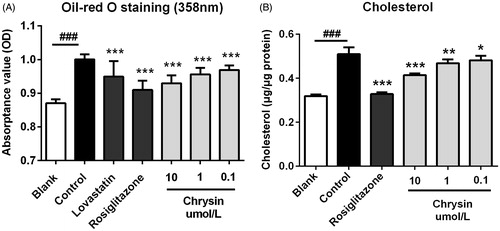
Figure 2. The inhibitive effect of chrysin on ox-LDL-elicited lipid accumulation in RAW264.7 cells. (A) Spectrophotometry at 358 nm of RAW264.7 macrophages after oil-red O staining. (B) Intracellular concentration of cholesterol. RAW264.7 cells were treated with lovastatin (1 μM), rosiglitazone (1 μM) or indicated concentration of chrysin in serum-free DMEM containing ox-LDL (50 μg/mL) for 24 h then subjected to oil red O staining and intracellular cholesterol quantification. Values represent mean ± SD. Results are representative of four different experiments. ###p < 0.001 versus the blank group, *p < 0.05, **p < 0.01, ***p < 0.001 versus the control group.
Chrysin enhances cholesterol efflux from RAW264.7 macrophages
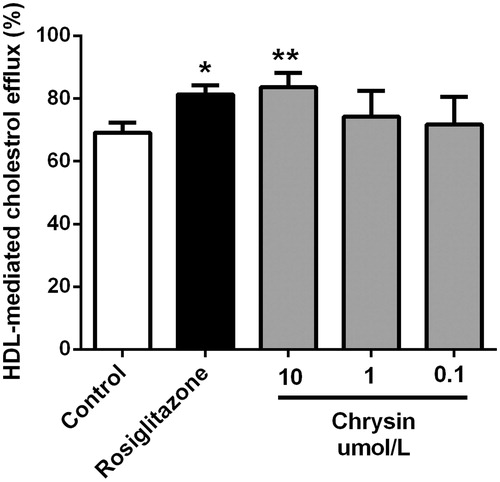
Cholesterol efflux from macrophages is the first and potentially most important step in macrophages reverse cholesterol transport (RCT) which has been demonstrated as a good anti-atherogenic strategy by accumulating evidence (Tall et al., Citation2008; Yvan-Charvet et al., Citation2010). We assessed the effect of chrysin on cholesterol efflux in RAW264.7 macrophages. As shown in , supplementation with rosiglitazone, a marketed cholesterol efflux stimulator, enormously increased HDL-mediated cholesterol efflux. Treatment with chrysin significantly and dose dependently increases cholesterol efflux with efficiency comparable with that of rosiglitazone. Chrysin showed higher cholesterol efflux rate than that of rosiglitazone (1 µM) at 10 µM but lower at 1 µM.
Figure 3. Chrysin enhances HDL-mediated cholesterol efflux from RAW264.7 macrophages. Cells were treated with rosiglitazone (1 μM) or indicated concentration of chrysin for 6 h after incubation with 25-NBD cholesterol for 24 h. Values represent mean ± SD. Results are representative of four different experiments. *p < 0.05, **p < 0.01 versus the control group.
Chrysin increases transcription of cholesterol efflux-associated genes
Real-time quantitative PCR showed that treatment with chrysin significantly increased the mRNA level of PPARγ, LXR, ABCA1, and ABCG1 (). At the same time, luciferase assay also demonstrated the stimulating effect of chrysin on the transcriptional activity of PPARγ (). Correspondingly, chrysin significantly enhanced cholesterol efflux mediated by HDL () but showed no effect on ApoA-1-mediated cholesterol efflux (data not shown).
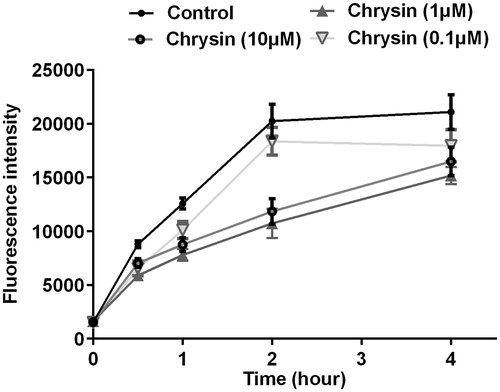
Chrysin inhibits ox-LDL uptake in RAW264.7 macrophages
To verify the effect of chrysin on cholesterol uptake, we performed cholesterol uptake assay using NBD-cholesterol as a fluorescence indicator. As shown in , chrysin significantly and dose dependently inhibits cholesterol uptake in RAW264.7 macrophages, and the strongest efficiency happened at 2 h after treated with chrysin.
Chrysin prevents transcription of cholesterol uptake-associated genes
Many critical scavenger receptors, especially CD36 (Silverstein, Citation2009), SR-A1, and SR-A2 (Webb et al., Citation1998), have the selective ability to uptake cholesterol from HDL or cells (Brodeur et al., Citation2008). As shown in , real-time quantitative PCR showed that treatment with chrysin significantly decreased the mRNA level of SR-A1 and SR-A2, while chrysin has no effect on CD36.
Discussion
Among chrysin pharmacological functions, anti-atherosclerosis-related effects and associated target spot have been presented (Anandhi et al., Citation2014; Feng et al., Citation2014). However, the exact mechanism of anti-atherogenic potential has not been uncovered. In this study, we investigated the effect of chrysin on foam cell formation, cholesterol accumulation, cholesterol efflux, and cholesterol uptake in RAW264.7 cells, which all play critical role in the occurrence and development of atherosclerosis.
The accumulation of foam cells (cholesterol-laden macrophages) in the arterial wall is an indication of plaque-build up or atherosclerosis. Therefore, we first assessed the inhibitive effect of chrysin on foam cells formation. Supplement with ox-LDL (50 µg/mL) can enormously elicit RAW264.7 macrophages transform into foam cells with their surface areas largely expand (). Treatment with chrysin (10 µM) significantly reduced the elevated surface area () with no influence on cell visibility in our previous studies (date not shown). This suggests that chrysin may be effective for the inhibiting foam cell formation induced by ox-LDL in RAW264.7 macrophage. It has been reported that chrysin (200 mg/kg) significantly lowered mean serum level of lipid profile parameters (total cholesterol, triglycerides, low-density, and very low-density lipoprotein cholesterol, except for HDL-cholesterol) in Wistar rats (Anandhi et al., Citation2014). Additionally, foam cell formation is closely related to intracellular cholesterol level, which inspired us to explore whether chrysin can lower intracellular cholesterol to prevent foam cells formation. So we test the intracellular cholesterol accumulation induced by ox-LDL in RAW264.7 macrophage. As shown in , treatment with chrysin significantly and dose dependently decreases intracellular cholesterol level with an efficiency comparable with the popular hypocholesterol drug lovastatin, but weaker than the marketed cholesterol efflux stimulator rosiglitazone, which suggest that chrysin can efficiently inhibit ox-LDL-induced cholesterol accumulation and thus prevent foam cell formation in RAW264.7 macrophages. As shown in , the inhibitive effect of chrysin on intracellular cholesterol is comparable with lovastatin. But in our previous studies, the cholesterol-lowering potency of chrysin is weaker than lovastatin (25.2% and 36.5%, both at 10 µM). These results suggest that decreasing ox-LDL-induced cholesterol accumulation in RAW264.7 cells may involve another mechanism rather than just hypolipidemic action only.
Stimulation of cholesterol efflux from macrophages is a most likely mechanism adding to the hypocholesterol activity of chrysin in preventing foam cell formation. As shown in , treatment with chrysin (10 µM) significantly increase cholesterol efflux mediated by HDL from RAW264.7 macrophages, which is superior to rosiglitazone (1 µM). But, we can see in , the inhibitive effect of chrysin on cholesterol accumulation is weaker than rosiglitazone. A reasonable explanation for this contradiction is that cholesterol-lowering activity and cholesterol efflux are at least in part, if not wholly, dependent on each other, which confirms our conjecture that cholesterol efflux is a mechanism adding to the hypochesterol activity in preventing foam cell formation. Among various regulating factors in cholesterol efflux from macrophages, ABCA1 and ABCG1 play a pivotal role (Liu et al., Citation2014). ABCA1 is regulated by a PPARγ-LXR pathway independently, and ABCG1 is regulated by a PPARγ-LXR pathway partially (Ozasa et al., Citation2011). ABCA1, ABCG1, and their transcriptional factors LXRα and PPARγ are essential regulators in cholesterol efflux in macrophages. It has been reported that chrysin could activate PPARγ (Feng et al., Citation2014). Therefore, we applied real-time PCR (RT-PCR) to analyze cholesterol efflux-associated genes, such as PPARγ, LXRα, ABCA1, and ABCG1. As shown in , chrysin significantly up-regulates the expression of PPARγ, LXRα, ABCA1, and ABCG1, and the transcription activity of PPARγ is correspondingly increased as determined by luciferase assay (). These data indicate that chrysin may promote cholesterol efflux from RAW264.7 macrophages through PPARγ–LXRα–ABCA1/ABCG1 pathway. Nevertheless, other influential factors on cholesterol efflux and ABCG1 regulation are needed to be clarified in the future.
The formation of foam cells is usually caused either by the uncontrolled uptake of cholesterol or by the impaired cholesterol efflux. Therefore, we also detected the effect of chrysin on cholesterol uptake. As shown in , chrysin time dependently and dose dependently inhibits cholesterol uptake in RAW264.7 macrophages, which can partially account for the reason of decreased cholesterol level (). However, chrysin significantly prevents cholesterol uptake and increases cholesterol efflux which is superior to rosiglitazone, while the cholesterol accumulation level is still bigger than rosiglitazone. Cholesterol endogenous synthesis might be the most reasonable explanation for this contradiction, which, however, needs to be further studied in the future. We can also conjecturable conclude that decreasing cholesterol accumulation, increasing cholesterol efflux, and preventing cholesterol uptake are dependent on each other and all of them make contribution to inhibiting foam cell formation. Cholesterol uptake by macrophages is regulated by surface scavenger receptors, especially SR-A and CD36, which control the majority (75–90%) of the modified LDL uptake in macrophages (Tomasini et al., Citation2013). Therefore, we applied RT-PCR to analyze the mRNA expression level of CD36, SR-A1, and SR-A2. The results showed that chrysin significantly prevents the transcription of SR-A1 and SR-A2, but has no effect on CD36 (). These dates indicate that chrysin inhibiting cholesterol may uptake through down-regulating the transcription of SR-A1 and SR-A2. However, the pathway of regulation on cholesterol uptake needs further study in the future.
Conclusions
This work demonstrated that chrysin isolated from the fruits of Pandanus tectorius is able to inhibit foam cell formation through inhibiting cholesterol accumulation induced by ox-LDL, stimulating cholesterol efflux, and preventing cholesterol uptake in RAW264.7 macrophages. Up-regulation of PPARγ–LXRα–ABCA1/ABCG1 pathway may involve in the promoting effect of chrysin on cholesterol efflux and down-regulation of SR-A1 and SR-A2 may contribute to the inhibitive effect of chrysin on cholesterol uptake. These results provide in vitro evidence that chrysin and chrysin-containing fruit like Pandanus tectorius can be favorably used for the prevention and treatment of atherosclerosis.
Declaration of interest
The authors declare that they have no conflicts of interest. The financial assistance given by the Natural Sciences Foundation of China (Nos. 81402983 and 81000135) is gratefully acknowledged.
References
- Ahad A, Ganai AA, Mujeeb M, et al. (2014). Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol 279:1–7
- Anandhi R, Thomas PA, Geraldine P. (2014). Evaluation of the anti-atherogenic potential of chrysin in Wistar rats. Mol Cell Biochem 385:103–13
- Brodeur MR, Brissette L, Falstrault L, et al. (2008). Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J Bone Miner Res 23:326–37
- Dean M, Hamon Y, Chimin G. (2001). The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res 42:1007–17
- Feng X, Qin H, Shi Q, et al. (2014). Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARgamma. Biochem Pharmacol 89:503–14
- Glass CK, Witztum JL. (2001). Atherosclerosis: The road ahead. Cell 104:503–16
- Kandhare AD, Shivakumar V, Rajmane A, et al. (2014). Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med 68:586–603
- Li R, Zang A, Zhang L, et al. (2014). Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol Sci 35:1527–32
- Liu N, Wu C, Sun L, et al. (2014). Sesamin enhances cholesterol efflux in RAW264.7 macrophages. Molecules 19:7516–27
- Mantawy EM, El-Bakly WM, Esmat A, et al. (2014). Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–18
- Ohashi R, Mu H, Wang X, et al. (2005). Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 98:845–56
- Ozasa H, Ayaori M, Iizuka M, et al. (2011). Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARgamma/LXRalpha pathway: Findings from in vitro and ex vivo studies. Atherosclerosis 219:141–50
- Pennings M, Meurs I, Ye D, et al. (2006). Regulation of cholesterol homeostasis in macrophages and consequences for atherosclerotic lesion development. FEBS Lett 580:5588–96
- Pietta PG. (2000). Flavonoids as antioxidants. J Nat Prod 63:1035–42
- Rashid S, Ali N, Nafees S, et al. (2014). Mitigation of 5-fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in Wistar rats. Food Chem Toxicol 66:185–93
- Rehman MU, Tahir M, Khan AQ, et al. (2013). Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: Plausible role of NF-kappaB. Toxicol Lett 216:146–58
- Reiss AB, Cronstein BN. (2012). Regulation of foam cells by adenosine. Arterioscler Thromb Vasc Biol 32:879–86
- Silverstein RL. (2009). Inflammation, atherosclerosis, and arterial thrombosis: Role of the scavenger receptor CD36. Cleve Clin J Med 76:S27–30
- Sultana S, Verma K, Khan R. (2012). Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol 64:872–81
- Tabas I. (2004). Apoptosis and plaque destabilization in atherosclerosis: The role of macrophage apoptosis induced by cholesterol. Cell Death Differ 11:12–16
- Tall AR, Charvet L, Terasaka N, et al. (2008). HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab 7:365–75
- Tomasini MD, Zablocki K, Petersen LK, et al. (2013). Coarse grained molecular dynamics of engineered macromolecules for the inhibition of oxidized low-density lipoprotein uptake by macrophage scavenger receptors. Biomacromolecules 14:2499–509
- Wang J, Zhang T, Du J, et al. (2014). Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS One 9:e89668
- Webb NR, Connell PM, Graf GA, et al. (1998). SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J Biol Chem 273:15241–8
- Wu C, Guo Y, Su Y, et al. (2014). Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the gamma1 subunit. J Cell Mol Med 18:293–304
- Wu C, Luan H, Wang S, et al. (2013a). Modulation of lipogenesis and glucose consumption in HepG2 cells and C2C12 myotubes by sophoricoside. Molecules 18:15624–35
- Wu C, Zhang X, Zhang X, et al. (2013b). The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J Nutr Biochem 25:412–19
- Yu XH, Fu YC, Zhang DW, et al. (2013a). Foam cells in atherosclerosis. Clin Chim Acta 424:245–52
- Yu XM, Phan T, Patel PN, et al. (2013b). Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 119:774–81
- Yvan CL, Wang N, Tall AR. (2010). Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 30:139–43
- Zanoli P, Avallone R, Baraldi M. (2000). Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 71:117–23
- Zhang M, Wu JF, Chen WJ, et al. (2014). MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 234:54–64
- Zhang X, Wu C, Wu H, et al. (2013). Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS One 8:e61922